COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress †
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis: SARS-CoV-2 Infection
2.2. Laboratory-Based Analyses: Antibodies and Inhibitors
2.2.1. Primary Antibodies
2.2.2. Secondary Antibodies
2.2.3. Inhibitors
2.3. Cell Culture
2.4. Immunocytochemistry
2.5. Western Blot Analysis
2.6. MTT Assay
2.7. TUNEL Assay
2.8. Detection and Statistical Analysis
3. Results
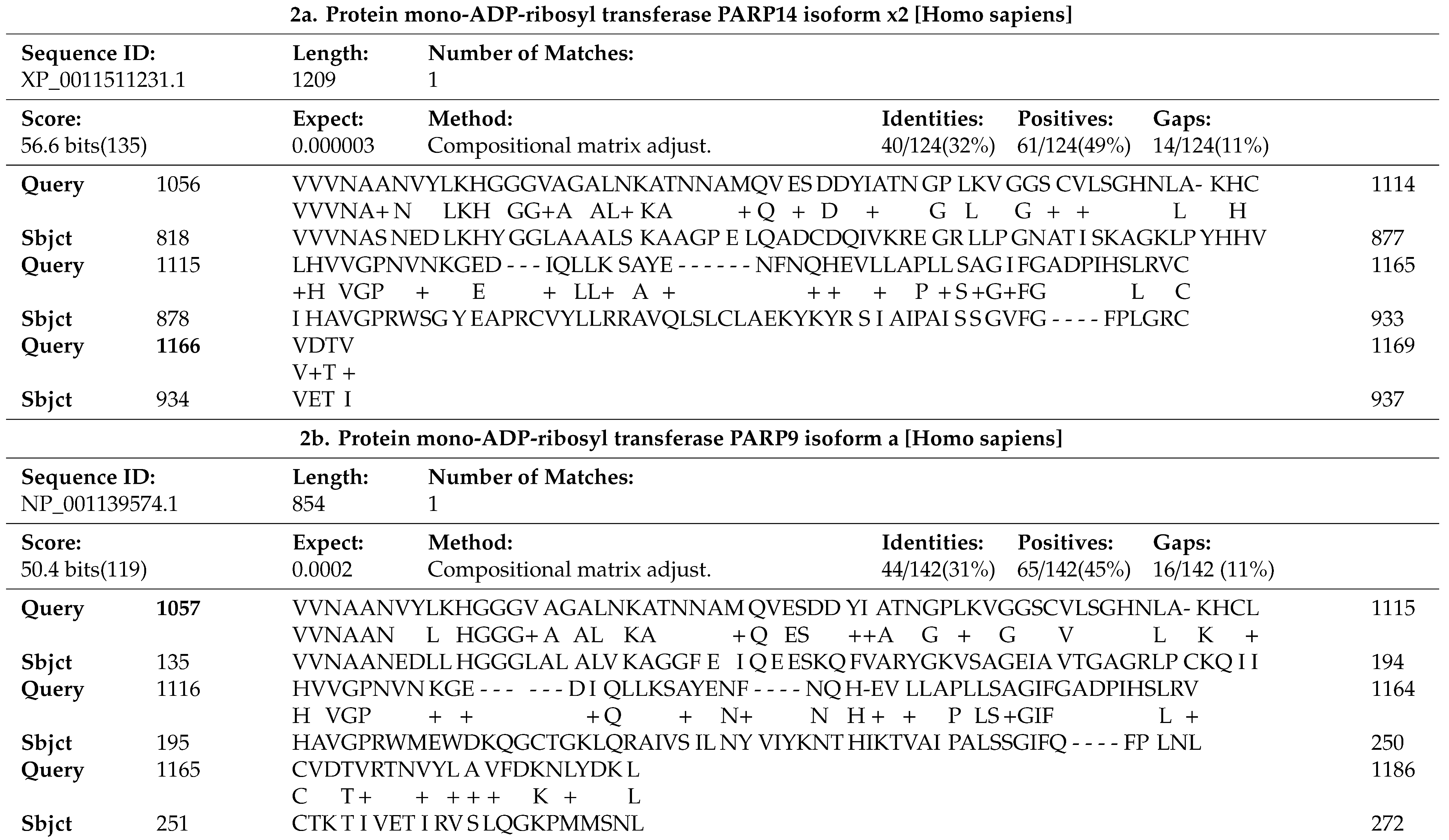
3.1. SARS-CoV-2 Infection Induces Pro-Apoptotic Responses
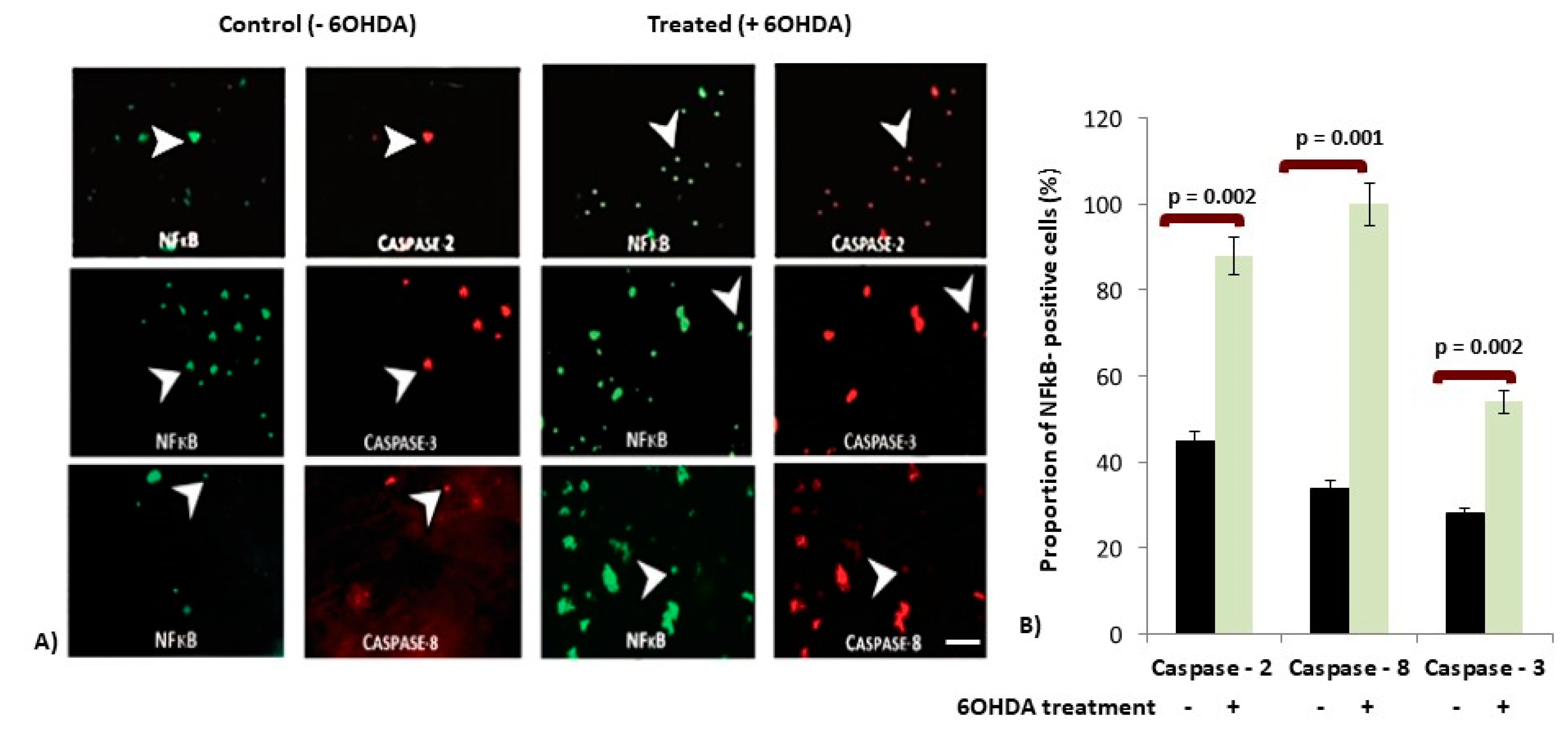
3.2. 6OHDA-Induced Toxicity Amplifies Activation of NFκB in dDCNs
3.3. Increased Expression of Caspases in p65-NFκB Expressed dDCNs Following 6OHDA-Induced Stress
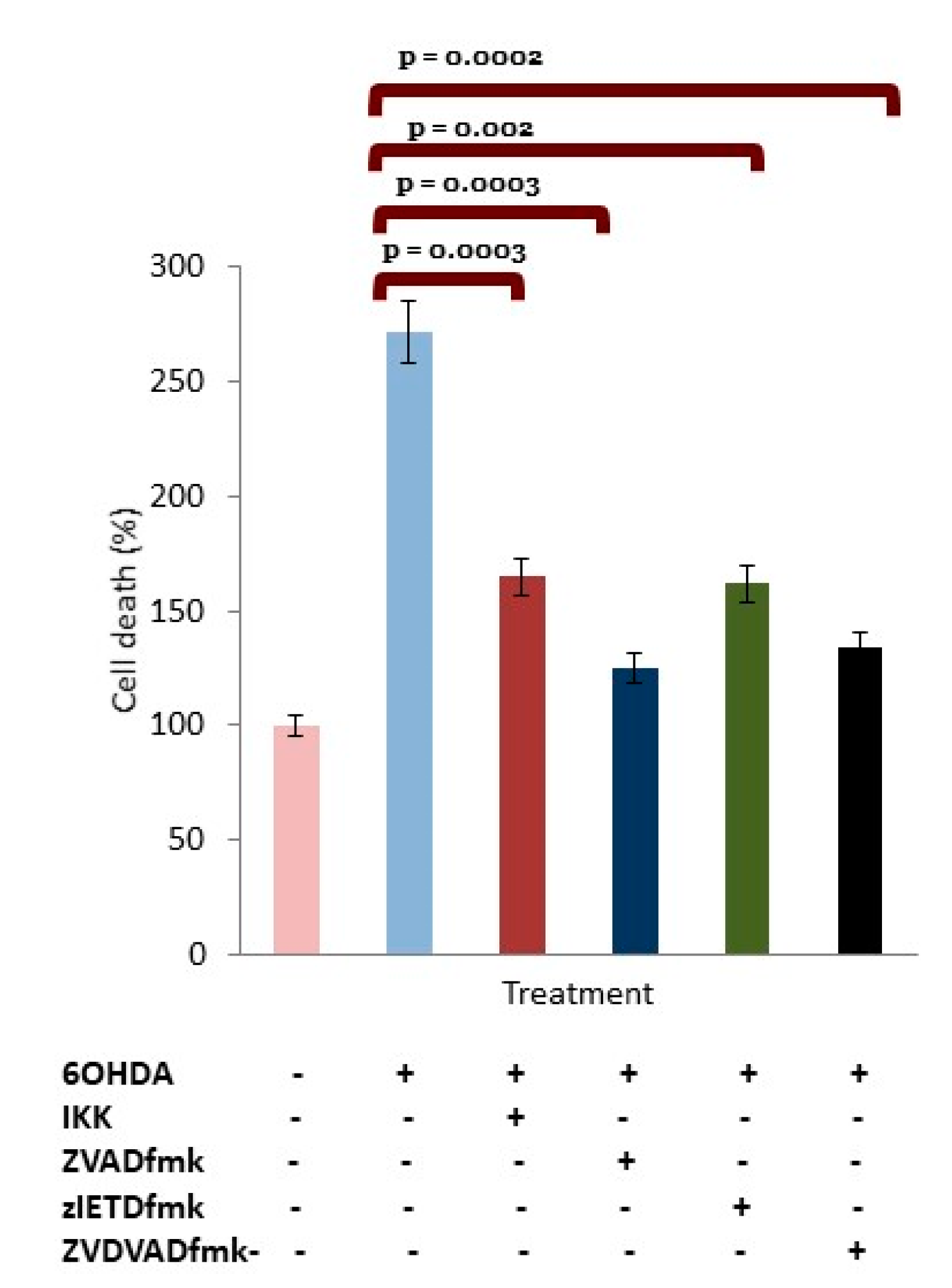
3.4. IKK Suppressed Activation of p65-NFκB in 6OHDA-Treated dDCNs
3.5. OHDA Provokes Apoptotic Death in dDCNs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Nile, S.H.; Arti Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020, 53, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Murakami, M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Chen, S.; Ye, J.; Chen, X.; Shi, J.; Wu, W.; Lin, W.; Lin, W.; Li, Y.; Fu, H.; Li, S. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J. Neuroinflamm. 2018, 15, 150–164. [Google Scholar] [CrossRef]
- Sinkovics, J.G. The cnidarian origin of the proto-oncogenes NF-κB/STAT and WNT-like oncogenic pathway drives the ctenophores. Int. J. Oncol. 2015, 47, 1211–1229. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.; Gogulamudi, V.R.; Periasamy, R.; Raghavaraju, G.; Subramanian, U.; Pandey, K.N. Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am. J. Physiol. Ren. Physiol. 2017, 313, F781–F795. [Google Scholar] [CrossRef] [PubMed]
- Claverie, J.M. A Putative Role of de-Mono-ADP-Ribosylation of STAT1 by the SARS-CoV-2 Nsp3 Protein in the Cytokine Storm Syndrome of COVID-19. Viruses 2020, 12, 646. [Google Scholar] [CrossRef]
- Cha, B.; Lim, J.W.; Kim, H. Jak1/Stat3 is an upstream signaling of NF-κB activation in Helicobacter pylori-induced IL-8 production in gastric epithelial AGS cells. Yonsei Med J. 2015, 56, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Misra, H.P. Reactive oxygen species in in vitro pesticide-induced neuronal cell (SH-SY5Y) cytotoxicity: Role of NFκB and caspase-3. Free Radic. Biol. Med. 2007, 42, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Tornatore, L.; Thotakura, A.K.; Bennett, J.; Moretti, M.; Franzoso, G. The nuclear factor kappa B signaling pathway: Integrating metabolism with inflammation. Trends Cell Biol. 2012, 22, 557–566. [Google Scholar] [CrossRef]
- Flood, P.M.; Qian, L.; Peterson, L.J.; Zhang, F.; Shi, J.S.; Gao, H.M.; Hong, J.S. Transcriptional factor NF-κB as a target for therapy in Parkinson’s disease. Parkinson’s Dis. 2011, 2011, 216298. [Google Scholar] [CrossRef]
- Midwinter, R.; Cheah, F.; Moskovitz, J.; Vissers, M.; Winterbourn, C. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: Inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem. J. 2006, 396, 71–78. [Google Scholar] [CrossRef]
- Krappmann, D.; Scheidereit, C. A pervasive role of ubiquitin conjugation in activation and termination of IkappaB kinase pathways. EMBO Rep. 2005, 6, 321–326. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Dong, S.; Liu, P.; Luo, Y.; Cui, Y.; Song, L.; Chen, Y. Pathophysiology of SARS-CoV-2 infection in patients with intracerebral hemorrhage. Aging 2020, 12, 13791. [Google Scholar] [CrossRef]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmannm, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Izquierdo-Álvareza, A.; Sánchez Gómez, F.J.; Ramos, E.; Villa-Piña, T.; Lamas, S.; Bogdanovae, A.; Martínez-Ruiz, A. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014, 71, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Niizuma, K.; Endo, H.; Chan, P.H. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurosci. 2009, 109 (Suppl. S1), S133–S138. [Google Scholar] [CrossRef]
- Gazewood, J.D.; Richards, D.R.; Clebak, K. Parkinson disease: An update. Am. Fam. Physician 2013, 87, 267–273. [Google Scholar] [PubMed]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Okami, N.; Chan, P.H. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochimica Biophysica Acta 2010, 1802, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochimica Biophysica Acta 2009, 1787, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory pattern and phrenic and hypoglossal nerve activity during normoxia and hypoxia in 6-OHDA-induced bilateral model of Parkinson’s disease. 25. J. Physiol. Sci. 2020, 70, 16. [Google Scholar] [CrossRef]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s disease in rats: An evaluation of 6-OHDA lesions of the Nigrostriatal pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef]
- Prieto-Lloret, J.; Donnelly, D.F.; Rico, A.J.; Moratalla, R.; González, C.; Rigual, R.J. Hypoxia transduction by carotid body chemoreceptors in mice lacking dopamine D(2) receptors. J. Appl. Physiol. 2007, 103, 1269–1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tirmenstein, M.A.; Hu, C.X.; Scicchitano, M.S.; Narayanan, P.K.; McFarland, D.C.; Thomas, H.C.; Schwartz, L.W. Effects of 6-hydroxydopamine on mitochondrial function and glutathione status in SH-SY5Y human neuroblastoma cells. Toxicol. In Vitro 2005, 19, 471–479. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Glinka, Y.Y.; Youdium, M.B. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur. J. Pharmacol. 1995, 292, 329–332. [Google Scholar] [CrossRef]
- Lalley, P.M. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1829–R1836. [Google Scholar] [CrossRef]
- Baille, G.; De Jesus’, A.M.; Perez, T.; Devos, D.; Dujardin, K.; Charley, C.M.; Defebvre, L.; Moreau, C. Ventilatory dysfunction in Parkinson’s disease. J. Parkinson’s Dis. 2016, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Seccombe, L.M.; Giddings, H.L.; Rogers, P.G.; Corbett, A.J.; Hayes, M.W.; Peters, M.J.; Veitch, E.M. Abnormal ventilatory control in Parkinson’s disease- further evidence for non-motor dysfunction. Respir. Physiol. Neurobiol. 2011, 179, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kalivendi, S.V.; Yedlapudi, D.; Hillard, C.J.; Kalyanaraman, B. Oxidants induce alternative splicing of α-synuclein: Implications for Parkinson’s disease. Free Radic. Biol. Med. 2010, 48, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Deng, F.; Zhang, W.; Ji, J.; Liu, J.; Sun, Y.; Hu, J. Tectorigenin attenuates the MPP+-induced SH-SY5Y cell damage, indicating a potential beneficial role in Parkinson’s disease by oxidative stress inhibition. Exp. Ther. Med. 2017, 14, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Paek, S.H. Mitochondrial dysfunction in Parkinson’s disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Halvorsen, E.M.; Swerdlow, R.H.; Abramova, N.N.; Parker, W.D., Jr.; Sturgill, T.W.; Bennett, J.P., Jr. Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson’s disease. J. Neurochem. 2000, 74, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Z.L.; Ahmed, B.Y. The role of Caspase in Parkinson’s disease pathogenesis: A brief look at the mitochondrial pathway. Austin J. Alzheimer’s Parkinson’s Dis. 2014, 4, 1–5. [Google Scholar]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef]
- Erekat, N.S.; Al-Jarrah, M.D. Association of Parkinson disease induction with cardiac upregulation of apoptotic mediators P53 and active caspase-3: An immunohistochemistry study. Med. Sci. Monit. Basic Res. 2018, 24, 120. [Google Scholar] [CrossRef]
- Ghosh, A.; Roy, A.; Liu, X.; Kordower, J.H.; Mufson, E.J.; Hartley, D.M.; Ghosh, S.; Mosley, R.L.; Gendelman, H.E.; Pahan, K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 18754–18759. [Google Scholar] [CrossRef]
- Henn, I.H.; Bouman, L.; Schlehe, J.S.; Schlierf, A.; Schramm, J.E.; Wegener, E.; Nakaso, K.; Culmsee, C.; Berninger, B.; Krappmann, D.; et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappa B signalling. J. Neurosci. 2007, 27, 1868–1878. [Google Scholar] [CrossRef]
- Xiang, B.; Fei, X.; Zhuang, W.; Fang, Y.; Qin, Z.; Liang, Z. Cathepsin L is involved in 6-hydroxydopamine induced apoptosis of SH-SY5Y neuroblastoma cells. Brain Res. 2011, 1387, 29–38. [Google Scholar] [CrossRef]
- Andersen, J.K. Does neuronal loss in Parkinson’s disease involve programmed cell death? Bioessays 2001, 23, 640–646. [Google Scholar] [CrossRef]
- Cookson, M.R. The Biochemistry of Parkinson’s Disease. Annu. Rev. Biochem. 2005, 74, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Hyun, Y.J.; Kim, H.M.; Ryu, Y.S.; Hyun, J.W. Shikonin exerts cytotoxic effects in human colon cancers by inducing apoptotic cell death via the endoplasmic reticulum and mitochondria-mediated pathways. Biomol. Ther. 2019, 27, 41–47. [Google Scholar] [CrossRef] [PubMed]
- McStay, G.P.; Green, D.R. Measuring apoptosis: Caspase inhibitors and activity assays. Cold Spring Harb. Protoc. 2014, 2014, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Van-Raam, B.J.; Salvesen, G.S. Proliferative versus apoptotic functions of caspase-8 Hetero or homo: The caspase-8 dimer controls cell fate. Biochimica Biophysica Acta 2012, 824, 113–122. [Google Scholar] [CrossRef]
- Mehta, K.J.; Ahmed, B.Y.; Farnaud, S.J.C. A novel human neuronal cell model to study iron accumulation in Parkinson’s disease. J. Alzheimer’s Dis. Parkinsonism 2019, 9461. [Google Scholar] [CrossRef]
- Ahmed, B.Y.; Hasnain, O.; Satfford, R.; Gujar, A.; Sihotra, S.; Howard, M.; Moradiya, V.; Patel, K. Hyperphosphorylation of CREB induced by 6 OHDA treatment in human dopaminergic neurons: A kinetic study of distribution of tCREB and pCREB following oxidative stress. Neuroreport 2013, 24, 757–762. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Iwata, H.; Goettsch, C.; Sharma, A.; Ricchiuto, P.; Goh, W.W.; Halu, A.; Yamada, I.; Yoshida, H.; Hara, T.; Wei, M.; et al. PARP9 and PARP14 cross-regulate macrophage activation via STAT1 ADP-ribosylation. Nat. Commun. 2016, 7, 12849. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, D.; Roswit, W.T.; Jin, X.; Patel, A.C.; Patel, D.A.; Agapov, E.; Wang, Z.; Tidwell, R.M.; Atkinson, J.J.; et al. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nature Immunol. 2015, 16, 1215–1227. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. IFNγ sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene 2002, 21, 2295–2308. [Google Scholar] [CrossRef]
- Apelbaum, A.; Yarden, G.; Warszawski, S.; Harari, D.; Schreiber, G. Type I interferons induce apoptosis by balancing cFLIP and caspase-8 independent of death ligands. Mol. Cell. Biol. 2013, 33, 800–814. [Google Scholar] [CrossRef]
- Sironi, J.J.; Ouchi, T. STAT1-induced apoptosis is mediated by caspase 2, 3, and 7. J. Biol. Chem. 2004, 279, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shu, T.; Wu, D.; Mu, J.; Wang, C.; Huang, M.; Han, Y.; Zhang, X.Y.; Zhou, W.; Qiu, Y.; et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020, 8, 881–883. [Google Scholar] [CrossRef]
- Butturini, E.; Boriero, D.; Carcereri de Prati, A.; Mariotto, S. STAT1 drives M1 microglia activation and neuroinflammation under Hypoxia. Arch. Biochem. Biophys. 2019, 669, 22–30. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, D.-J.; Jeong, H.-K.; Kim, J.; Kim, D.W.; Choi, S.Y.; Park, S.-M.; Suh, Y.H.; Ilo, J.; Joe, E.-H. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 2013, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Park, E.J.; Joe, E.-H.; Jou, I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J. Biol. Chem. 2002, 277, 40594–40601. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Ziemiecki, A.; Wilks, A.F.; Harpur, A.G.; Sadowski, H.B.; Gilman, M.Z.; Darnell, J.E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature 1993, 366, 580–583. [Google Scholar] [CrossRef]
- Venderova, K.; Park, D.S. Programmed cell death in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Jiang, H.; He, P.; Adler, C.H.; Shill, H.; Beach, T.G.; Li, R.; Shen, Y. Bid signal pathway components are identified in the temporal cortex with Parkinson disease. Neurology 2012, 79, 1767–1773. [Google Scholar] [CrossRef]
- Masumoto, J.; Dowds, T.A.; Schaner, P.; Chen, F.F.; Ogura, Y.; Li, M.; Zhu, L.; Katsuyama, T.; Sagara, J.; Taniguchi, S.; et al. ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem. Biophys. Res. Commun. 2003, 303, 69–73. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Z.; Wei, J.; Lu, L.; Huang, Y.; Luo, L.; Xie, H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci. Lett. 2013, 553, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Festjens, N.; Declercq, W.; Vanden-Berghe, T.; Vandenabeele, P. Caspase in cell survival, proliferation and differentiation. Cell Death Differ. 2007, 14, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Rideout, H.J.; Ribe, E.; Troy, C.M.; Dauer, W.T. The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration. J. Neurosci. 2009, 29, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- De Erausquin, G.A.; Hyrc, K.; Dorsey, D.A.; Mamah, D.; Dokucu, M.; Masco, D.H.; Walton, T.; Dikranian, K.; Soriano, M.; Garcia-Verdugo, J.M.; et al. Nuclear translocation of nuclear transcription factor-kappa B by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors leads to transcription of p53 and cell death in dopaminergic neurons. Mol. Pharmacol. 2003, 63, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, M.; Miyazaki, I.; Diaz-Corrales, F.J.; Ogawa, N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic parkinson’s disease and neurotoxin-induced parkinsonism. Acta Medica Okayama 2004, 58, 221–234. [Google Scholar]



| Primary Antibody | Secondary Antibody |
|---|---|
| anti-NFκB-p65 (1:5000) | donkey anti-mouse IgG-HRP (1:6000) |
| anti-caspase-2 antibody (1:2500) | goat anti-rabbit IgG-HRP (1:1000) |
| anti-caspase-8 antibody (1:1000) | goat anti-rabbit IgG-HRP (1:1000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhry, Z.L.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B.Y. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sci. 2020, 10, 807. https://doi.org/10.3390/brainsci10110807
Chaudhry ZL, Klenja D, Janjua N, Cami-Kobeci G, Ahmed BY. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sciences. 2020; 10(11):807. https://doi.org/10.3390/brainsci10110807
Chicago/Turabian StyleChaudhry, Zahara L., Donika Klenja, Najma Janjua, Gerta Cami-Kobeci, and Bushra Y. Ahmed. 2020. "COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress" Brain Sciences 10, no. 11: 807. https://doi.org/10.3390/brainsci10110807
APA StyleChaudhry, Z. L., Klenja, D., Janjua, N., Cami-Kobeci, G., & Ahmed, B. Y. (2020). COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sciences, 10(11), 807. https://doi.org/10.3390/brainsci10110807




