Abstract
Olive micropropagation is nowadays possible but knowing if it induces juvenile traits and how juvenility, vigor and fruit productivity are affected is pivotal. Three trials were carried out during micropropagation and afterwards in the field. Three varieties were characterized during multiplication in vitro, after several subcultures. ‘Arbequina’ revealed higher shoot miniaturization than ‘Coratina’ and ‘Frantoio’, and likely-juvenile shoots with three or four leaves per node. The ‘Arbequina’ trees obtained from two- and three-leaves-per-node in vitro plantlets were compared to cuttings in the field. Two years after planting, flower-differentiated shoots were found in the apical part of the canopy in all tested trees while in this position the ramification was more intense on three-leaves-per-node trees. Architecture of ‘Arbequina’ trees from micropropagation and cuttings was finally characterized in a high-density commercial grove. Micropropagated trees showed a well distributed and deep root system, a regular conical shape of the canopy, a higher number of primary branches, and a reproductive ability equivalent to cuttings. In conclusion, some juvenile traits and vigor may appear in vitro and last after ex vitro acclimation, but no more than two years in the field.
1. Introduction
1.1. Intensification of Olive Cultivation
Olive (Olea europaea L.) is one of the oldest and most important fruit tree species cultivated in the Mediterranean region. According to the International Olive Council [1], olive oil production amounts to about 3.2 Mt/year worldwide, of which 2.3 Mt are produced in Europe. Olive production according to FAO [2] is classified as follows; world 20.9 Mt, EU 12.9 Mt of which, Spain 6.5 Mt, Greece 2.7 Mt, Italy 2.6 Mt, and Portugal 0.8 Mt. However, traditional olive groves are becoming less competitive in Europe due to the lack of mechanization matched by a high demand for intensive labor. For these reasons, in the four leading EU countries, Spain, Italy, Greece, and Portugal, olives have been planted as high-density hedgerow systems (also known as super-high-density orchards, SHDO) since the early 1990s [3]. Currently, these represent the most common irrigated orchard design in new plantings. They rely on mechanization and increased trees density, spreading exponentially, to accommodate an increasing olive oil and olive consumption in Europe and in several countries in the world (3% increase every year). In SHDO, tree size must be limited without altering leaves exposure to sunlight [4], while harvesting and pruning are managed on hedgerows and not on individual widely spaced trees as in traditional systems. Therefore, proper balance between vegetative and reproductive growth such as high ramification and a consistent rate of flowers’ differentiation are required standards to predict success of high-density olive plantations also according to the cultivar’s characteristics [5,6,7,8].
Successful mechanical harvest depends on trees’ shape/architecture [9,10,11,12,13,14] shoot growth and architecture [10,15,16], selective pruning integrated with mechanical topping, hedging and trimming [17,18,19,20,21] with branch renovation near the scaffold [22], and a specific control of irrigation and nutrition [23]. In addition, in high-density olive groves, an early onset of production is related to a rapid achievement of the continuity of vegetation along the row (hedgerow systems), with mature reproductive trees of small dimensions. Therefore, during the very rapid training period, vigorous growth on a single axis and a strong ramification on the primary branches are encouraged to maintain a conical hierarchy, which is accomplished by limiting pruning to a minimum. From the 4–5th year, after the first abundant productions, the canopy is flattened as espalier until the individual shape of the tree blends in with the hedgerow of reduced vigor and dimensions, making it compatible with the use of over-the-row harvesting machines. In addition, the development of an early strong root architecture can be pivotal to obtain an efficient management of SHDO [24,25].
The risk of a long unproductive period combined with the possibility to apply in continuous mechanical harvest are important concerns in high-density olive groves, as very early, abundant, and consistent fruit productions are the main objectives, at this time. Production of olive plants in nurseries is nowadays possible via micropropagation. Consequently, it is very important to know whether or not micropropagated trees express juvenile traits, how long juvenility lasts, and whether this may reduce the potential of fruit productivity and the vigor of micropropagated olive trees.
1.2. Micropropagation
Rootstocks and deciduous fruit tree species are extensively produced through tissue culture (micropropagation) in Europe [26]. The collaboration with research laboratories has been effective to develop innovations in micropropagation such as the search for new growth regulators, antibiotics, natural biocides, techniques of sterilization, disposable and gas-permeable containers, and culture systems. This has achieved remarkable outcomes about the morphogenetic effect of light, as well as rooting, acclimation and conservation of stock cultures at low temperatures, for several fruit tree species, including olive too, with a high number of cultivars that showed an easy-to-medium suitability to in vitro growing conditions [27].
Nevertheless, because of the long juvenility which naturally occurs in seedlings of this species, it is important to verify the value of micropropagation on this unproductive growth stage and describe possible epigenetic variations in micropropagated olive trees. In strawberries, the epigenetic differences induced by modern in vitro propagation were very limited [28], while in other species the stress of in vitro culture may induce more variability [29]. On tree species, the influence of in vitro multiplication in some specific cases may induce a temporary and greater vegetative behavior; rarely, it may induce a full rejuvenation, or even somaclonal variability, with the appearance of specific juvenile traits and delay of fruit production [30]. It is well known that, during the first years of growth in the field, the origin of the material or the propagation technique can affect the development and the reproductive attitude of the plant [31]. For olive trees, the use of plantlets from ovules and from suckers can delay the onset of fruit production [32].
The role of the in vitro permanence of olive and the number of subcultures on the ex vitro phenotypic behavior is still being debated. Hence, the need to verify the behavior in the first years after planting of the olive varieties suitable for high-density cultivation in response to micropropagation, describing the impact of the multiplication technique on the tree architecture and the onset of fruit production compared with trees propagated from cuttings.
1.3. Juvenility
Juvenility is a rigid physiological stage in the life cycle of seedlings. In this period, this may last even several years, the plant is characterized by the inability to induce flower formation, nor bear fruits, while showing a strong tendency for vigorous, vegetative growth [33]. During juvenility, meristems acquire reproductive capacity and become able to respond to the signals that induce flowering [34,35]. In olive, juvenile trees differ both morphologically and physiologically from mature ones, showing specific characteristics that are well recognizable: vigorous and irregular growth of the shoot with variable, internodal length; variable number of axillary meristems per node and leaves per node (greater than two); small, thickened, darker green and rounded leaves, when compared to the leaves of mature branches [36].
These morphological traits affect the plant during the first years of seedling development (juvenility and/or epigenetic effects), but they can also occur from adventitious meristems on specific portions of mature plants. As a consequence, juvenile traits and vegetative vigor are erroneously used as synonyms because of the common morphological characteristics, yet these terms possess different meanings.
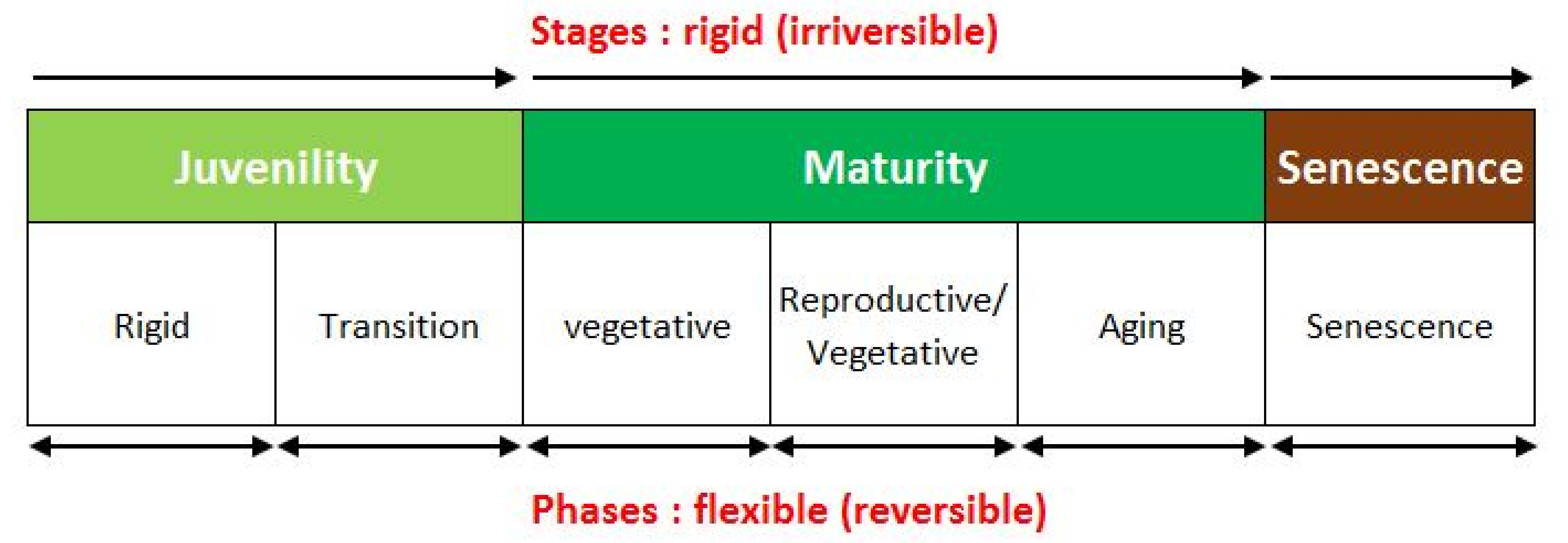
From the physiological point of view (Figure 1), the stages of juvenility, maturity, and senescence are considered rigid, while the different phases within the individual stages (in maturity: vegetative, vegetative-reproductive balance and senility) appear flexible and reversible (phenotypic plasticity).
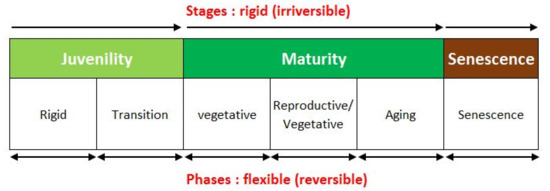
Figure 1.
Physiological ontogenetic stages of the olive trees.
In olive, juvenility is a marked and persistent phenomenon during the whole life cycle of the tree. The reappearance of juvenile traits on micropropagated plants seems to be linked to in vitro acclimation, and, in olive trees, it is still an open problem. However, the hypothesis that micropropagation in olive always induces a regression to a juvenile stage with a delay for the onset of fruit production in the field is unfounded, whereas it has been demonstrated that this delay may be strongly linked to the physiological stage of the starting point material (Zuccherelli, personal communication).
The transition to the stage of physiological maturity does not refer to a fixed mass of the tree, but to a threshold value defined by the shoot-to-root ratio and by the epigeous-hypogeous transport, called critical mass [37,38]. Once this physiological threshold is exceeded, the plant sets its fruits, regardless of its physical size and age [39].
Some authors [40] reported that ’Maurino’ micropropagated trees did not show any difference in comparison to self-rooted in vivo plants (by cuttings) in their architecture and morphology after plantation in the field. In literature [41], it was observed that micropropagated plants of ‘Nocellara etnea’ and ‘Carolea’ did not exhibit juvenile traits, showing a short unproductive period (set of production in the second and third growing season in the field, respectively). Acclimated plants of ‘Frantoio’, ‘Canino’, ‘Leccino’, and ‘Moraiolo’ were able to differentiate flowers even during their in-pot growth [42]. From the tests carried out in different experimental studies [24,25,43,44,45], it can be deduced that trees propagated in vitro do not have a rigid juvenile phase, amid delaying fruit production. In fact, micropropagated plants delay the onset of flowering capacity by only one year. These results showed that the micropropagated trees did not regress to the juvenile stage, but only to a higher level of vigor that ensures a very rapid growth in the post-implant phase with times of onset into production, comparable to those propagated with cuttings.
The purpose of our study consisted of understanding if micropropagation in olives induces a regression to juvenility. For this reason, three consequential trials were carried out to check the presence of morphological juvenile traits in an early stage of in vitro propagation, their evolution during the growth in the field, and the differences with trees obtained from cutting in terms of architecture and fruit production.
2. Materials and Methods
The three experimental trials were carried out during in vitro olive propagation and during the first years of growth after plantation in the field of the in vitro propagated olive trees, according to the specific methodologies described below.
2.1. Trial 1—‘Arbequina’, ‘Coratina’ and ‘Frantoio’ Varieties Multiplied In Vitro for Several Years
Biometric measurements of micropropagated plantlets of three cultivars (‘Arbequina’, ‘Coratina’ and ‘Frantoio’) coming from different explant dates (2000, 2007, 2010) were executed on 2012 to identify morphological traits easily recognizable for a possible early selection according to juvenility/vigor level. Plantlets were produced by the company Vitroplant S.p.A. of Cesena (Italy) according to the conventional protocol used for the in vitro propagation of the olive and using nodal explants with vegetative buds collected during vegetative growth [27]. The assessed plantlets, for each explant dates, were originated from the 7th, 5th, and 4th subculture per tested cultivar.
Basal diameter and total length, number of nodes, number of leaves per node, the presence of anticipated shoots, and fresh and dry weights were measured per each elongated shoot after multiplication phase, before rooting [46] (Figure 2).

Figure 2.
‘Arbequina’ shoots from micropropagation during the elongation phase.
For the measurement of the basal diameter, total length and fresh and dry weights a micrometric caliper, a manual ruler and a precision balance were used. One-way ANOVA and the Tukey–Kramer HSD post-hoc test (p ≤ 0.05) were performed by using JMP software (Release 8; SAS Institute Inc., Cary, NC, USA, 2009).
2.2. Trial 2—Orchard Establishment with ‘Arbequina’ Trees
In May 2012, a field experiment was carried out using the cv. ‘Arbequina’, setting up a comparison between micropropagated plantlets having a different number of leaves per node (assuming that a higher number indicate a deeper degree of juvenility) and plants obtained from semi hard-wood cuttings in a new high-density commercial olive grove (1250 tree ha−1, 4.0 m × 2.0 m) in Montignano di Senigallia (Ancona province: latitude 43°67′17′′ N; longitude 13°27′64″ E; altitude 90 m a.s.l.; Marche Region, central Italy).
Yearly average precipitation and temperatures are 770 mm and 13.9 °C, respectively. The olive grove was drip irrigated from mid-May to the end of September supplying water according to evapotranspiration requirements, whereas fertigation was applied to enhance trees vegetative growth.
Micropropagated plants were grown in 5 × 5 × 7 cm pots during acclimation ex vitro and were sorted in two groups according to the number of leaves per node (2 or 3) along the main axis. Then, the plants were transplanted in the field and compared with standard plants obtained from cuttings of similar size (Figure 3). Each treatment (in vitro propagated 2 and 3 leaves per node and standard cuttings) consisted of 443 plants for a total of 1329 plants. The experimental design consisted of three randomized blocks, each one constituted by three adjacent rows (one per treatment). For each row, two plots of 7 homogeneous trees were selected for measurements, for a total of 126 trees.

Figure 3.
Elongated shoots of ‘Arbequina’ with 2, 3, and 4 leaves per node.
For each plant, the canopy architecture and the fertility were evaluated between May and June 2013 through the following measurements: stem diameter at 20 cm from the ground using a manual caliper, total height of the plant using a manual ruler, number of primary branches inserted along the central leader of the plant (dividing the main axis into three portions: basal, median, and distal) and number of fertile primary branches (having at least one inflorescence) along the central leader of the plant. In addition, one branch was selected per each of the three portions along the central leader of the canopy in three plants of each plot. For each branch, the total length, the number of nodes, the number of shoots, and the number of inflorescences were recorded, dividing the ramified portions from the non-branched one, besides the second order ramifications.
On 7 June 2013, the small shoots present on the selected branches were counted. Moreover, the number of inflorescences, perfect and male flowers were counted in two branches of the three previously selected plants, during full bloom.
ANOVA analysis and Tukey–Kramer HSD post-hoc test (p ≤ 0.05) were performed by using the JMP software (Release 8; SAS Institute Inc., Cary, NC, USA, 2009).
2.3. Trial 3—Field Comparison of ‘Arbequina’ Trees from Cuttings and from Micropropagation
Canopy architecture and fruit production of 2-year-old micropropagated ‘Arbequina’ olive trees were studied in comparison with those obtained from standard cuttings that had been planted in 2008. Measurements were taken two, three, and four years after planting (2010, 2011, 2012), in a high-density olive grove (1515 tree ha−1, 4.0 m × 1.65 m) located in La Almunia de Dona Godina (latitude 41°28′ N; longitude 1°22′ W; altitude 390 m a.s.l.; Zaragoza, Spain).
The soil was sandy, slightly alkaline and rich in skeleton (600 g kg−1 of soil). Yearly average precipitation in this region is 357 mm, and 14.7 °C, is the annual mean temperature. The olive grove was drip-irrigated, and the seasonal total water irrigation was around 20 L per plant, supplied every other day for the months of April, May, June, and September, while the trees were irrigated every day in July and August.
During the first three years after planting, pruning was limited to remove small branches in the first 60 cm from the ground in the central leader and few possible primary branches growing towards the inter-row.
From each propagation system, evaluations were done to estimate production on 100 trees in July 2010 and June 2011. The load of flowers and/or fruits was used to estimate the reproductive attitude and the absence of juvenile characters, using a “fertility index” value scale which was assigned a minimum (0) to absence of flowers and a maximum (5) to plants with a very high load.
At harvest (November), the fruit yield per plant was recorded on 60 trees from micropropagation and from cuttings in 2010 and on 40 trees for each type of propagation in 2011 and 2012. Then, all the olives from the two propagation systems were harvested independently with an over-the-row harvesting machine, and the fruit production was expressed as yield per hectare (t ha−1).
An architectural screening was performed on 100 trees per propagation type using a visual index based on the relationship between the canopy shape to the high-density system. The index values ranged from 0 (not well-adapted architecture with a globe shape, absence of central leader and top, not regular hierarchic arrangement of the primary branches along the stem) to 5 (optimal architecture with the presence of the top and excellent conical gradient of the primary branches along the stem). Therefore, high values corresponded to a better shape of the canopy with evident conical hierarchy, suitable for high-density plantation (Figure 4).

Figure 4.
Visual index for the canopy shape. (a) worst suitability (index 0) and (b) best suitability (index 5).
Furthermore, a series of biometric measurements were taken (total height, longitudinal, and transverse diameter and volume of the canopy) were calculated on 50 trees per each type of propagation system, in July 2010 and November 2011.
In addition, the distribution of primary branches in the canopy was measured on 5 trees per each type of propagation recording the insertion height from the ground, the basal caliber, and the length of every branch on the central leader.
In November 2011, the fresh and dry weight of all the leaves, the woody skeleton (epigeal portion) and the roots lengths on 3 trees from micropropagation and cutting were measured.
ANOVA analysis and Tukey–Kramer HSD post-hoc test (p ≤ 0.05) were performed by using the JMP software (Release 8; SAS Institute Inc., Cary, NC, USA, 2009).
3. Results
3.1. Trial 1
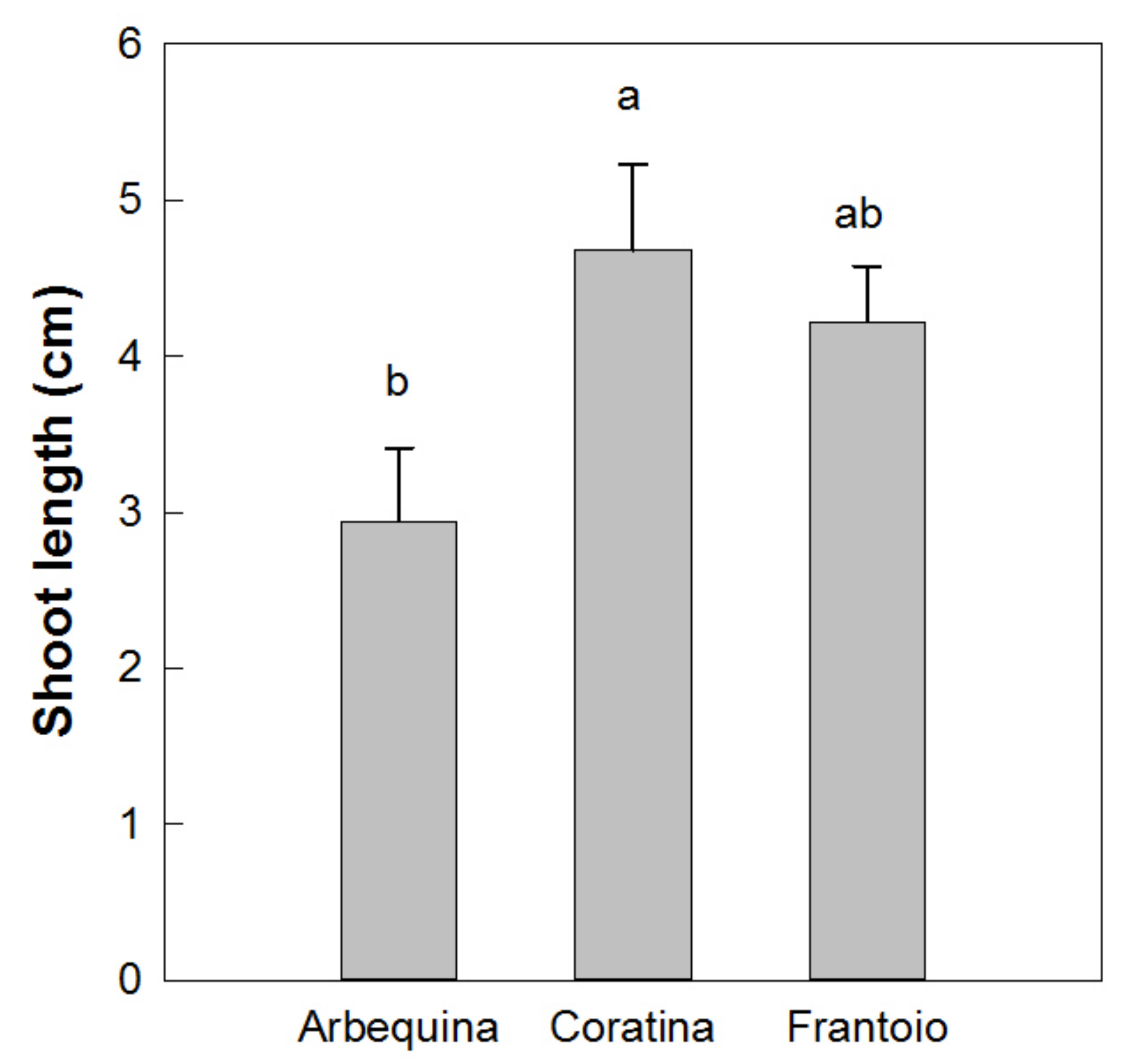
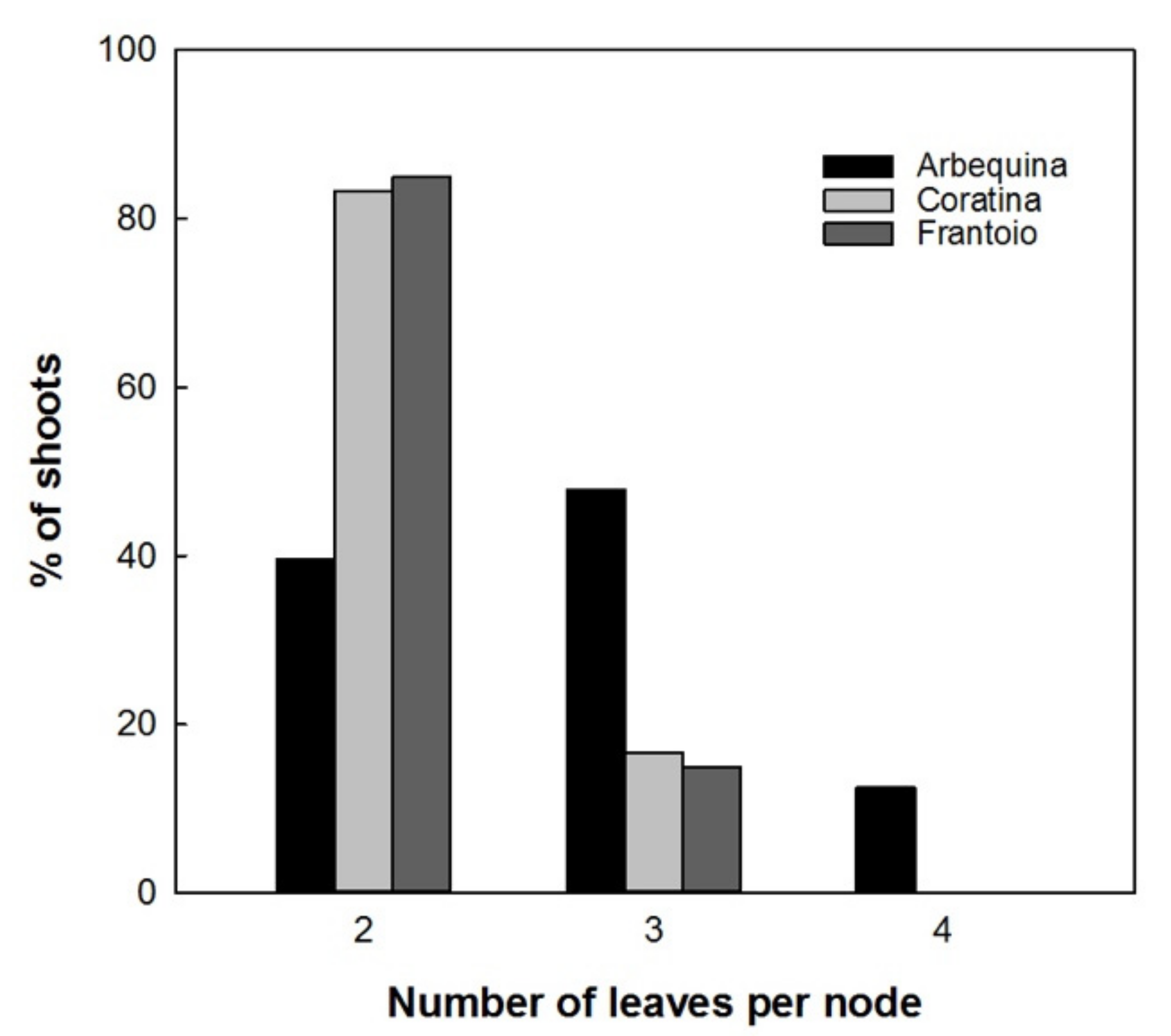
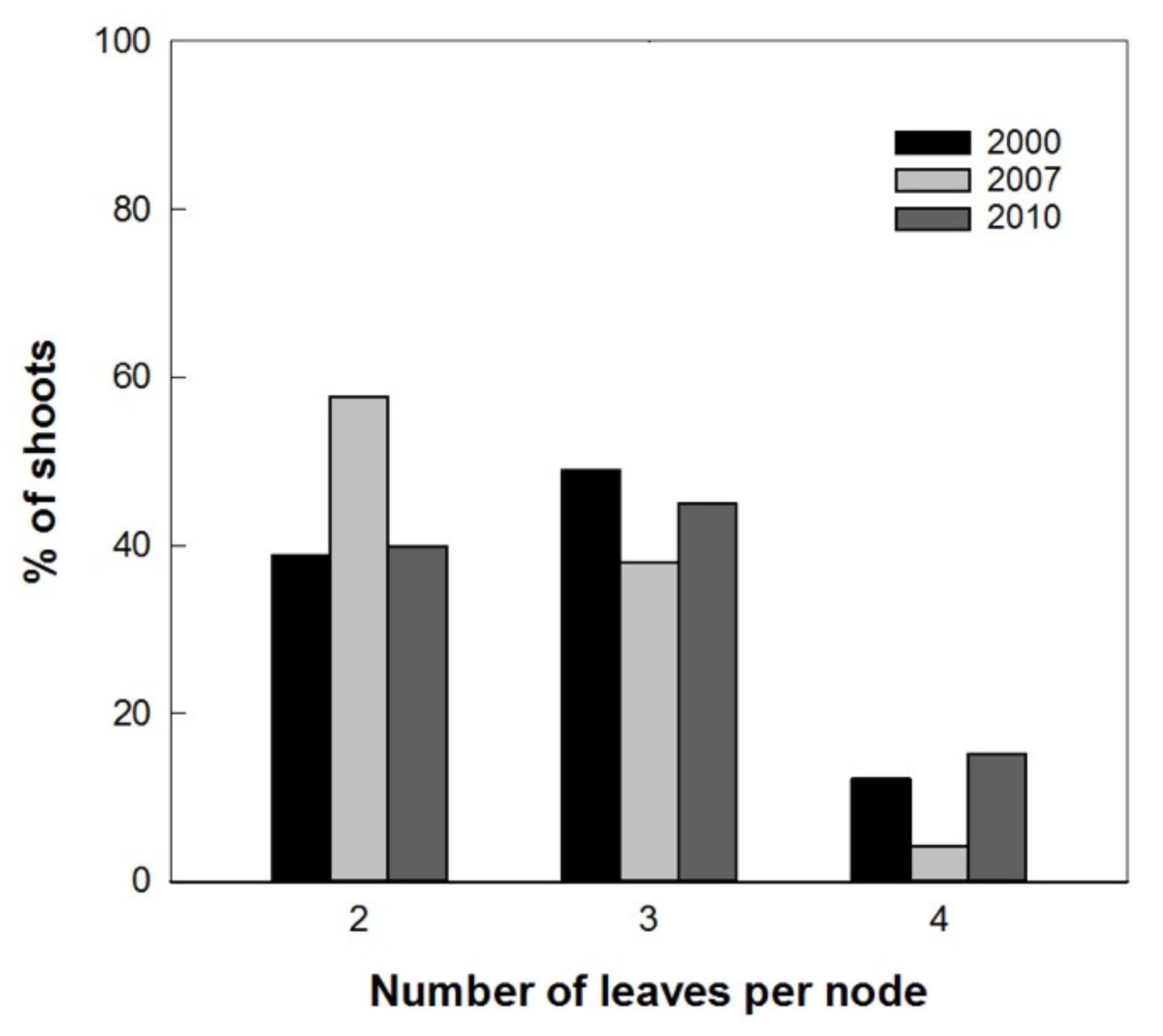
Results showed a normal distribution for the different measured parameters of the plantlets with different in vitro permanence. The total height and basal diameter showed a different vigor for the studied varieties: ‘Arbequina’ was the smallest and ‘Coratina’ the highest one (Figure 5). The expression of the juvenile morphological traits, as three or more leaves per node, within the explant dates was not significantly different. ‘Coratina’ and ‘Frantoio’ did not have elongated shoots with four leaves per node in any explant year and less than 20% of the shoots with three leaves per node (Figure 6). On the contrary, when the explant dates were compared in ‘Arbequina’, the range of frequency of shoots with two leaves per node (typical maturity trait) was included between 40 and 58%. This range was comparable with that one registered for the shoots with three leaves per node (assumed as juvenile characteristic): values between 38–48%. The range for elongated shoots with four leaves per node was included between 5 and 15% (Figure 7).
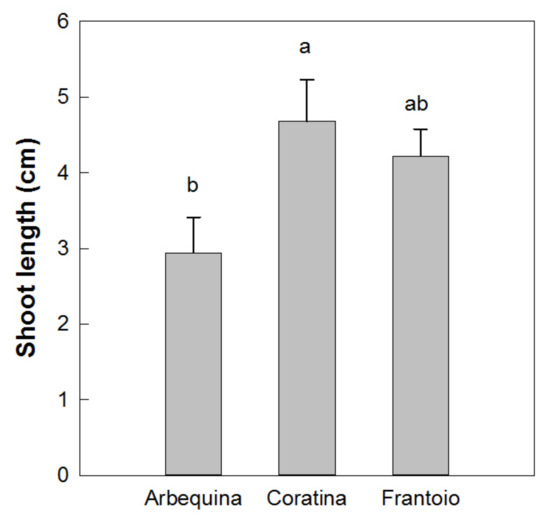
Figure 5.
Shoot length at the end of the elongation phase in vitro. Different letters indicate statistically significant differences between cultivars (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
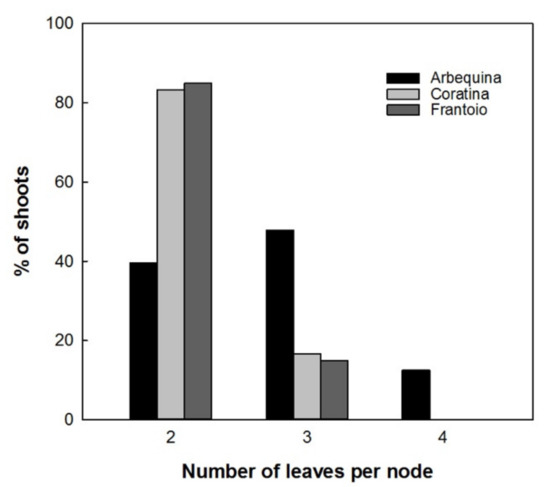
Figure 6.
Frequencies of shoots with 2, 3, and 4 leaves per node in ‘Arbequina’, ‘Coratina’, and ‘Frantoio’ cultivars.
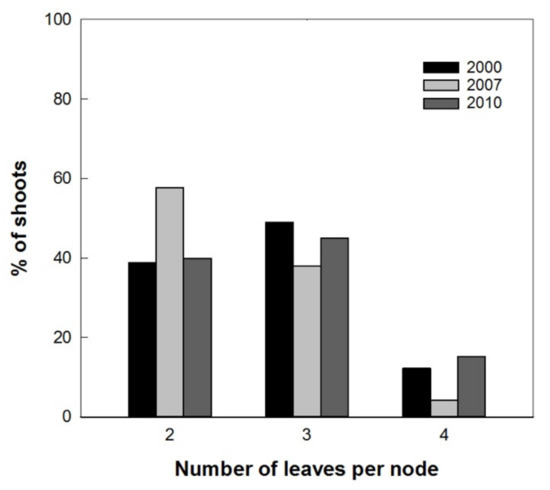
Figure 7.
Frequencies of shoots with 2, 3, and 4 leaves per node in ‘Arbequina’ with different explant dates (2000, 2007, 2010).
3.2. Trial 2
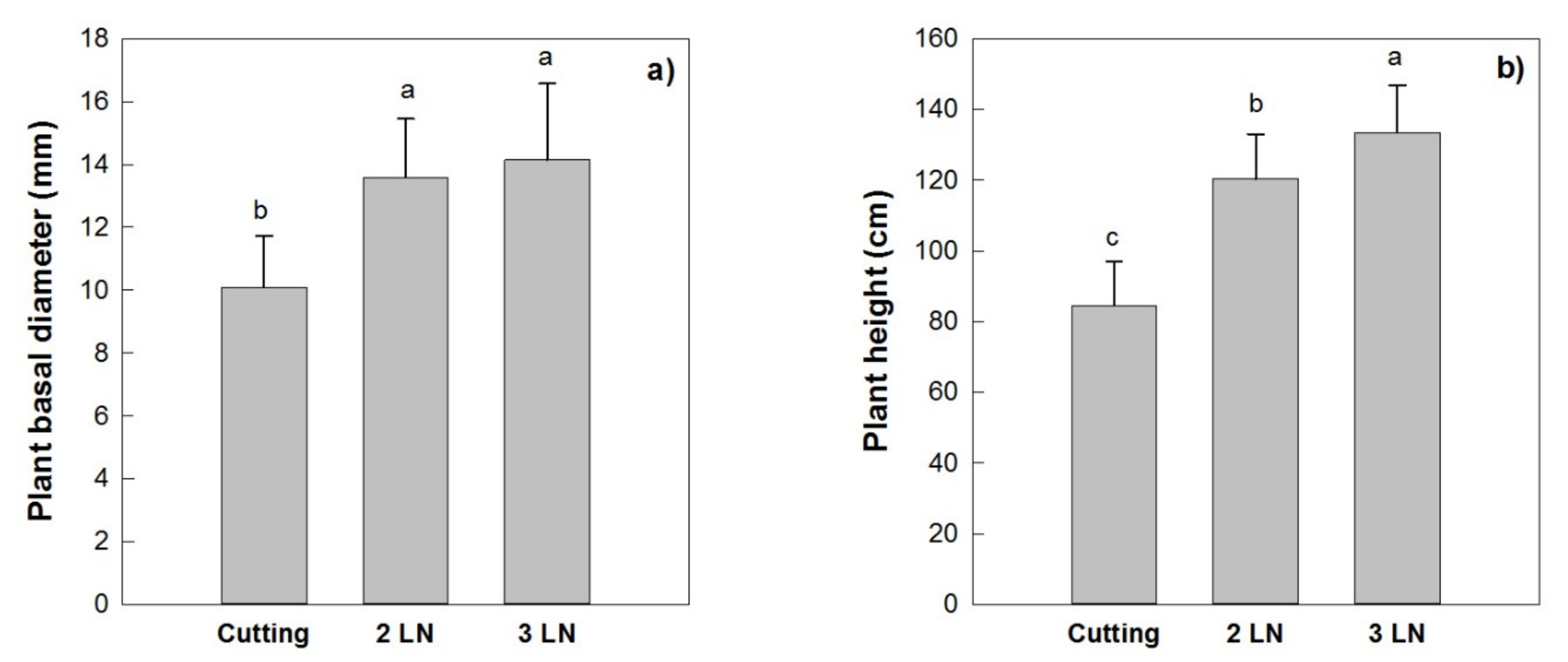
The mean basal diameter of the trees obtained by cutting measured 10 mm at the end of the first growing season, showing significant lower values when compared to the micropropagated trees with two and three leaves per node, whose diameter ranged between 13.6 and 14.1 mm (Figure 8a). Similar results were recorded for the total trees’ height where those obtained by cuttings were shorter than those obtained by micropropagation, and where the trees with three leaves per node were taller than those with two leaves per node (Figure 8b).
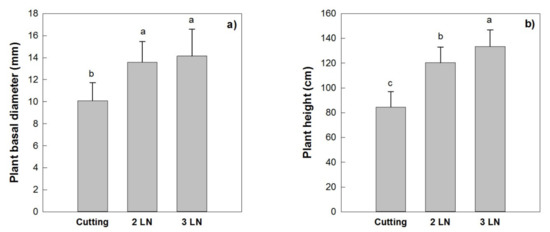
Figure 8.
Trees’ basal diameter (a) and height (b) of micropropagated plants of ‘Arbequina’ with two leaves per node (2 LN) and three leaves per node (3 LN), compared with trees from cuttings. Measurements were taken one year after planting in Montignano di Senigallia (AN, central Italy). Different letters indicate statistically significant differences between tested plant types (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
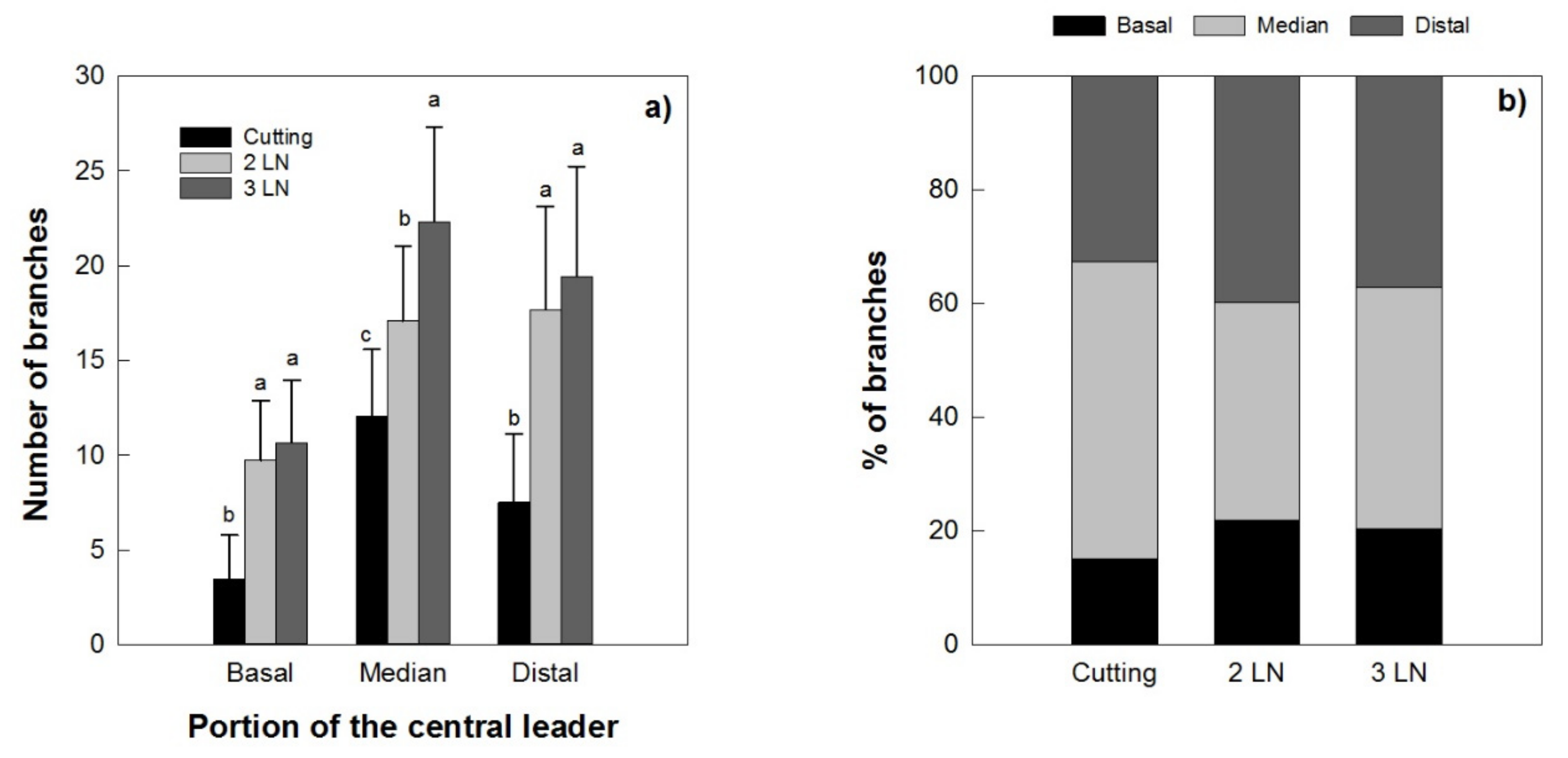
A higher number of primary branches was recorded for the micropropagated trees than those produced by cuttings (Figure 9a). A tendency for a higher number of branches per portion was observed in young trees with three leaves per node when micropropagated trees were compared to those obtained through cutting; however, these differences were seen only in the median portion of the central leader. The distribution of the branches along the main axis was more uniform in the micropropagated trees in comparison with those from cutting, which had mostly higher percentage of branches in the median portion (Figure 9b).
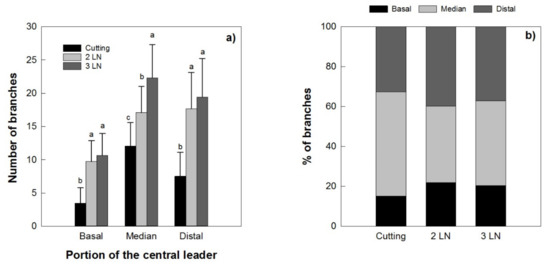
Figure 9.
Number of branches (a) and % of branches (b) in different portions of the central leader of Arbequina micropropagated trees with two leaves per node (2 LN) and three leaves per node (3 LN), compared with those from cuttings. Measurements were taken one year after planting in the field. Different letters indicate statistically significant differences between tested plant types (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
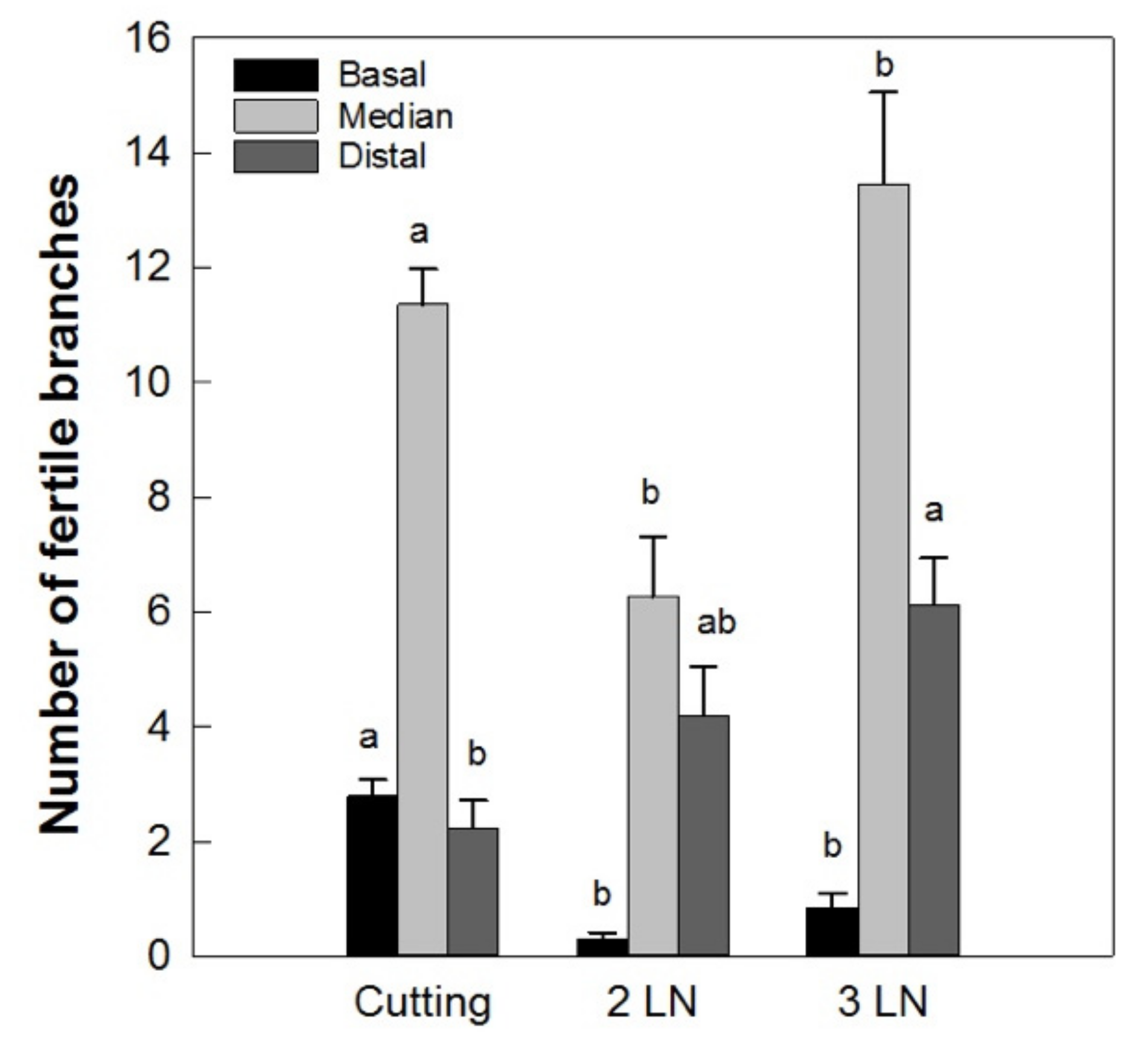
In addition to this, the branches of the micropropagated trees had a greater number of second-order ramifications, especially in the basal portion (data not shown). As a consequence, a lower number of fertile branches in the basal portion of the micropropagated trees with respect to those obtained from cuttings was recorded (Figure 10). In the median and distal portions of the central leader, the number of fertile shoots was higher in plants with three leaves per node.
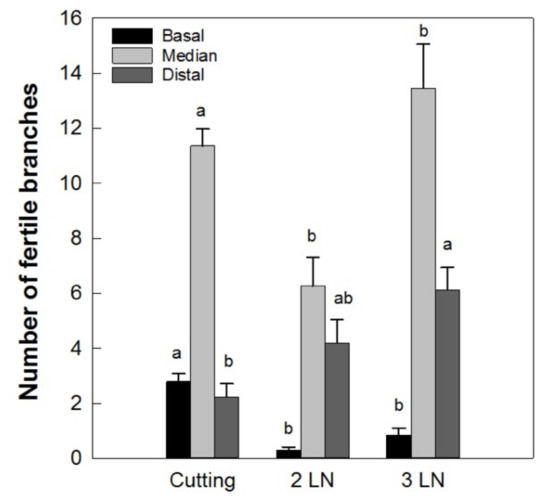
Figure 10.
Distribution of fertile branches along the central leader of ‘Arbequina’ micropropagated trees with two leaves per node (2 LN) and three leaves per node (3 LN), compared with trees propagated from cuttings. Measurements were taken one year after planting in the field. Different letters indicate statistically significant differences between tested plant types (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
3.3. Trial 3
3.3.1. Flowering and Fruit Production
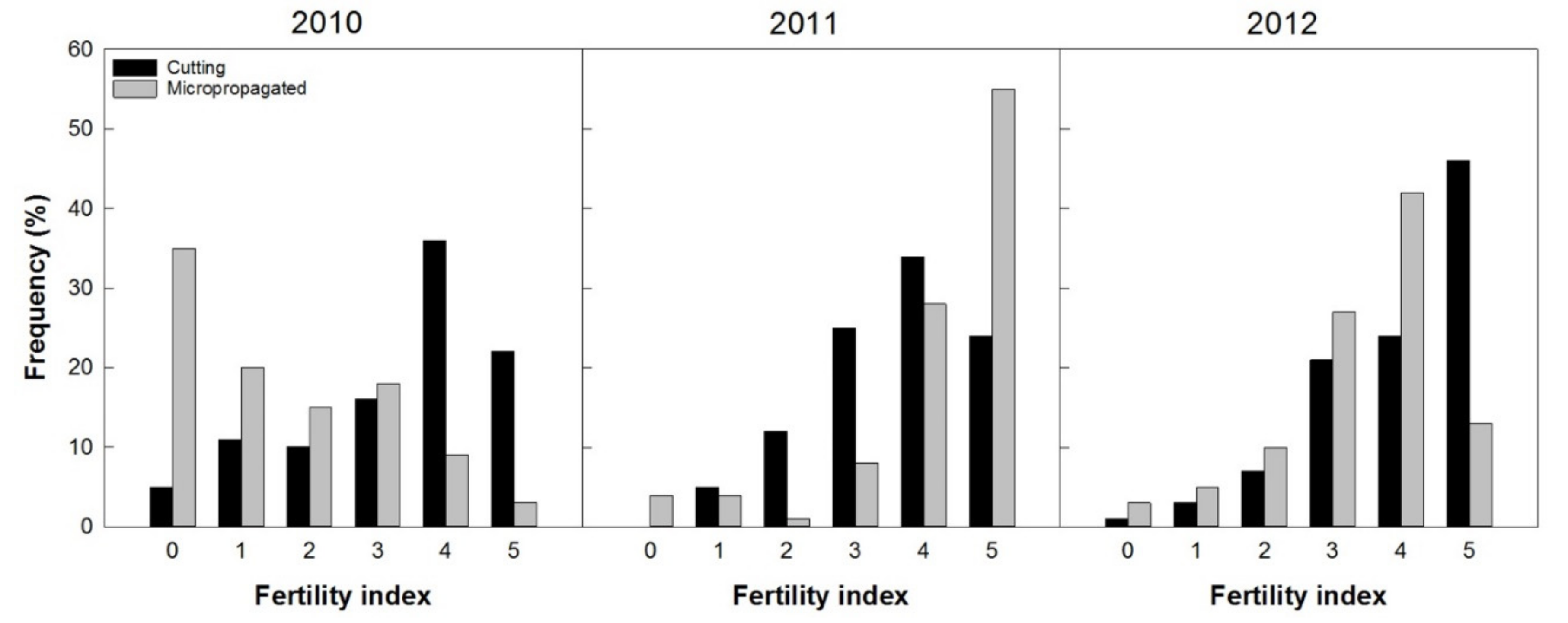
The first flower onset for both propagation techniques (cuttings and micropropagation in vitro) was recorded two years from planting (2010). Through the fertility index it was possible to describe the general conditions of the trees regarding the flowering level, or fruit setting and the timelines of fruit production in 2010, 2011, and 2012 (Figure 11).
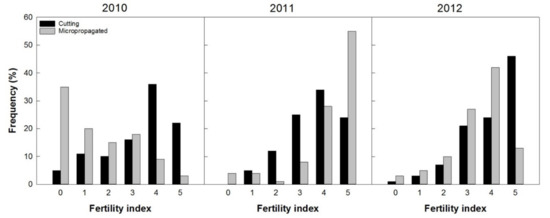
Figure 11.
Trees’ frequency distribution based on visual fertility index (presence of inflorescences, or fruit set), from 0 (absence of flowers) to 5 (very high load), recorded from 100 plants per treatment (2, 3, and 4 years after planting).
In 2010, ‘Arbequina’ trees from cuttings were very fertile (more than 70% of the trees recorded a “fertility index” between the classes 3 to 5), with few unproductive specimens, while the micropropagated trees were distributed mainly in classes 0 to 3 with about 1/3 of the unproductive specimens (35%). This situation changed during the fourth year since planting (2011), when over 55% of the micropropagated trees bloomed more copiously (visual index 5) and more than 90% flowers were included in the classes with the highest production potential (3 to 5). In 2011, the trees obtained from cuttings had about 35% flowers in class 4 and only 23% in class 5. It should be noted that, in 2011, the presence of inflorescences in trees from micropropagation was very high with more than 50% of the trees in class 5. Only 3% of the trees showed very little, or no inflorescences (class 0 and 1). Similar results were recorded in 2012, when more than 50% of all the trees had a presence of inflorescences classified in classes 4 and 5, even though a prevalence of micropropagated trees was recorded in class 4 and those from cuttings in class 5.
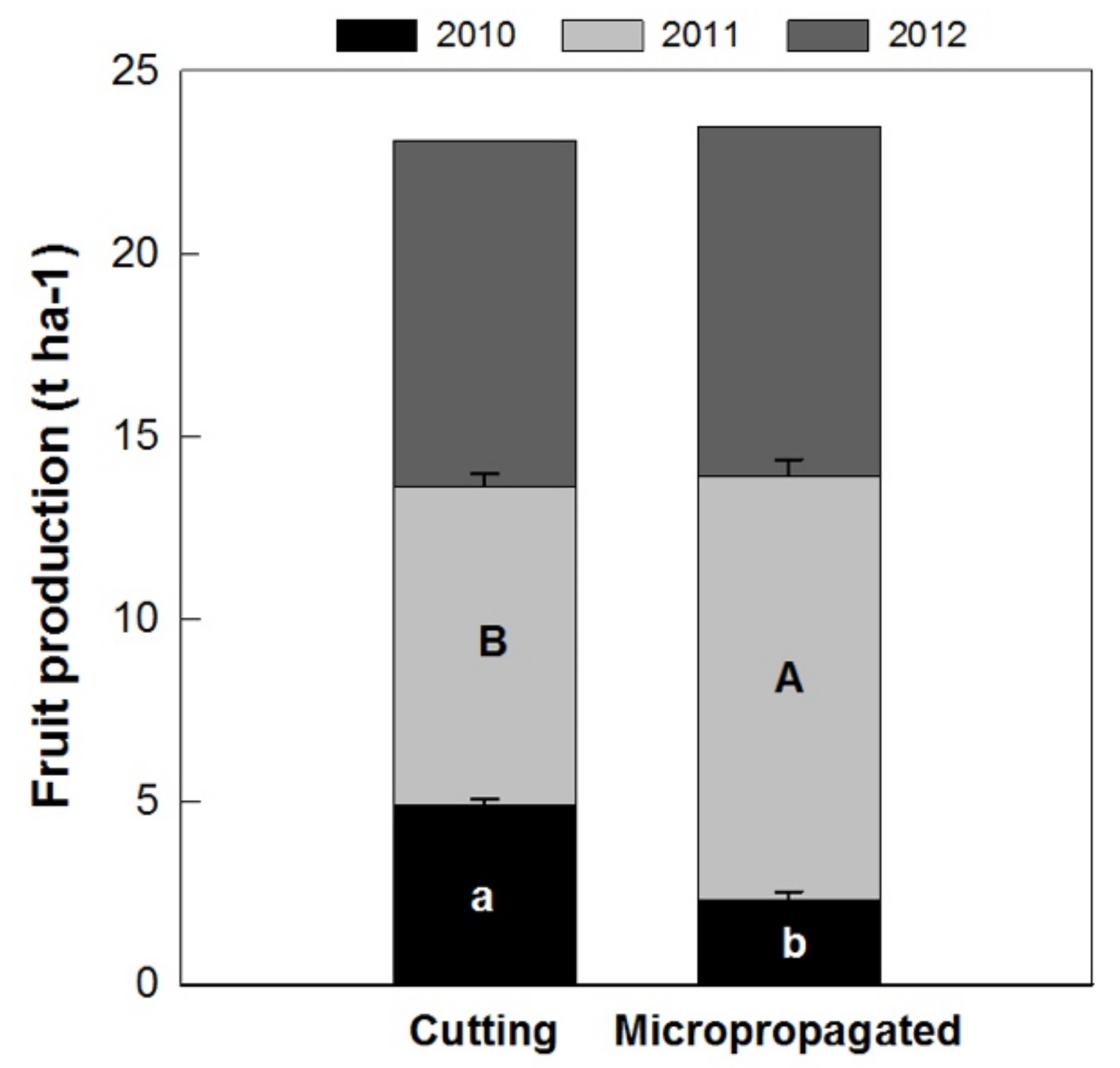
The mean fruit yield per hectare at harvest confirmed the estimations performed during flowering and fruit setting. In 2010, the micropropagated trees did not yield as many fruits as those from cuttings; however, in 2011, the micropropagated trees yielded higher fruit production per hectare. In 2012, no differences in production per hectare were detected in the two tree types. Consequently, the cumulative fruit production in the three-year period (2010–2011–2012) was very similar in trees obtained from micropropagation (14.2 t ha−1) and from cuttings (14.0 t ha−1) (Figure 12).
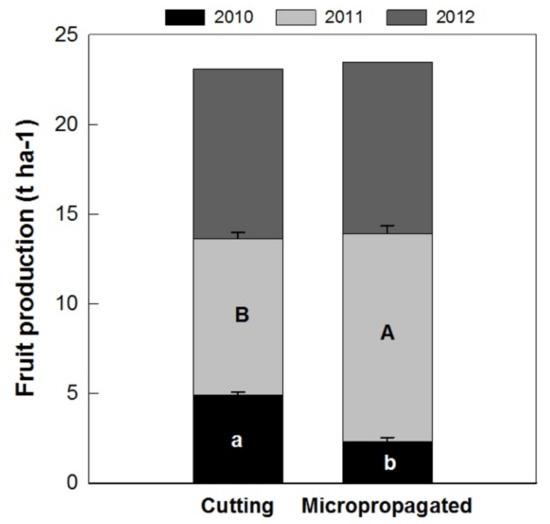
Figure 12.
Fruit production during the three-year period of experimentation on ‘Arbequina’ trees, obtained from cuttings and micropropagation. Bars represent the standard error and different letters indicate significant differences between propagation method, through the years (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
3.3.2. Canopy Architecture
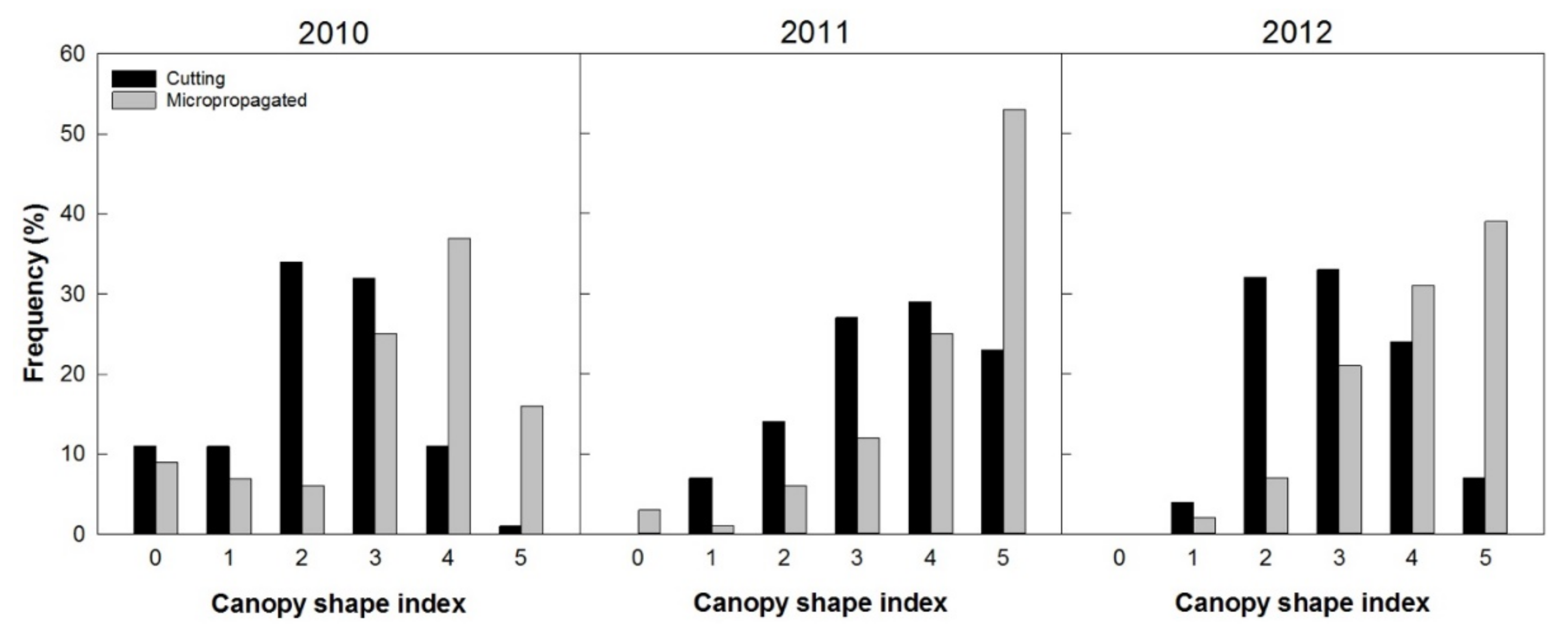
The canopy shape index highlighted that, in 2010, about 80% of trees from micropropagation already belonged to classes 3 to 5, which indicated almost the optimal form. On the other hand, an inverse behavior was observed for trees derived from cuttings that had about 70% classified in classes 2 and 3 (Figure 13).
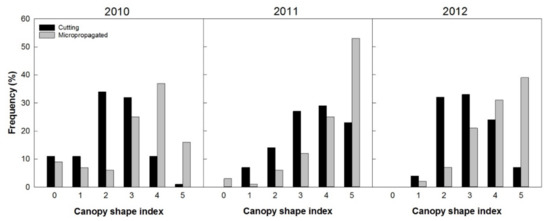
Figure 13.
Trees’ frequency distribution based on the canopy shape index (correspondence of the canopy shape to the high-density system), from 0 (not well-adapted architecture) to 5 (optimal architecture), recorded from 100 trees per treatment in three consecutive years (2, 3, and 4 years after planting).
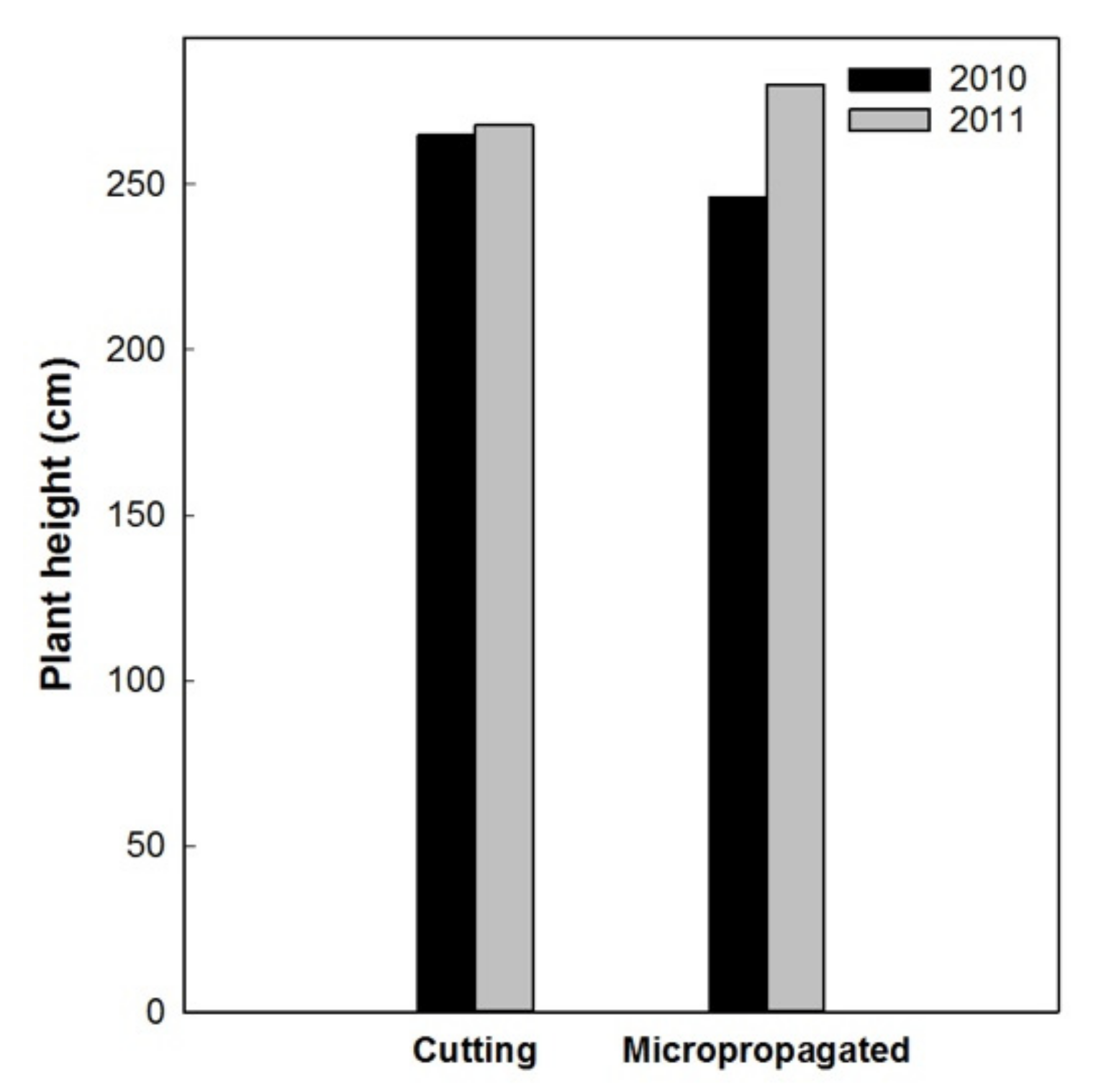
Results in 2011 and 2012 confirmed an overall better conical shape and homogeneity of the canopy for the micropropagated trees. Two years after planting (2010), all the trees had a total height higher than 2 m, but those from cuttings were about 0.2 m taller than those from micropropagation (Figure 14). Micropropagated trees recovered from this growth delay in the following year (2011), reaching a plant height of about 2.8 m. We concede that this might be attributable to a superior vigor of the trees from micropropagation, when compared to those from cuttings.
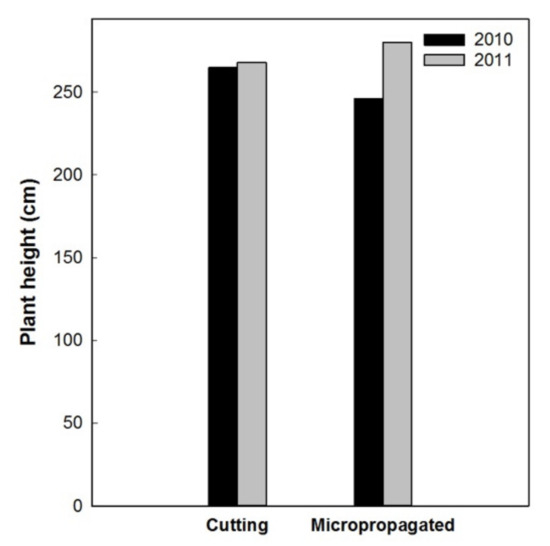
Figure 14.
Total trees’ height in 2010 and 2011.
No statistically significant differences were found between micropropagated trees and cuttings regarding the longitudinal (in the direction of the row) and transversal (perpendicular to the row) diameters of the canopy in 2010 and 2011, as both types of trees had already exceeded their fixed limits (meaning that both types of plants have occupied the available space towards the inter-row and along the row in a similar way). If we consider the growth along the row, we can add that the ‘Arbequina’ trees in the specific pedo-climatic conditions of the experiment closed the intra-row spaces very quickly (three years), showing an excellent disposition to create a continuous hedgerow. On the other hand, canopy enlargement across the row can become a problem for the over-the-row harvesting machine when the width of 0.8 m is exceeded, and the branches are not very flexible.
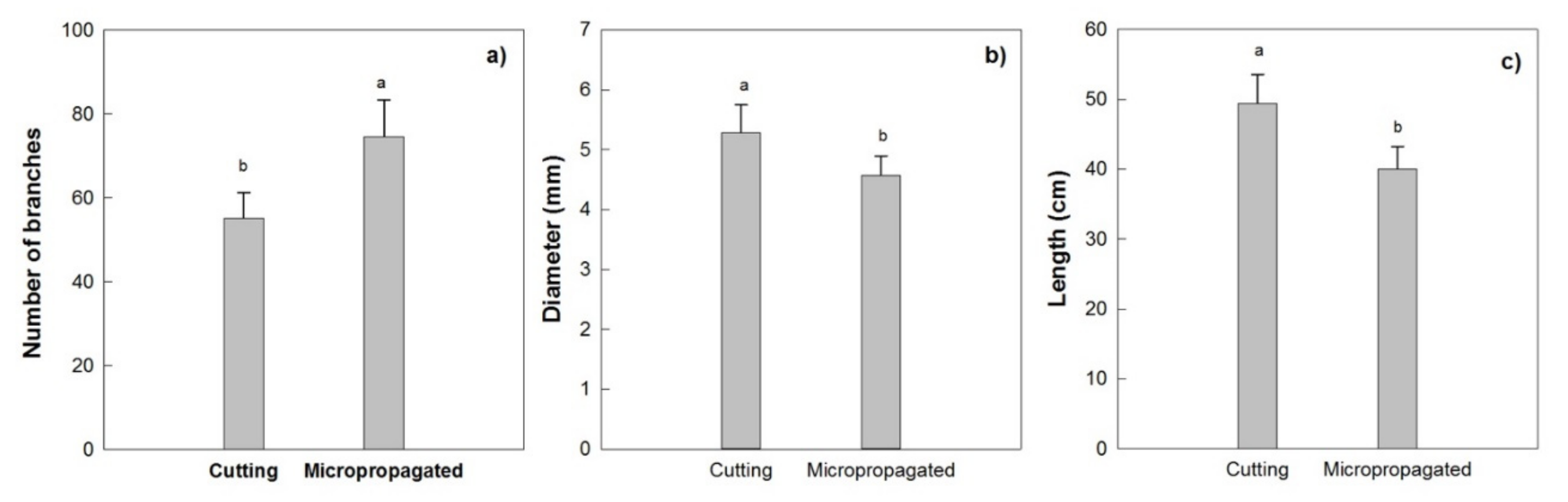
This canopy architecture for trees derived from cuttings showed a lower number of branches along the central leader, higher average basal diameter, and length of the primary branches (Figure 15a–c), indicating a higher susceptibility to mechanical damages (breakages or injuries) during the harvest with the over-the row machines, in the following years.
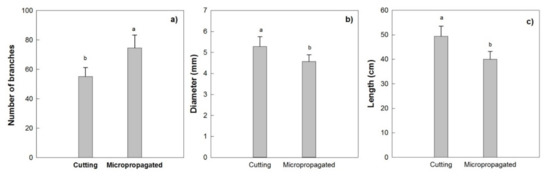
Figure 15.
Mean number of branches (a), basal diameter (b) and length (c) of the primary branches along the central leader (main axis), for the two type of trees, in 2010 (2 years after planting). Different letters indicate statistically significant differences between trees obtained from micropropagation and cutting (Tukey–Kramer HSD post hoc tests, p ≤ 0.05).
The “conical hierarchy” was assumed to be the most suitable for the high-density system in the first years by both types of trees. However, trees from cuttings showed a tendency to have an excessive load of vegetation in the apical portion and this could cause an excessive shading of the basal area of the canopy with loss of functionality of the lower branches, in future years. As illustrated in Figure 16, the micropropagated trees had a greater number of branches along the whole central leader (main axis), with a predominance in the central portion of the canopy.

Figure 16.
Primary branch distribution along the central leader of ‘Arbequina’ trees from cutting (a) and micropropagation (b).
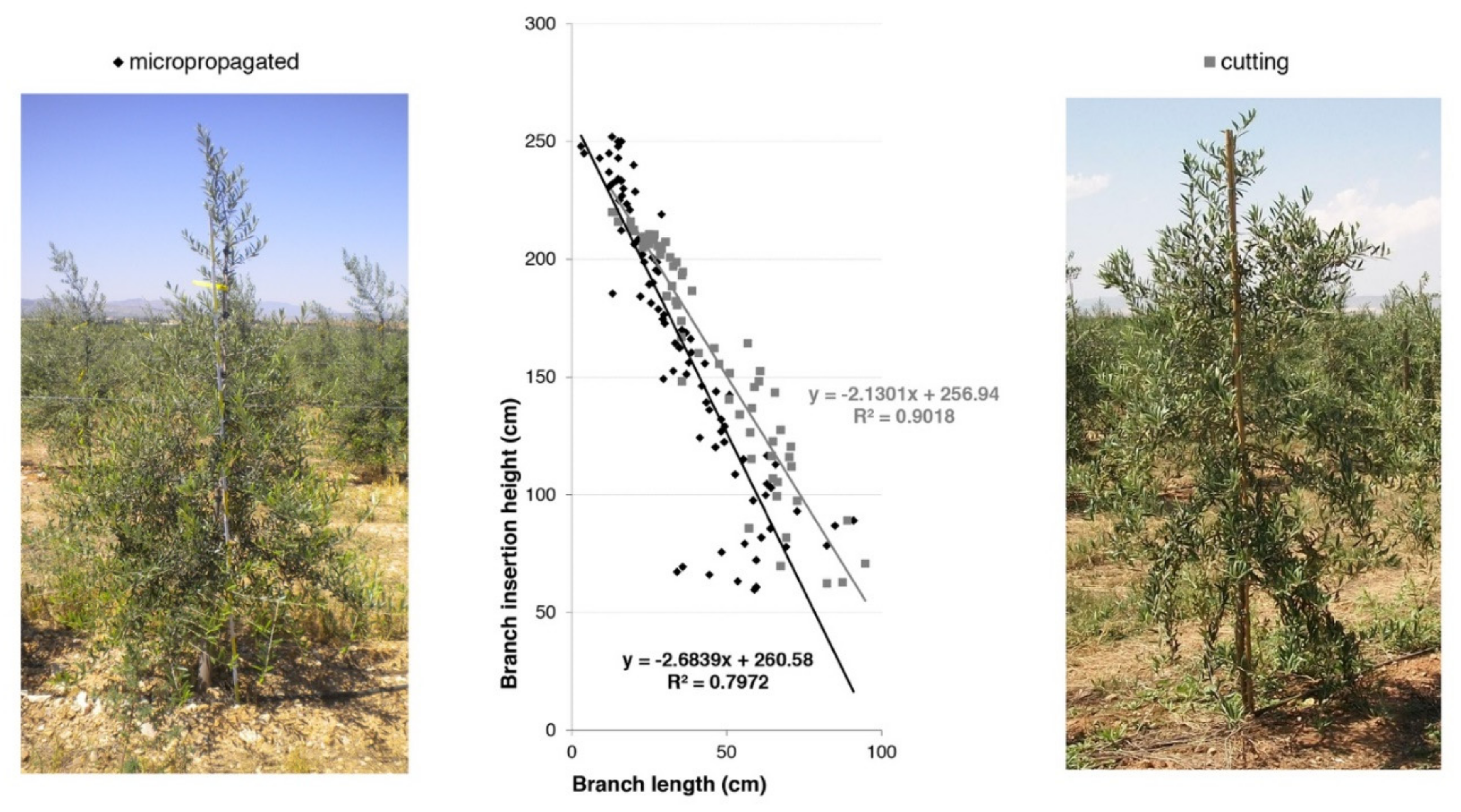
The distribution of the primary branches along the central leader according to their length is reported (Figure 17). This parameter is very important to supply information about the architectural model of the canopy in both tree types. Data collected two years after planting showed that the primary branches were more expanded in the basal portion of the central leader in cuttings when compared to micropropagated trees. On the contrary, plants obtained by micropropagation possessed more primary branches along the central leader, creating a higher ramification density (Figure 17). Moreover, such kind of trees (from in vitro propagation) acquired a more conical shape of the canopy, becoming more suitable for high-density olive groves.
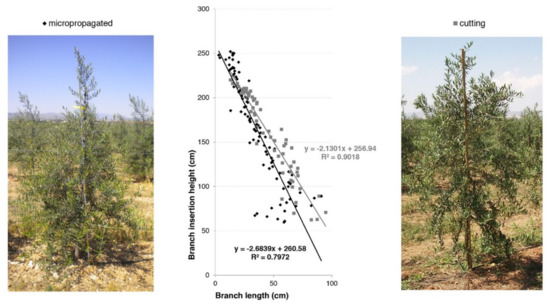
Figure 17.
Conical shape of the canopy in cutting and micropropagated trees (cv. ‘Arbequina’) in 2010.
Destructive measurements (fresh and dry weight of the epigeal scaffold of the canopy) did not highlight significant differences between the two types of trees (data not shown), but rather in the trees’ root systems. Micropropagated trees showed a completely different root system architecture when compared to that of trees from cuttings, as recorded from a higher total fresh weight (data not shown). In these trees, several primary roots were found (on average 5 per plant), with a positive geotropical insertion angle of about 30°. Moreover, the roots in micropropagated trees were more uniformly distributed (360° in a horizontal plane), originating a higher number of laterals with more regular calibers (Figure 18a). On the contrary, roots in trees obtained from cuttings showed a growth mainly in two directions (along the row where the irrigation drippers were located), determining a lesser number of primary roots with thicker diameters (Figure 18b).

Figure 18.
Spatial arrangement of the root system of trees from micropropagation (a) and cutting (b) in November 2011.
4. Discussion
Results from trial 1 showed very limited effects on possible juvenile characters related to the duration of in vitro permanence, in all the studied cultivars. ‘Arbequina’ demonstrated a superior morphological plasticity in vitro, presenting higher incidence of possible juvenile characters, i.e., 3–4 leaves per node instead of the standard two per node of mature shoots and shorter plantlets in comparison with ‘Coratina’ and Frantoio’ as previously reported [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Moreover, the juvenile characters were more related to varietal genetic characteristics than to the permanence of the explants in vitro, with possible consequences on the vegetative-reproductive behavior during the first years after field plantation (ex vitro).
It is worth noting that, during trials 2 and 3 in the field, ‘Arbequina’ quickly overcame the possible juvenile phase, showing significant fruit productions already in the second year from planting on at least 40% of the trees and on almost all of the trees during the third year. It presented a high vigor, superior to that of trees derived from cuttings, which, on the contrary, produced abundantly already in the second year from planting. This study suggests a transient level of juvenility of ‘Arbequina’ trees during the multiplication phase with possible consequences on the vegetative-reproductive behavior during the first years after planting. The early selection of the micropropagated material for a different level of juvenility based on some morphological characters resulted in not being feasible because no improvements of the tree management in high-density orchards were achieved, not for earlier onset of fruit production, nor for higher yields and contrasting with results discussed in previous studies [45].
In the comparison of selected shoots from in vitro multiplication carried out in trial 2 with trees from cuttings, the results confirmed that taller plants, obtained by micropropagation, had a greater tendency to vegetative growth (higher vigor) and thus confirming previous results [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. From the field results, it can be inferred that micropropagated trees with three leaves per node had a greater diameter and height and greater vigor when compared to the cuttings, but not significantly higher than micropropagated trees with two leaves per node. Different basal diameters between the two types of trees could be due to a different growth rate, depending on the sap flow between the epigeal and hypogeal tree organs.
These results suggest that a marked juvenility and vigor at the beginning of the first year and then a rapid plant maturation during the late growing season occurred in micropropagated trees. This difference can be traced back to juvenility that manifests itself mostly in the lower parts of the plant, while the differences in the upper portion are due to a greater vigor. In fact, both the number of fertile branches and the flower density in the median and distal branch portions did not show significant differences in flowers’ abundance.
Comparable results were obtained in plants from cutting and from micropropagation for the number of inflorescences and flowers (perfect and imperfect flowers) per branch, showing that the quality of flowering does not depend from the different type of planting material, where a mean of 5.5 inflorescences per branch, with 85% of perfect flowers being yielded. These findings can be traced back to a higher level of vigor in microprogated trees with a rapid transition in less than two years from in vitro characters (i.e., three leaves per node), to standard architecture and fertility (i.e., two leaves per node and flower differentiation) as reported by other authors [40].
These results were confirmed by the outcome of trial 3, where about the same percentages of ‘Arbequina’ trees selected for the juvenile character were devoid of production in the second year. On the other hand, the micropropagated trees with 2 leaves per node also had dimensions and branching aptitude clearly superior to those of the cuttings, although they had the possibility to produce already in their third growing season. In this case, it was hypothesized that the in vitro culture did evoke characters of juvenility, but ex vitro the trees rapidly matured showing a greater vigor that was expressed with a greater branching and tapering of the canopy. In fact, the strong vegetative growth, combined with the high ramification ability in ‘Arbequina’ micropropagated trees, induced a wider insertion angle of the primary branches along the central leader and a better conical shape of the canopy, compared to trees from cuttings. This behavior can lead to a higher production efficiency of the tree, as indicated in previous studies [10,11,12,13,14]. These interpretations were confirmed 11 years after planting (data not shown) when differences between the two types of trees were not visible, except for a more regular branch distribution along the trunk of micropropagated trees.
Trial 3 yielded interesting data about the deep and well distributed root systems of the micropropagated trees, whereas the central leader supported a tree structure with an outstanding conical hierarchy of the canopy which was characterized by high and uniform branching along its axis. The remarkable vigor of the micropropagated ‘Arbequina’ trees in the early years without the appearance of juvenile characters and a more aggressive root explorative behavior represent the most interesting traits for designing intensive olive growing systems. The fast and regular vegetative growth prepares trees for a high production, starting already from the fourth growing season and confirming previous results [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Consequently, the cumulative fruit production in the three years experiment was very high.
Therefore, it is possible to take advantage of the greater vigor of micropropagated plants without delaying the onset of production, when this occurs already in the third year and a high cumulated yield in the fourth year. ‘Arbequina’ from in vitro propagation could be used in poor and dry soils, where a greater autonomy of the plants and a stronger root system are required.
5. Conclusions
The tree propagation technique appears to be one of the factors involved in the olive cultivation intensification process. In particular, the in vitro propagation seems to represent an interesting solution for standardizing the plant material. In ‘Arbequina’, limited juvenile characters and positive vigor increase promoted an architecture of the canopy and of the root system highly suitable for high-density growing systems. A delay of only one year in the onset into production for the in vitro propagation can be considered negligible but the better ramification and suitability to high density olive orchards represent greater advantages in comparison to cuttings. Further studies are needed to check the effect of micropropagation in other olive varieties. A standardization of the propagation material can promote an intensification process in olive even for local germplasm, combining mechanization increase with valorization of local productions.
Author Contributions
Conceptualization, D.N., T.C., E.M.L., G.Z., O.N.; methodology and formal analysis T.C., E.M.L., D.N.; data curation, V.G., T.C., E.M.L.; writing—original draft preparation, D.N., E.M.L., T.C., V.G.; writing—review and editing, D.N., E.M.L., V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ricerca locale di Ateneo 5534-2010, UNIVPM; Dottorato di ricerca in Scienze agrarie, alimentari ed ambientali, 2009, UNIVPM.
Acknowledgments
We thank Vitroplant S.P.A. (Cesena, IT) for providing micropropagated olive trees, Massimo Bastianelli for the nursery help, Alessandro Luigi Ancora and Elisa Laurenzi for the help in the field measurements, the Viveros Mariano Soria (Zaragoza, SP) for having made available the experimental olive grove, and Vicente Perez for the help in the field operations during trial 3, and the Polytechnic University of Marche for supporting the Ph.D. program of Tonino Cioccolanti.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IOC (International Olive Council). Available online: https://www.internationaloliveoil.org (accessed on 14 October 2020).
- FAO (Food and Agriculture Organization of the United Nations). Available online: http://www.fao.org/faostat/en/#home (accessed on 12 October 2020).
- Tous, J.; Romero, A.; Hermoso, J.F. New trends in olive orchard design for continuous mechanical harvesting. Adv. Hortic. Sci. 2010, 24, 43–52. [Google Scholar]
- Neri, D.; Sansavini, S. Planting and training systems, pruning and fruiting control. Theoretical and practical principles–Physiological. In Principles of Modern Fruit Science-The Ultimate Textbook: Understanding the Fundamentals of Plant Science; Sansavini, S., Costa, G., Gucci, R., Inglese, P., Ramina, A., Xiloyannis, C., Desjardins, Y., Eds.; ISHS: Leuven, Belgium, 2019; pp. 271–293. [Google Scholar]
- Godini, A.; Vivaldi, G.A.; Camposeo, S. Olive cultivars field-tested in super high-density system in southern Italy. Calif. Agric. 2011, 65, 39–40. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’ and four Italian olive cultivars under super high-density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- Marino, G.; Macaluso, L.; Marra, F.P.; Ferguson, L.; Marchese, A.; Campisi, G.; Volo, P.; Laudicina, V.A.; Caruso, T. Horticultural performance of 23 Sicilian olive genotypes in hedgerow systems: Vegetative growth, productive potential and oil quality. Sci. Hortic. 2017, 217, 217–225. [Google Scholar] [CrossRef]
- Marino, G.; Macaluso, L.; Grilo, F.; Marra, F.P.; Caruso, T. Toward the valorization of olive (Olea europaea var. europaea L.) biodiversity: Horticultural performance of seven Sicilian cultivars in a hedgerow planting system. Sci. Hortic. 2019, 256, 108583. [Google Scholar] [CrossRef]
- Moutier, N.; Garcia, G.; Lauri, P.E. Shoot architecture of the olive tree: Effect of cultivar on the number and distribution of vegetative and reproductive organs on branches. Acta Hortic. 2004, 635, 689–694. [Google Scholar] [CrossRef]
- Rosati, A.; Paoletti, A.; Caporali, S.; Perri, E. The role of tree architecture in super high-density olive orchards. Sci. Hortic. 2013, 161, 24–29. [Google Scholar] [CrossRef]
- Rosati, A.; Paoletti, A.; Al Hariri, R.; Morelli, A.; Famiani, F. Partitioning of dry matter into fruit explains cultivar differences in vigor in young olive (Olea europaea L.) trees. HortScience 2018, 53, 491–495. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Reale, L.; Caruso, T.; Ferranti, F. Productive and vegetative behavior of olive cultivars in super high-density olive grove. Sci. Agric. 2015, 72, 20–27. [Google Scholar] [CrossRef]
- Proietti, P.; Filippucci, M.; Nasini, L.; Regni, L.; Brunori, A. Generative trees: Architectural modelling of an olive to estimate morphology and radiation relationship. In Environmental Information Systems: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2018; Volume 1, pp. 399–425. [Google Scholar]
- Lodolini, E.M.; Tarragoni, A.; Cioccolanti, T.; Pollastri, L.; Neri, D. Architectural characteristics of six olive cultivars with respect to their suitability for high density orchards. Acta Hortic. 2017, 1160, 127–133. [Google Scholar] [CrossRef]
- Strippoli, G.; Vivaldi, G.A.; Camposeo, S.; Contò, F. Sprouts seasonal elongation of two olive cultivars in high-density orchard. Sci. Res. 2013, 4, 376–381. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Tarragoni, A.; Cioccolanti, T.; Massetani, F.; Pollastri, L.; Neri, D. Reproductive patterns of 1-year-old mixed shoots in different olive cultivars in Central Italy. Acta Hortic. 2017, 1160, 119–126. [Google Scholar] [CrossRef]
- Vivaldi, G.A.; Strippoli, G.; Pascuzzi, S.; Stellacci, A.M.; Camposeo, S. Olive genotypes cultivated in an adult high-density orchard respond differently to canopy restraining by mechanical and manual pruning. Sci. Hortic. 2015, 192, 391–399. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Polverigiani, S.; Grossetti, D.; Neri, D. Pruning management in a high-density olive orchard in central Italy. Acta Hortic. 2018, 1199, 385–390. [Google Scholar] [CrossRef]
- Albarracin, V.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of vegetative growth and fruit yield to winter and summer mechanical pruning in olive trees. Sci. Hortic. 2017, 225, 185–194. [Google Scholar] [CrossRef]
- Albarracin, V.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Impact of simulated mechanical hedge pruning and wood age on new shoot demography and return flowering in olive trees. Trees 2018, 32, 1767–1777. [Google Scholar] [CrossRef]
- Albarracin, V.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of shoot growth, return flowering, and fruit yield to post-pruning practices and growth regulator application in olive trees. Sci. Hortic. 2019, 254, 163–171. [Google Scholar] [CrossRef]
- Famiani, F.; Proietti, P.; Lodolini, E.M.; Neri, D. Gestione della chioma. In L’ulivo e l’olio; Ed. Script: Bologna, Italy, 2009; pp. 390–411. [Google Scholar]
- Tombesi, S.; Farinelli, D. Canopy management in super high-density olive orchards: Relationship between canopy light penetration, canopy size and productivity. Acta Hortic. 2017, 1177, 87–91. [Google Scholar] [CrossRef]
- Cioccolanti, T.; Lodolini, E.M.; Neri, D.; Bastianelli, M.; Zuccherelli, G.; Capaccio, V.; Navacchi, O. Giovanilità dell’Olivo in micropropagazione (Juvenility in micropagated olive trees). Acta ItalusHortus 2013, 10, 102–105. [Google Scholar]
- Cioccolanti, T.; Lodolini, E.M.; Neri, D.; Perez, V.; Zuccherelli, G. Modello architetturale in piante micropropagate e da talea (cv Arbequina). Acta ItalusHortus 2013, 10, 50–54. [Google Scholar]
- Lambardi, M.; Ozudogru, A.E. La propagazione in vitro dei portinnesti e delle specie da frutto. Frutticoltura 2010, 12, 18–25. [Google Scholar]
- Zuccherelli, G.; Zuccherelli, S. In vitro propagation of fifty olive cultivars. Acta Hortic. 2002, 586, 931–934. [Google Scholar] [CrossRef]
- Capocasa, F.; Balducci, F.; Marcellini, M.; Bernardini, D.; Navacchi, O.; Mezzetti, B. Comparing nursery behavior, field plant yield and fruit quality of in vitro and in vivo propagated strawberry mother plants. Plant Cell Tiss. Organ. Cult. 2019, 136, 65–74. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orlowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ. Cult. 2019, 140, 245–257. [Google Scholar] [CrossRef]
- Neri, D. Giovanilità e ringiovanimento nelle piante arboree. Frutticoltura 1990, 12, 69–73. [Google Scholar]
- Hackett, W.P. Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Fabbri, A.; Bartolini, G.; Lambardi, M.; Kailis, S. Olive Propagation Manual; Landlinks Press: Collingwood, Australia, 2004; p. 144. [Google Scholar]
- Goldschmidt, E.E.; Samach, A. Aspects of flowering in fruit trees. Acta Hortic. 2004, 653, 23–27. [Google Scholar] [CrossRef]
- Merkle, S.A.; Battle, P.J. Enhancement of embryogenic culture initiation from tissues of mature sweetgum trees. Plant Cell Rep. 2000, 19, 268–273. [Google Scholar] [CrossRef]
- Araki, T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 2001, 4, 63–68. [Google Scholar] [CrossRef]
- Gucci, R.; Cantini, C. Pruning and Training Systems for Modern Olive Growing; CSIRO Publishing: Collingwood, Australia, 2000; p. 147. [Google Scholar]
- Zucconi, F. Nanizzazione Delle Piante Arboree; Spazio Verde: Padova, Italy, 1996; p. 128. [Google Scholar]
- Zucconi, F. Nuove Tecniche per i Fruttiferi: Fisiologia e Metodi Innovative Nell’allevamento dei Fruttiferi; Edagricole: Bologna, Italy, 2003; p. 246. [Google Scholar]
- Lodolini, E.M.; Neri, D. How growth and reproduction cycles affect alternate bearing in olive. Acta Hortic. 2012, 949, 191–198. [Google Scholar] [CrossRef]
- Leva, A.R.; Petruccelli, R.; Montagni, G.; Muleo, R. Field performance of micropropagated olive plants (cv Maurino): Morphological and molecular features. Acta Hortic. 2002, 586, 891–894. [Google Scholar] [CrossRef]
- Briccoli Bati, C.; Godino, G.; Nuzzo, V. Preliminary agronomic evaluation of two olive cultivars obtained from micropropagation methods. Acta Hortic. 2002, 586, 867–870. [Google Scholar] [CrossRef]
- Lambardi, M.; Rugini, E. Micropropagation of olive (Olea europaea L.). In Micropropagation of Woody Trees and Fruits; Jain, S.M., Ishii, K., Eds.; Springer: Basel, Switzerland, 2003; pp. 621–646. [Google Scholar]
- Bastianelli, M.; Cioccolanti, T. Tutte le declinazioni del mondo spagnolo–reportage da Valdejalon dove l’Arbequina ad alta densità è rapidamente proliferata grazie alla precoce entrata in produzione e ai bassi costi di gestione. Ma ogni produttore gestisce l’allevamento “in libertà”. Le prospettive della micropropagazione. Olivo e Olio 2012, 2, 54–57. [Google Scholar]
- Lodolini, E.M.; Cioccolanti, T.; Bastianelli, M.; Zuccherelli, G.; Capaccio, V.; Navacchi, O.; Neri, D. Giovanilità e vigoria in piante di olivo micropropagato. Acta ItalusHortus 2012, 6, 106. [Google Scholar]
- Cioccolanti, T.; Lodolini, E.M.; Bastianelli, M.; Zuccherelli, G.; Capaccio, V.; Navacchi, O.; Neri, D. Selezione precoce di germogli di olivo micropropagato in base al diverso livello di vigoria. Acta ItalusHortus 2012, 6, 110. [Google Scholar]
- Kumar, N.; Reddy, M.P. In vitro plant propagation: A review. J. For. Sci. 2011, 27, 61–72. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).