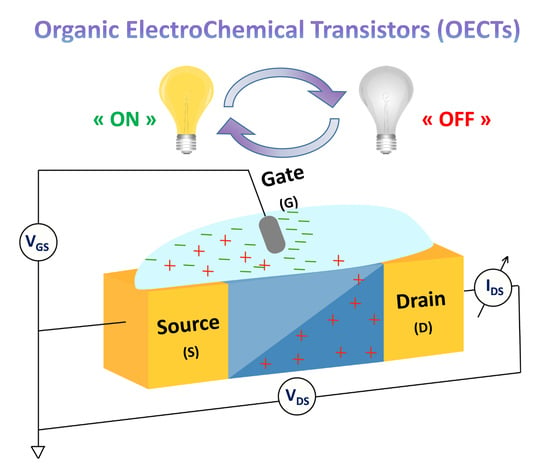
Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics
Abstract
:1. Introduction
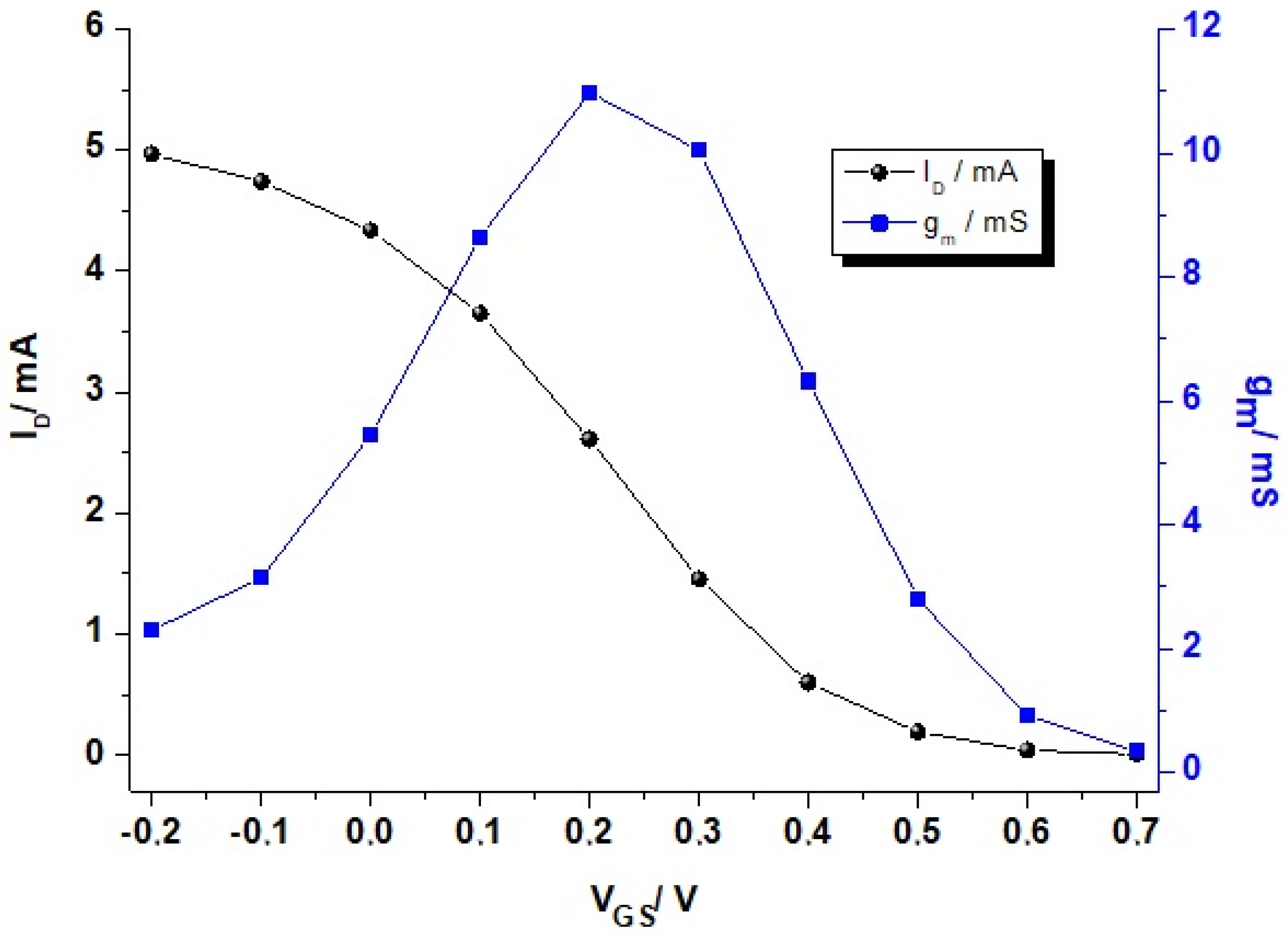
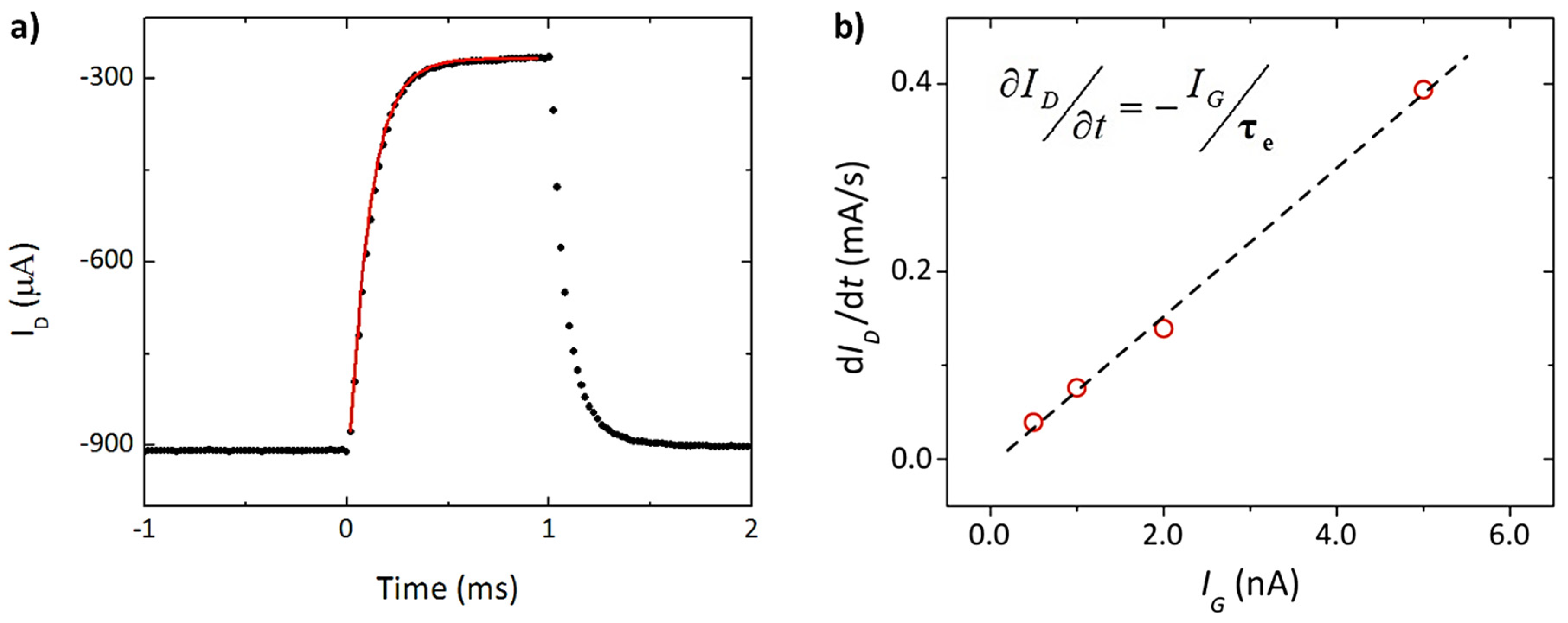
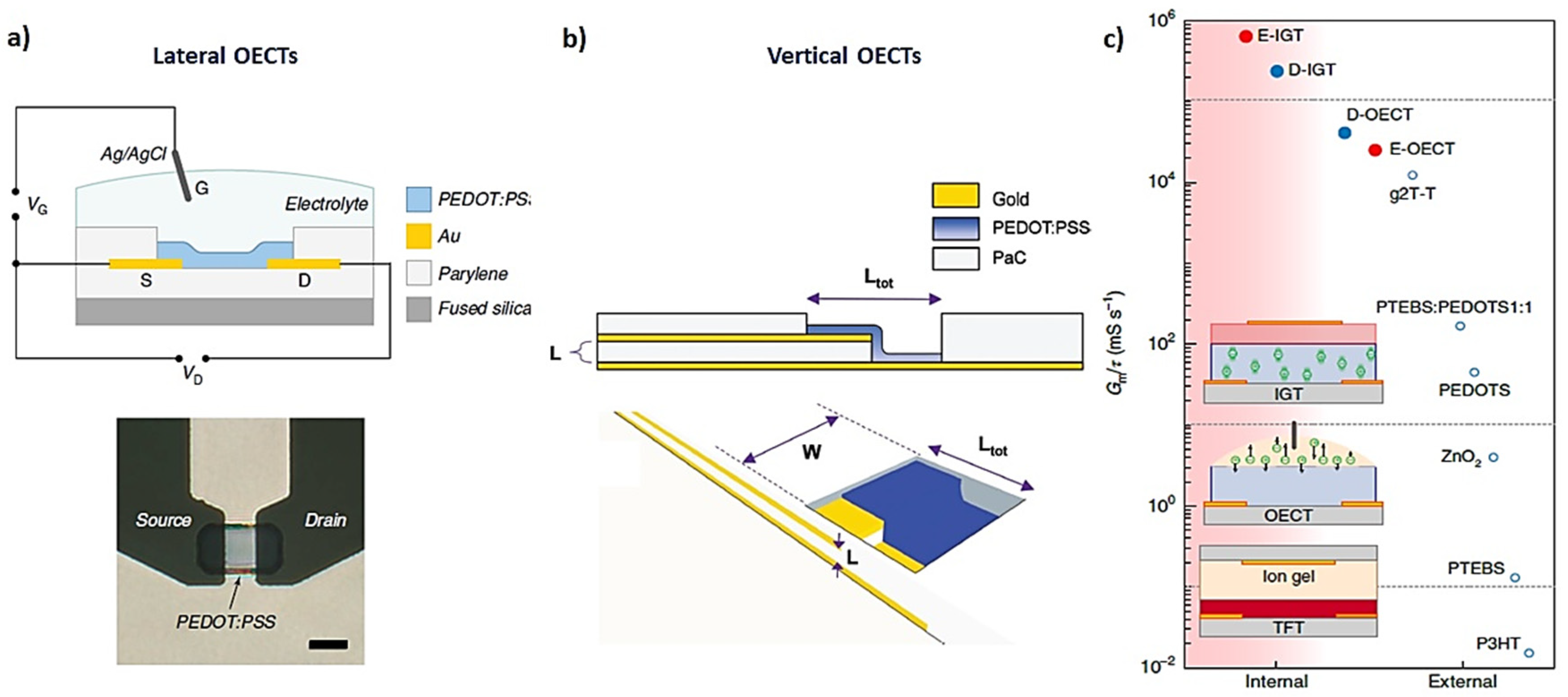
2. Physical Characterization: Figure of Merit (FoM), Modeling, and Performance Design
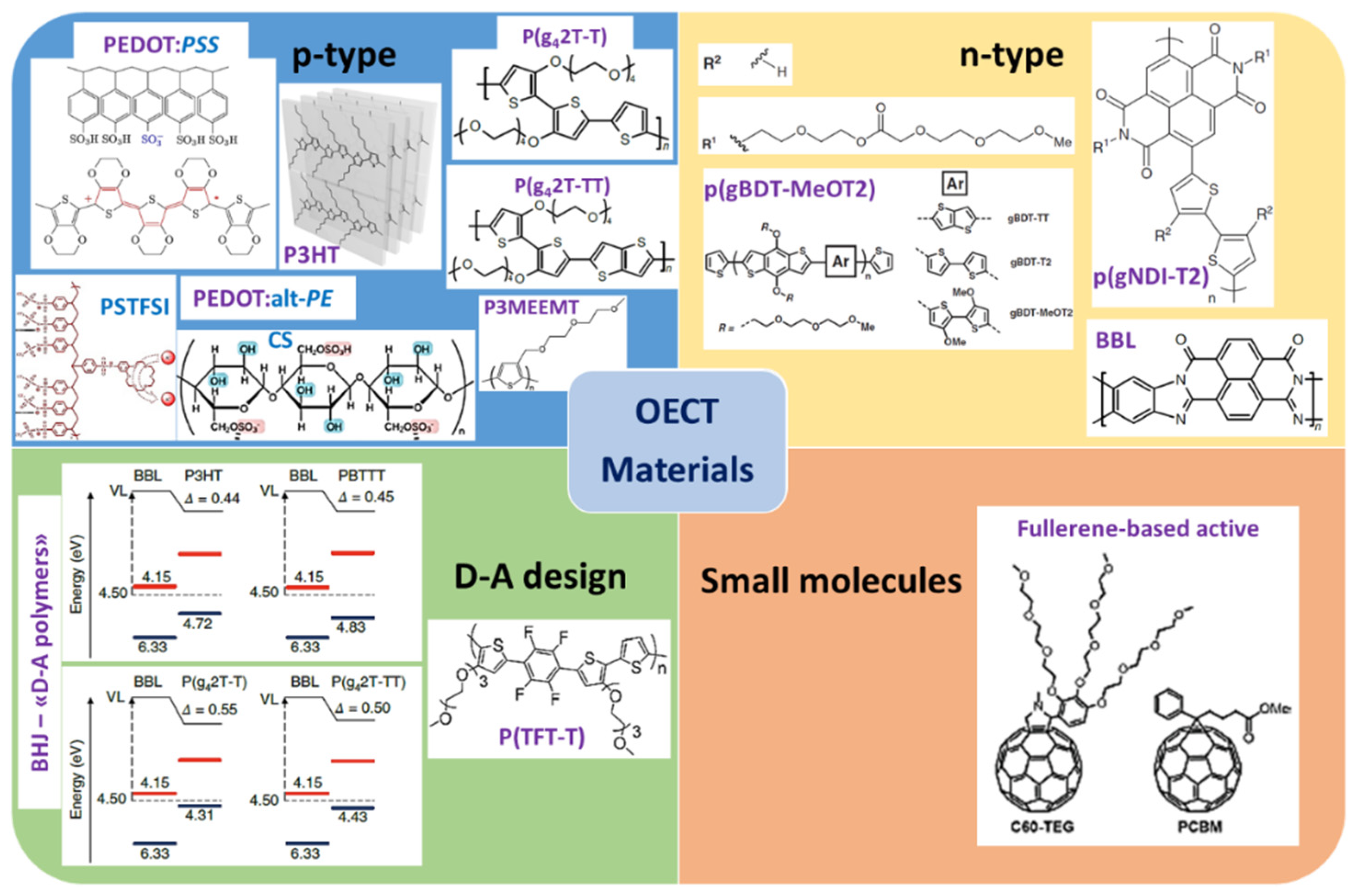
3. Organic Semiconductor Materials for OECT
3.1. Conducting Polymers—p-Type
3.1.1. PEDOT and Some Derivatives
3.1.2. Other Thiophene Derivatives
- P3HT Family: Integration of Functions
- Donor–Acceptor (D–A) Design
3.2. Conducting Polymers—n-Type
3.3. Small Molecules
4. Applications
4.1. Biology and Sensing
4.2. Future in Wearable Devices
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponder, J.F., Jr.; Osterholm, A.M.; Reynolds, J.R. Conjugated Polyelectrolytes as Water Processable Precursors to Aqueous Compatible Redox Active Polymers for Diverse Applications: Electrochromism, Charge Storage, and Biocompatible Organic Electronics. Chem. Mater. 2017, 29, 4385–4392. [Google Scholar] [CrossRef]
- Malti, A.; Edberg, J.; Granberg, H.; Khan, Z.U.; Andreasen, J.W.; Liu, X.; Zhao, D.; Zhang, H.; Yao, Y.; Brill, J.W.; et al. An Organic Mixed Ion–Electron Conductor for Power Electronics. Adv. Sci. 2016, 3, 1500305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Someya, T.; Bao, Z.; Malliaras, G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Arbring Sjostrom, T.; Berggren, M.; Gabrielsson, E.O.; Janson, P.; Poxson, D.J.; Seitanidou, M.; Simon, D.T. A Decade of Iontronic Delivery Devices. Adv. Mater. Technol. 2018, 3, 1700360. [Google Scholar] [CrossRef]
- Strakosas, X.; Bongo, M.; Owens, R.M. The Organic Electrochemical Transistor for Biological Applications. J. Appl. Polym. Sci. 2015, 132, 41735. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic Electrochemical Transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- White, H.S.; Kittlesen, G.P.; Wrighton, M.S. Conjugated Polymer-Based Chemical Sensors Chemical Derivatization of an Array of Three Gold Microelectrodes with Polypyrrole: Fabrication of a Molecule-Based Transistor. J. Am. Chem. Soc. 1984, 106, 5375–5377. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-State and Transient Behavior of Organic Electrochemical Transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Inal, S.; Malliaras, G.G.; Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 2017, 8, 1767. [Google Scholar] [CrossRef] [Green Version]
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Herve, T.; Sanaur, S.; Owens, R.M.; et al. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133. [Google Scholar] [CrossRef]
- Friedlein, J.T.; Donahue, M.J.; Shaheen, S.E.; Malliaras, G.G.; McLeod, R.R. Microsecond Response in Organic Electrochemical Transistors: Exceeding the Ionic Speed Limit. Adv. Mater. 2016, 28, 8398–8404. [Google Scholar] [CrossRef] [PubMed]
- Sessolo, M.; Khodagholy, D.; Rivnay, J.; Maddalena, F.; Gleyzes, M.; Steidl, E.; Buisson, B.; Malliaras, G.G. Easy-to-Fabricate Conducting Polymer Microelectrode Arrays. Adv. Mat. 2013, 25, 2135. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Leleux, P.; Ferro, M.; Sessolo, M.; Williamson, A.; Koutsouras, D.A.; Khodagholy, D.; Ramuz, M.; Strakosas, X.; Owens, R.M.; et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 2015, 1, e1400251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, M.J.; Williamson, A.; Strakosas, X.; Friedlein, J.T.; McLeod, R.R.; Gleskova, H.; Malliaras, G.G. High-performance vertical organic electrochemical transistors. Adv. Mater. 2018, 30, 1705031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Chen, Q.; Wu, X.; Wang, X.; Li, E.; Ke, Y.; Liu, Y.; Chen, H.; Guo, T. High-Performance Organic Electrochemical Transistors with Nanoscale Channel Length and Their Application to Artificial Synapse. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Spyropoulos, G.D.; Gelinas, J.N.; Khodagholy, D. Internal ion-gated organic electrochemical transistor: A building block for integrated bioelectronics. Sci. Adv. 2019, 5, eaau7378. [Google Scholar] [CrossRef] [Green Version]
- Cea, C.; Spyropoulos, G.D.; Jastrzebska-Perfect, P.; Ferrero, J.J.; Gelinas, J.N.; Khodagholy, D. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 2020, 5, 679–686. [Google Scholar] [CrossRef]
- Choi, H.H.; Cho, K.; Frisbie, C.D.; Sirringhaus, H.; Podzorov, V. Critical assessment of charge mobility extraction in FETs. Nat. Mater. 2018, 17, 2–7. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [Green Version]
- Podzorov, V. Organic single crystals: Adressing the fundamentals of organic electronics. MRS Bull. 2013, 38, 15–24. [Google Scholar] [CrossRef]
- Stavrinidou, E.; Leleux, P.; Rajaona, H.; Khodagholy, D.; Rivnay, J.; Lindau, M.; Sanaur, S.; Malliaras, G.G. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 2013, 25, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Koutsouras, D.A.; Bihar, E.; Fairfield, J.A.; Saadaoui, M.; Malliaras, G.G. Fabrication Approaches for Conducting Polymer Devices. In Green Materials and Applications; Wiley-VCH: Weinheim, Germany, 2017; ISBN 978-3-527-33865-8. [Google Scholar] [CrossRef]
- Yi, Z.; Natale, G.; Kumar, P.; Mauro, E.D.; Heuzey, M.-C.; Soavi, F.; Perepichka, I.I.; Varshney, S.K.; Santato, C.; Cicoira, F. Ionic liquid–water mixtures and ion gels as electrolytes for organic electrochemical transistors. J. Mater. Chem. C 2015, 3, 6549–6553. [Google Scholar] [CrossRef]
- Yu, K.; Rich, S.; Lee, S.; Fukuda, K.; Yokota, T.; Someya, T. Organic Photovoltaics: Toward Self-Powered Wearable Electronics. Proc. IEEE 2019, 107, 2137–2154. [Google Scholar] [CrossRef]
- Spindler, J.; Kondakova, M.; Boroson, M.; Büchel, M.; Eser, J.; Knipping, J. Advances in High Efficacy and Flexible OLED Lighting. SID Symp. Dig. Tech. Pap. 2018, 49, 1135. [Google Scholar] [CrossRef]
- Bai, L.; García, C.; Li, W.; Yu, P.; Fei, J.; Mao, L. Biological Applications of Organic Electrochemical Transistors: Electrochemical Biosensors and Electrophysiology Recording. J. Front. Chem. 2019, 7, 313. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.C. The Early History of Polyaniline: Discovery and Origins. Substantia 2017, 1, 99–109. [Google Scholar] [CrossRef]
- McNeill, R.; Siudak, R.; Wardlaw, J.H.; Weiss, D.E. Electronic Conduction in Polymers. I. The Chemical Structure of Polypyrrole. Aust. J. Chem. 1963, 16, 1056–1075. [Google Scholar] [CrossRef]
- Contat-Rodrigo, L.; Pérez-Fuster, C.; Lidón-Roger, J.V.; Bonfiglio, A.; García-Breijo, E. Characterization of Screen-Printed Organic Electrochemical Transistors to Detect Cations of Different Sizes. Sensors 2016, 16, 1599. [Google Scholar] [CrossRef] [Green Version]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers- Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- PEDOT: Principles and Applications of an Intrinsically Conductive Polymer. Available online: https://www.crcpress.com/PEDOT-Principles-and-Applications-of-an-Intrinsically-Conductive-Polymer/Elschner-Kirchmeyer-Lovenich-Merker-Reuter/p/book/9781420069112 (accessed on 13 August 2018).
- Nielsen, C.B.; Giovannitti, A.; Sbircea, D.-T.; Bandiello, E.; Niazi, M.R.; Hanifi, D.A.; Sessolo, M.; Amassian, A.; Malliaras, G.G.; Rivnay, J.; et al. Molecular design of semiconducting polymers for high-performance organic electrochemical transistors. J. Am. Chem. Soc. 2016, 138, 10252–10259. [Google Scholar] [CrossRef] [Green Version]
- Groenendaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Luscombe, C.K. The future of organic photovoltaics. Chem. Soc. Rev. 2015, 44, 78. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Morin, J.-F. Design and Synthesis of Conjugated Polymers; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527629787. [Google Scholar]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical-chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Flagg, L.Q.; Bischak, C.Q.; Quezada, R.J.; Onorato, J.W.; Luscombe, C.K.; Ginger, D.S. P-Type Electrochemical Doping Can Occur by Cation Expulsion in a High-Performing Polymer for Organic Electrochemical Transistors. ACS Mater. Lett. 2020, 2, 254–260. [Google Scholar] [CrossRef]
- Malliaras, G.; Friend, R. An Organic Electronics Primer. Phys. Today 2005, 58, 53–58. [Google Scholar] [CrossRef]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The Rise of Organic Bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qud, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring PEDOT properties for applications in bioelectronics. Mater. Sci. Eng. R 2020, 140, 100546. [Google Scholar] [CrossRef] [Green Version]
- Park, T.; Park, C.; Kim, B.; Shin, H.; Kim, E. Flexible PEDOT electrodes with large thermoelectric power factors to generate electricity by the touch of fingertips. Energy Environ. Sci. 2013, 6, 788–792. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Thaning, E.M.; Asplund, M.L.M.; Nyberg, T.A.; Inganas, O.W.; von Holst, H. Stability of poly(3,4-ethylene dioxythiophene) materials intended for implants. J. Biomed. Mater. Res. 2010, 93B, 407–415. [Google Scholar] [CrossRef]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J. In vitro and In vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE. Trans. Neural Syst. Rehabil. Eng. 2011, 3, 307–316. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; Breiby, D.W.; West, K. Base inhibited oxidative polymerization of 3,4-ethylenedioxythiophene with iron(III)tosylate. Synth. Met. 2005, 152, 1–4. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; West, K. Vapor-phase polymerization of 3,4-ethylenedioxythiophene: A route to highly conducting polymer surface layers. Macromolecules 2004, 37, 4538–4543. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kwon, M.-H.; Min, Y.-K.; Kwon, S.; Ihm, D.-W. Self-assembly and crystalline growth of poly(3,4-ethylenedioxythiophene) nanofilms. Adv. Mater. 2007, 19, 3501–3506. [Google Scholar] [CrossRef]
- Im, S.G.; Gleason, K.K. Systematic control of the electrical conductivity of poly(3,4-ethylenedioxythiophene) via oxidative chemical vapor deposition. Macromolecules 2007, 40, 6552–6556. [Google Scholar] [CrossRef]
- Cho, B.; Park, K.S.; Baek, J.; Oh, H.S.; Koo Lee, Y.-E.; Sung, M.M. Single-crystal poly(3,4-ethylenedioxythiophene) nanowires with ultrahigh conductivity. Nano Lett. 2014, 14, 3321–3327. [Google Scholar] [CrossRef]
- Yamato, H.; Kai, K.-I.; Ohwa, M.; Asakura, T.; Koshiba, T.; Wernet, W. Synthesis of free-standing poly (3,4-ethylenedioxythiophene) conducting polymer films on a pilot scale. Mater. Synth. Met. 1996, 5, 125–130. [Google Scholar] [CrossRef]
- Berggren, M.; Crispin, X.; Fabiano, S.; Jonsson, M.P.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K.; Zozoulenko, I. Ion Electron–Coupled Functionality in Materials and Devices Based on Conjugated Polymers. Adv. Mater. 2019, 31, 1805813. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT-PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 15000517. [Google Scholar] [CrossRef]
- Aijan, F.N.; Casado, N.; Rębiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganas, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Mantione, D.; del Agua, I.; Schaafsma, W.; Diez-Garcia, J.; Castro, B.; Sardon, H.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene):GlycosAminoGlycan aqueous dispersions: Toward electrically conductive bioactive materials for neural interfaces. Macromol. Biosci. 2016, 16, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Harman, D.G.; Gorkin, R.; Stevens, L.; Thompson, B.; Wagner, K.; Weng, B.; Chung, J.H.Y.; Panhuis, M.; Wallace, G.G. Poly(3,4-ethylenedioxythiophene): Dextran sulfate (PEDOT:DS)—A highly processable conductive organic biopolymer. Acta Biomater. 2015, 14, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Liu, H.; Jiang, C.; Zhu, Y.; Wan, M.; Dai, L.; Jiang, L. Biomolecule-doped PEDOT with three-dimensional nanostructures as efficient catalyst for oxygen reduction reaction. Small 2014, 10, 2087–2095. [Google Scholar] [CrossRef]
- Ner, Y.; Invernale, M.A.; Grote, J.G.; Stuart, J.A.; Sotzing, G.A. Facile chemical synthesis of DNA-doped PEDOT. Synth. Met. 2010, 160, 351–353. [Google Scholar] [CrossRef]
- Horikawa, M.; Fujiki, T.; Shirosaki, T.; Ryu, N.; Sakurai, H.; Nagaoka, S.; Ihara, H. The development of a highly conductive PEDOT system by doping with partially crystalline sulfated cellulose and its electric conductivity. J. Mater. Chem. C 2015, 3, 8881–8887. [Google Scholar] [CrossRef]
- Mawad, D.; Lauto, A.; Wallace, G.G. Conductive Polymer Hydrogels, Polymeric Hydrogels As Smart Biomaterials; Springer: Cham, Switzerland, 2016; pp. 19–44. ISBN 9783319253206. [Google Scholar]
- Teshima, T.; Nakashima, H.; Kasai, N.; Sasaki, S.; Tanaka, A.; Tsukada, S.; Sumitomo, K. Mobile silk fibroin electrode for manipulation and electrical stimulation of adherent cells. Adv. Funct. Mat. 2016, 26, 8185–8193. [Google Scholar] [CrossRef]
- Sasaki, M.; Karikkineth, B.C.; Nagamine, K.; Kaji, H.; Torimitsu, K.; Nishizawa, M. Highly conductive stretchable and biocompatible electrode-hydrogel hybrids for advanced tissue engineering. Adv. Healthc. Mater. 2014, 3, 1919–1927. [Google Scholar] [CrossRef]
- Green, R.A.; Hassarati, R.T.; Goding, J.A.; Baek, S.; Lovell, N.H.; Martens, P.J.; Poole-Warren, L.A. Conductive hydrogels: Mechanically robust hybrids for use as biomaterials. Macromol. Biosci. 2012, 12, 494–501. [Google Scholar] [CrossRef]
- Hofmann, A.I.; Smaal, W.T.T.; Mumtaz, M.; Katsigiannopoulos, D.; Brochon, C.; Schütze, F.; Hild, O.R.; Cloutet, E.; Hadziioannou, G. An Alternative Anionic Polyelectrolyte for Aqueous PEDOT Dispersions: Toward Printable Transparent Electrodes. Angew. Chem. Int. Ed. 2015, 54, 8506–8510. [Google Scholar] [CrossRef]
- Hofmann, A.I.; Cloutet, E.; Hadziioannou, G. Materials for Transparent Electrodes: From Metal Oxides to Organic Alternatives. Adv. Electron. Mater. 2018, 54, 1700412. [Google Scholar] [CrossRef]
- Villarroel Marquez, A.; Salinas, G.; Abarkan, M.; Idir, M.; Brochon, C.; Hadziioannou, G.; Raoux, M.; Kuhn, A.; Lang, J.; Cloutet, E. Design of potassium-selective mixed ion/electron conducting polymers. Macromol. Rapid Commun. 2020, 41, 2000134. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Vurro, F.; Picelli, F.; Bettelli, M.; Zappettini, A.; Coppedè, N. A mathematical model of OECTs with variable internal geometry. Sens. Actuators A 2020, 304, 111894. [Google Scholar] [CrossRef]
- Friedlein, J.T.; Shaheen, S.E.; Malliaras, G.G.; McLeod, R.R. Optical Measurements Revealing Nonuniform Hole Mobility in Organic Electrochemical Transistors. Adv. Electron. Mater. 2015, 1, 1500189. [Google Scholar] [CrossRef]
- Friedlein, J.T.; McLeod, R.R.; Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Okamoto, K.; Luscombe, C.K. Controlled polymerizations for the synthesis of semiconducting conjugated polymers. Polym. Chem. 2011, 2, 2424. [Google Scholar] [CrossRef]
- So, R.C.; Carreon-Asok, A.C. Molecular design, synthetic strategies, and applications of cationic polythiophenes. Chem. Rev. 2019, 119, 11442–11509. [Google Scholar] [CrossRef]
- Bousquet, A.; Awada, H.; Hiorns, R.C.; Dagron-Lartigau, C.; Billon, L. Conjugated-polymer grafting on inorganic and organic substrates: A new trend in organic electronic materials. Prog. Pol. Sci. 2014, 39, 1847–1877. [Google Scholar] [CrossRef]
- Leger, J.; Berggren, M.; Carter, S. Iontronics: Ionic Carriers in Organic Electronic Materials and Devices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–28. ISBN 9781138116504. [Google Scholar]
- Sekine, C.; Tsubata, Y.; Yamada, T.; Kitano, M.; Doi, S. Recent progress of high performance polymer OLED and OPV materials for organic printed electronics. Sci. Technol. Adv. Mater. 2014, 15, 034203–034218. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Guardado, J.O.; Salleo, A. Structural Effects of Gating Poly(3-hexylthiophene) through an Ionic Liquid. Adv. Funct. Mater. 2017, 27, 1–12. [Google Scholar] [CrossRef]
- Pacheco-Moreno, C.M.; Schreck, M.; Scaccabarozzi, A.D.; Bourgun, P.; Wantz, G.; Stevens, M.M.; Dautel, O.J.; Stingelin, N. The Importance of Materials Design to Make Ions Flow: Toward Novel Materials Platforms for Bioelectronics Applications. Adv. Mater. 2017, 29, 1604446. [Google Scholar] [CrossRef]
- Flagg, L.Q.; Bischak, C.G.; Onorato, J.W.; Rashid, R.B.; Luscombe, C.K.; Ginger, D.S. Polymer Crystallinity Controls Water Uptake in Glycol Side-Chain Polymer Organic Electrochemical Transistors. J. Am. Chem. Soc. 2019, 141, 4345–4354. [Google Scholar] [CrossRef]
- Giovannitti, A.; Sbircea, D.-T.; Inal, I.; Nielsen, C.B.; Bandiello, E.; Hanifi, D.A.; Sessolo, M.; Malliaras, G.G.; McCulloch, I.; Rivnay, J. Controlling the mode of operation of organic transistors through side-chain engineering. Proc. Natl. Acad. Sci. USA 2016, 113, 12017–12022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cendra, C.; Giovannitti, A.; Savva, A.; Venkatraman, V.; McCulloch, I.; Salleo, A.; Inal, S.; Rivnay, J. Role of the Anion on the Transport and Structure of Organic Mixed Conductors. Adv. Funct. Mater. 2019, 29, 1807034. [Google Scholar] [CrossRef] [Green Version]
- Venkateshvaran, D.; Nikolka, M.; Sadhanala, A.; Lemaur, V.; Zelazny, M.; Kepa, M.; Hurhangee, M.; Kronemeijer, A.J.; Pecunia, V.; Nasrallah, I.; et al. Approaching disorder-free transport in high-mobility conjugated polymers. Nature 2014, 515, 384–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hurhangee, M.; Nikolka, M.; Zhang, W.; Kirkus, M.; Neophytou, M.; Cryer, S.J.; Harkin, D.; Hayoz, P.; Abdi-Jalebi, M.; et al. Dithiopheneindenofluorene (TIF) semiconducting polymers with very high mobility in field-effect transistors. Adv. Mater. 2017, 29, 1702523. [Google Scholar] [CrossRef] [Green Version]
- Nikolka, M.; Nasrallah, I.; Rose, B.; Ravva, M.K.; Broch, K.; Sadhanala, A.; Harkin, D.; Charmet, J.; Hurhangee, M.; Brown, A.; et al. High operational and environmental stability of high-mobility conjugated polymer field-effect transistors through the use of molecular additives. Nat. Mater. 2017, 16, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Parr, Z.S.; Halaksa, R.; Finn, P.A.; Rashid, R.B.; Kovalenko, A.; Weiter, M.; Rivnay, J.; Krajčovič, J.; Nielsen, C.B. Glycolated Thiophene-Tetrafluorophenylene Copolymers for Bioelectronic Applications: Synthesis by Direct Heteroarylation Polymerisation. ChemPlusChem 2019, 84, 1384–1390. [Google Scholar] [CrossRef]
- Xu, K.; Sun, H.; Ruoko, T.-P.; Wang, G.; Kroon, R.; Kolhe, N.B.; Puttisong, Y.; Liu, X.; Fazzi, D.; Shibata, K.; et al. Ground-state electron transfer in all-polymer donor-acceptor heterojunctions. Nat. Mater. 2020, 19, 738–744. [Google Scholar] [CrossRef]
- Koutsouras, D.A.; Perrier, R.; Villarroel Marquez, A.; Pirog, A.; Pedraza, E.; Cloutet, E.; Renaud, S.; Raoux, M.; Malliaras, G.G.; Lang, J. Simultaneous monitoring of single cell and of micro-organ activity by PEDOT:PSS covered multi-electrode arrays. Mater. Sci. Eng. C 2017, 81, 84–89. [Google Scholar] [CrossRef]
- Giovannitti, A.; Nielsen, C.B.; Sbircea, D.-T.; Inal, S.; Donahue, M.; Niazi, M.R.; Hanifi, D.A.; Amassian, A.; Malliaras, G.G.; Rivnay, J.; et al. N-type organic electrochemical transistors with stability in water. Nat. Commun. 2016, 7, 13066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannitti, A.; Thorley, K.J.; Nielsen, C.B.; Li, J.; Inal, S.; Donahue, M.J.; Malliaras, G.G.; Rivnay, J.; McCulloch, I. Redox-Stability of Alkoxy-BDT Copolymers and their Use for Organic Bioelectronic Devices. Adv. Funct. Mater. 2018, 28, 1706325. [Google Scholar] [CrossRef]
- Sun, H.; Vagin, M.; Wang, S.; Crispin, X.; Forchheimer, R.; Berggren, M.; Fabiano, S. Complementary Logic Circuits Based on High-Performance n-Type Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1704916. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Jing, Y.; Rong, Y.; Facchetti, A.; Yao, Y. Heavily n-dopable π-conjugated redox polymers with ultrafast energy storage capability. J. Am. Chem. Soc. 2015, 137, 4956–4959. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S.A. High electron mobility in ladder polymer field-effect transistors. J. Am. Chem. Soc. 2003, 125, 13656–13657. [Google Scholar] [CrossRef]
- Takeya, J.; Yamagishi, M.; Tominari, Y.; Hirahara, R.; Nakazawa, Y.; Nishikawa, T.; Kawase, T.; Shimoda, T.; Ogawa, S. Very High-Mobility Organic Single-Crystal Transistors with in-Crystal Conduction Channels. Appl. Phys. Lett. 2007, 90, 102120. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-Fullerene Acceptors for Organic Solar Cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Bischak, C.G.; Flagg, L.Q.; Yan, K.; Li, C.-Z.; Ginger, D.S. Fullerene Active Layers for n-Type Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2019, 11, 28138–28144. [Google Scholar] [CrossRef]
- Biosensors Market Size Worth $36.0 Billion By 2027 | CAGR: 7.9%. Available online: https://www.grandviewresearch.com/press-release/global-biosensors-market (accessed on 9 April 2020).
- Kassal, P.; Steinberg, M.D.; Murkovic, I. Wireless chemical sensors and biosensors: A review. Sens. Actuators B 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Bresadola, M. Medicine and science in the life of Luigi Galvani (1737–1798). Brain Res. Bull. 1998, 46, 367–380. [Google Scholar] [CrossRef]
- Bouton, C.E.; Shaikhouni, A.; Annetta, N.V.; Bockbrader, M.A.; Friedenberg, D.A.; Nielson, D.M.; Sharma, G.; Sederberg, P.B.; Glenn, B.C.; Mysiw, W.J.; et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016, 533, 247–250. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 2019, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Kao, C.; Konrad, P.; Milner, T.; Kim, J.; Mahadevan-Jansen, A.; Jansen, E.D. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Byophys. J. 2007, 93, 2567–2580. [Google Scholar] [CrossRef] [Green Version]
- Russa, M.F.L.; Qi, L.S. The New State of the Art: Cas9 for Gene Activation and Repression. Mol. Cell. Biol. 2015, 35, 3800–3809. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.Y.; Langer, R.; Ingber, D.E. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3201–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612. [Google Scholar] [CrossRef] [PubMed]
- Pappa, A.M.; Ohayon, D.; Giovannitti, A.; Maria, I.P.; Savva, A.; Uguz, I.; Rivnay, J.; McCulloch, I.; Owens, R.M.; Inal, S. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 2018, 4, eaat0911. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Yao, C.; Liu, Y.; Hsing, I.-M. 16-Channel Organic Electrochemical Transistor Array for In Vitro Conduction Mapping of Cardiac Action Potential. Adv. Healthc. Mater. 2016, 5, 2345–2351. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Hervé, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guo, T.; Zhou, L.; Offenhäusser, A.; Mayer, D. Label-Free Split Aptamer Sensor for Femtomolar Detection of Dopamine by Means of Flexible Organic Electrochemical Transistors. Materials 2020, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, Y.; Wang, N.; Yang, A.; Li, Y.; Wu, J.; Ju, H.; Yan, F. Organic Electrochemical Transistors for the Detection of Cell Surface Glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, N.; Yang, A.; Law, H.K.; Li, I.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Ali, M.A.; Mondal, K.; Jiao, Y.; Oren, S.; Xu, Z.; Sharma, A.; Dong, L. Microfluidic Immuno-Biochip for Detection of Breast Cancer Biomarkers Using Hierarchical Composite of Porous Graphene and Titanium Dioxide Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20570. [Google Scholar] [CrossRef]
- Schmoltner, K.; Kofler, J.; Klug, A.; List-Kratochvil, E.J.W. Electrolyte-Gated Organic Field-Effect Transistor for Selective Reversible Ion Detection. Adv. Mater 2013, 25, 6895. [Google Scholar] [CrossRef]
- Sessolo, M.; Rivnay, J.; Bandiello, E.; Malliaras, G.G.; Bolink, H.J. Ion-selective organic electrochemical transistors. Adv. Mater. 2014, 26, 4803–4807. [Google Scholar] [CrossRef]
- Ghittorelli, M.; Lingstedt, L.; Romele, P.; Crăciun, N.I.; Kovács-Vajna, Z.M.; Blom, P.W.M.; Torricelli, F. High-Sensitivity Ion Detection at Low Voltages with Current-Driven Organic Electrochemical Transistors. Nat. Commun. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Wustoni, S.; Combe, C.; Ohayon, D.; Hassan Akhtar, M.; McCulloch, I.; Inal, S. Membrane-Free Detection of Metal Cations with an Organic Electrochemical Transistor. Adv. Funct. Mater. 2019, 29, 1904403. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.M.; Yan, F. Organic Electrochemical Transistors Integrated in Flexible Microfluidic Systems and Used for Label-Free DNA Sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef]
- Attili, S.K.; Lesar, A.; McNeill, A.; Camacho-Lopez, M.; Moseley, H.; Ibbotson, S.; Samuel, I.D.; Ferguson, J. An open pilot study of ambulatory photodynamic therapy using a wearable lowirradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br. J. Dermatol. 2009, 161, 170–173. [Google Scholar] [CrossRef]
- Bansal, A.K.; Hou, S.; Kulyk, O.; Bowman, E.M.; Samuel, I.D. Wearable organic optoelectronic sensors for medicine. Adv. Mater. 2015, 27, 7638–7644. [Google Scholar] [CrossRef] [Green Version]
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-organic optoelectronic sensor for pulse oximetry. Nat. Commun. 2014, 5, 5745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Kim, D.-H. Flexible and stretchable electronics paving the way for soft robotics. Soft Robot. 2014, 1, 53–62. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.A.; Bao, Z.; Baldwin, K.; Dodabalapur, A.; Crone, B.; Raju, V.R.; Kuck, V.; Katz, H.; Amundson, K.; Ewing, J.; et al. Paperlike electronic displays: Large-area rubber-stamped plastic sheets of electronics and microencapsulated electrophoretic inks. Proc. Natl. Acad. Sci. USA 2001, 98, 4835–4840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawase, T.; Sirringhaus, H.; Friend, R.H.; Shimoda, T. Inkjet printed via-hole interconnections and resistors for all-polymer transistor circuits. Adv. Mater. 2001, 13, 1601–1605. [Google Scholar] [CrossRef]
- Knobloch, A.; Bernds, A.; Clemens, W. Printed polymer transistors. In Proceedings of the IEEE Conference on Polymers and Adhesives in Microelectronics and Photonics, Potsdam, Germany, 21–24 October 2001; pp. 84–90. [Google Scholar] [CrossRef]
- Rogers, J.A.; Bao, Z.; Dodabalapur, A.; Makhija, A. Organic smart pixels and complementary inverter circuits formed on plastic substrates by casting and rubber stamping. IEEE Electron Device Lett. 2000, 21, 100–103. [Google Scholar] [CrossRef]
- Someya, T.; Sakurai, T. Integration of organic field-effect transistors and rubbery pressure sensors for artificial skin applications. In Proceedings of the Technical Digest—International Electron Devices Meeting, Washington, DC, USA, 8–10 December 2003; pp. 203–206. [Google Scholar] [CrossRef]
- Someya, T.; Kawaguohi, H.; Sakurai, T. Cut-and-paste organic FET customized ICs for application to artificial skin. Dig. Technol. Pap. IEEE Int. Solid State Circuits Conf. 2003, 47, 232–233. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.; Malliaras, G.G.; Someya, T. Biomedical devices go wild. Sci. Adv. 2018, 4, eaav1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, G.; Tee, B.C.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.H.; Bao, Z. Highly skinconformal microhairy sensor for pulse signal amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Sekitani, T.; Kato, Y.; Iba, S.; Shinaoka, H.; Someya, T.; Sakurai, T.; Takagi, S. Bending experiment on pentacene field-effect transistors on plastic films. Appl. Phys. Lett. 2005, 86, 073511. [Google Scholar] [CrossRef]
- Trung, T.Q.; Ramasundaram, S.; Hwang, B.-U.; Lee, N.-E. An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 2016, 28, 502–509. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-cells electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Campana, A.; Cramer, T.; Simon, D.T.; Berggren, M.; Biscarini, F. Electrocardiographic recording with conformable organic electrochemical transistor fabricated on resorbable bioscaffold. Adv. Mater. 2014, 26, 3874–3878. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Eles, J.R.; Ludwig, J.P.; Seymour, N.J.; Michelson, W.E.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D.Y. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef] [PubMed]
- Gilletti, A.; Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 2006, 3, 189. [Google Scholar] [CrossRef] [PubMed]
- Kolarcik, C.L.; Luebben, S.D.; Sapp, S.A.; Hanner, J.; Snyder, N.; Kozai, T.D.Y.; Chang, E.; Nabity, J.A.; Nabity, S.T.; Lagenaur, C.F.; et al. Elastomeric and soft conducting microwires for implantable neural interfaces. Soft Matter. 2015, 11, 4847–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R.; Abidian, M.R. Conducting polymers for neural prosthetic and neural interface applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y. The history and horizons of microscale neural interface. Micromachines 2018, 9, 445. [Google Scholar] [CrossRef] [Green Version]
- Alba, N.; Du, Z.; Catt, K.; Kozai, T.; Cui, X. In vivo electrochemical analysis of a PEDOT/MWCNT neural electrode coating. Biosensors 2015, 5, 618–646. [Google Scholar] [CrossRef] [Green Version]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.S.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M. PEDOT-CNT composite microelectrodes for recording and electrostimulation applications: Fabrication, morphology, and electrical properties. Front. Neuroeng. 2012, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Williamson, A.; Ferro, M.; Leleux, P.; Ismailova, E.; Kaszas, A.; Doublet, T.; Quilichini, P.; Rivnay, J.; Rózsa, B.; Katona, G.; et al. Localized Neuron Stimulation with Organic Electrochemical Transistors on Delaminating Depth Probes. Adv. Mater. 2015, 27, 4405–4410. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for organic transistors. Mat. Today 2007, 10, 28–36. [Google Scholar] [CrossRef]
- McCoy, C.H.; Wrighton, M.S. Potential-Dependent Conductivity of Conducting Polymers Yields Opportunities for Molecule-Based Devices: A Microelectrochemical Push-Pull Amplifier Based on Two Different Conducting Polymer Transistors. Chem. Mater. 1993, 5, 914. [Google Scholar] [CrossRef]
- Andersson, P.; Nilsson, D.; Svensson, P.-O.; Chen, M.; Malmström, A.; Remonen, T.; Kugler, T.; Berggren, M. Active matrix displays based on all-organic electrochemical smart pixels printed on paper. Adv. Mater. 2002, 14, 1460. [Google Scholar] [CrossRef]
- Nilsson, D.; Robinson, N.; Berggren, M.; Forchheimer, R. Electrochemical logic circuits. Adv. Mater. 2005, 17, 353–358. [Google Scholar] [CrossRef]
- Braendlein, M.; Pappa, A.-M.; Ferro, M.; Lopresti, A.; Acquaviva, C.; Mamessier, E.; Malliaras, G.G.; Owens, R.M. Lactate detection in tumor cell cultures using organic transistor circuits. Adv. Mater. 2017, 29, 1605744. [Google Scholar] [CrossRef] [PubMed]
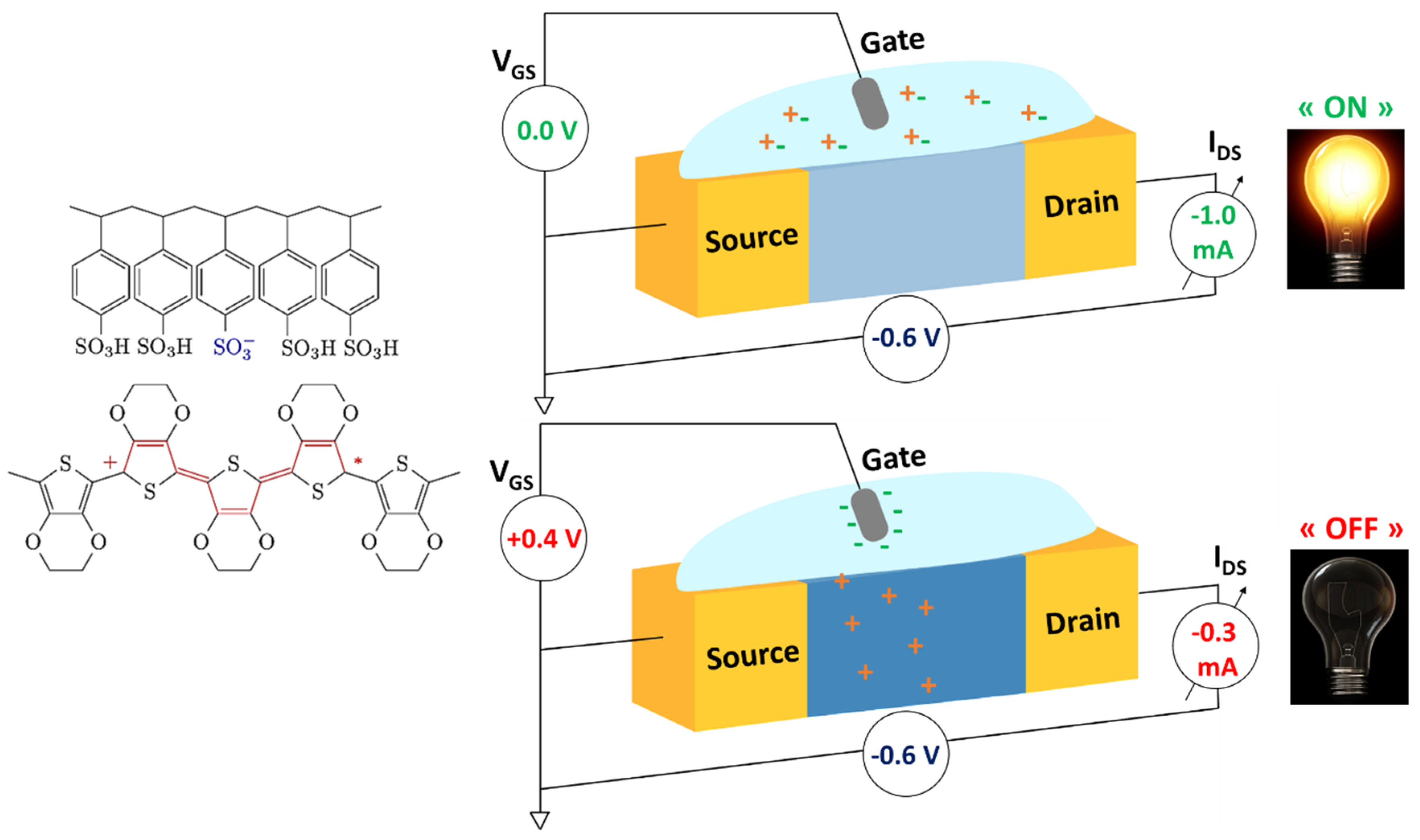


| Material | Ref. | Max gm (mS) | Max ID (mA) | ΔVgeff (mV·dec−1) | Device Sensitivity | Application | Analyte | LOD Sensitivity | Selectivity |
|---|---|---|---|---|---|---|---|---|---|
| PEDOT:PSS + SplitAptamers (Gate) | [108] | 4 | 2.5 | - | - | Neurotransmitter sensing | DA, AA, UA, GABA | 0.5 × 10−15 M; 10−15–10−9 M | Dopamine (DA) |
| PEDOT:PSS + Glycan + PDCNT (Gate) | [109] | 0.4 | 0.275 | 11.4 | - | Cancer cells detection | Mannose | 10 cells/uL; 104–105 cells/uL (sat) | MCF-7 |
| PEDOT:PSS + m-antiHER2 (Gate) | [110,111] | - | - | - | - | Cancer protein biomarkers detection | HER2 | 10−16 M; 10−7–10−14 M | HER2 biomarker (10 cells/uL) |
| P3HT | [112] * | - | 0.012 * | 62 * | 0.25-0.5 µA·dec−1 * | Ion detection * | Na+ | 10−6 M; 10−6–10−1 M | Na+ |
| PEDOT:PSS + Ion-selective membrane | [113] | 1.2 | 0.7 | 48 | 47 µA·dec−1; 120 mV·V−1·dec−1 | Ion sensing | K+, Na+ | 1.5·10−5 M; 10−4–10−1 M | K+ |
| PEDOT:PSS + K+-selective membrane | [114] | - | 414 | 1035 mV·V−1·dec−1 | Ion sensing | K+, Na+ | 10−4 M; 10−4–1 M | K+ | |
| PEDOT:PSS + P(T18cr6-ran-EDOT) (Gate) | [115] | 10–15 | - | 49 µA·dec−1 | Ion sensing | K+, Na+ | 10−4 M; 10−4–1 M | K+ | |
| PEDOT:PSS + P(T15cr5-ran-EDOT) (Gate) | [115] | 11–15 | - | 37 µA·dec−1 | Ion sensing | K+, Na+ | 2 × 10−5 M; 10−5–1 M | Na+ | |
| PEDOT:PSS + ssDNA (Gate) | [116] | 0.1 | 145–149 | - | Label-free DNA sensing | ssDNA | 10−12 M; 10−6–10−1 M | Hybridized DNA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, A.V.; McEvoy, N.; Pakdel, A. Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics. Molecules 2020, 25, 5288. https://doi.org/10.3390/molecules25225288
Marquez AV, McEvoy N, Pakdel A. Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics. Molecules. 2020; 25(22):5288. https://doi.org/10.3390/molecules25225288
Chicago/Turabian StyleMarquez, Ariana Villarroel, Niall McEvoy, and Amir Pakdel. 2020. "Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics" Molecules 25, no. 22: 5288. https://doi.org/10.3390/molecules25225288
APA StyleMarquez, A. V., McEvoy, N., & Pakdel, A. (2020). Organic Electrochemical Transistors (OECTs) Toward Flexible and Wearable Bioelectronics. Molecules, 25(22), 5288. https://doi.org/10.3390/molecules25225288