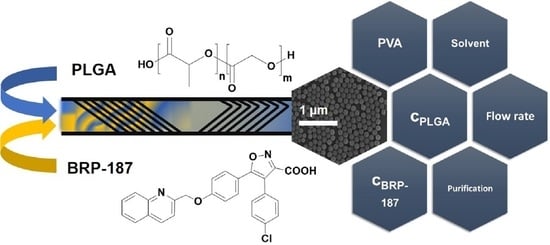
Optimized Encapsulation of the FLAP/PGES-1 Inhibitor BRP-187 in PVA-Stabilized PLGA Nanoparticles Using Microfluidics
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Nanoparticle Formulation
2.3. Nanoparticle Purification
2.4. Dynamic Light Scattering
2.5. UV-Vis Spectroscopy
2.6. PVA Assay
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Variation of the Flow Rates
3.2. Variation of the Solvent/Co-Solvent (Ratio)
3.3. The Importance of the Initial Polymer Concentration
3.4. The Crux with the Increasing Drug Feed
3.5. Removement of Free Drug Crystals
3.6. Variation of the Surfactant Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Acetone |
| ACN | Acetonitrile |
| DMSO | Dimethyl sulfoxide |
| EE | Encapsulation efficiency |
| EtOAc | Ethyl acetate |
| FLAP | 5-lipoxygenase-activating protein |
| GA | Glycolic acid |
| LA | Lactic acid |
| LO | lipoxygenase |
| LC | Loading capacity |
| mPGES-1 | Microsomal prostaglandin E2 synthase-1 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| NPs | Nanoparticles |
| PLGA | Poly(lactic-co-glycolic acid) |
| PTFE | Polytetrafluoroethylene |
| PVA | Poly(vinyl alcohol) |
| PGE2 | Prostaglandin E2 |
| SEM | Scanning electron microscopy |
| THF | Tetrahydrofuran |
References
- Kabanov, A.; Gendelman, H. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, M.; Kumar, A.; Malinee, M.; Sharma, T.K. Nanomedicine: Diagnosis, treatment, and potential prospects. In Nanoscience in Medicine; Daima, H.K., PN, N., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 297–331. [Google Scholar]
- El-Sayed, A.; Kamel, M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2019, 27, 19200–19213. [Google Scholar] [CrossRef] [PubMed]
- Shkodra-Pula, B.; Vollrath, A.; Schubert, U.S.; Schubert, S. Polymer-based nanoparticles for biomedical applications. In Colloids for Nanobiotechnology-Synthesis, Characterization and Potential Applications; Parak, W., Feliu, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 16, pp. 233–252. [Google Scholar]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Silva, M.E.S.R.E.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; De Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Shkodra-Pula, B.; Kretzer, C.; Jordan, P.M.; Klemm, P.; Koeberle, A.; Pretzel, D.; Banoglu, E.; Lorkowski, S.; Wallert, M.; Höppener, S.; et al. Encapsulation of the dual FLAP/mPEGS-1 inhibitor BRP-187 into acetalated dextran and PLGA nanoparticles improves its cellular bioactivity. J. Nanobiotechnol. 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol. Adv. 2018, 36, 1709–1723. [Google Scholar] [CrossRef]
- Garscha, U.; Voelker, S.; Pace, S.; Gerstmeier, J.; Emini, B.; Liening, S.; Rossi, A.; Weinigel, C.; Rummler, S.; Schubert, U.S.; et al. BRP-187: A potent inhibitor of leukotriene biosynthesis that acts through impeding the dynamic 5-lipoxygenase/5-lipoxygenase-activating protein (FLAP) complex assembly. Biochem. Pharmacol. 2016, 119, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Banoglu, E.; Çelikoğlu, E.; Völker, S.; Olgaç, A.; Gerstmeier, J.; Garscha, U.; Çalışkan, B.; Schubert, U.S.; Carotti, A.; Macchiarulo, A.; et al. 4,5-Diarylisoxazol-3-carboxylic acids: A new class of leukotriene biosynthesis inhibitors potentially targeting 5-lipoxygenase-activating protein (FLAP). Eur. J. Med. Chem. 2016, 113, 1–10. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainsford, K.D. Anti-Inflammatory Drugs in the 21st Century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Streck, S.; Neumann, H.; Nielsen, H.M.; Rades, T.; McDowell, A. Comparison of bulk and microfluidics methods for the formulation of poly-lactic-co-glycolic acid (PLGA) nanoparticles modified with cell-penetrating peptides of different architectures. Int. J. Pharm. X 2019, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Heuck, G.; Ip, S.; Ramsay, E. Microfluidics: A transformational tool for nanomedicine development and production. J. Drug Target. 2016, 24, 821–835. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.; Álvarez, F.H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, D.; Yuan, T.; Chen, X. Microfluidic PCR Chips. Nano Biomed. Eng. 2011, 3. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, C.; Riordon, J.; Sinton, D.; Moffitt, M.G. Microfluidic Manufacturing of Polymeric Nanoparticles: Comparing Flow Control of Multiscale Structure in Single-Phase Staggered Herringbone and Two-Phase Reactors. Langmuir 2016, 32, 12781–12789. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Controlled and tunable polymer particles’ production using a single microfluidic device. Appl. Nanosci. 2018, 8, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Tagami, T.; Hoshikawa, A.; Ozeki, T. The Use of an Efficient Microfluidic Mixing System for Generating Stabilized Polymeric Nanoparticles for Controlled Drug Release. Biol. Pharm. Bull. 2018, 41, 899–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Operti, M.C.; Dölen, Y.; Keulen, J.; Van Dinther, E.A.; Figdor, C.G.; Tagit, O. Microfluidics-Assisted Size Tuning and Biological Evaluation of PLGA Particles. Pharmaceutics 2019, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef]
- Chen, R.; Wulff, J.E.; Moffitt, M.G. Microfluidic Processing Approach to Controlling Drug Delivery Properties of Curcumin-Loaded Block Copolymer Nanoparticles. Mol. Pharm. 2018, 15, 4517–4528. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Wang, H.; Wilson, R.; Hui, Y.; Yu, L.; Wibowo, D.; Zhang, C.; Whittaker, A.K.; Middelberg, A.P.J.; et al. Bioinspired Core–Shell Nanoparticles for Hydrophobic Drug Delivery. Angew. Chem. Int. Ed. 2019, 58, 14357–14364. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Microfluidic-Chipshop, Fluidic 187. Available online: https://www.microfluidic-chipshop.com/catalogue/microfluidic-chips/polymer-chips/micro-mixer/micro-mixer-fluidic-187/ (accessed on 7 August 2020).
- Lim, J.-M.; Bertrand, N.; Valencia, P.M.; Rhee, M.; Langer, R.; Jon, S.; Farokhzad, O.C.; Karnik, R. Parallel microfluidic synthesis of size-tunable polymeric nanoparticles using 3D flow focusing towards in vivo study. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shkodra-Pula, B.; Grune, C.; Traeger, A.; Vollrath, A.; Schubert, S.; Fischer, D.; Schubert, U.S. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int. J. Pharm. 2019, 566, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From particle preparation to in vivo applications. In Polymer Nanoparticles for Nanomedicines: A Guide for their Design, Preparation and Development; Vauthier, C., Ponchel, G., Eds.; Springer: Cham, Switzerland, 2016; pp. 17–53. [Google Scholar]
- Spek, S.; Haeuser, M.; Schaefer, M.; Langer, K. Characterisation of PEGylated PLGA nanoparticles comparing the nanoparticle bulk to the particle surface using UV/vis spectroscopy, SEC, 1H NMR spectroscopy, and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2015, 347, 378–385. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Totten, J.D.; Johnston, B.F.; Seib, F.P. Microfluidic-assisted silk nanoparticle tuning. Nanoscale Adv. 2019, 1, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Perevyazko, I.Y.; Vollrath, A.; Hornig, S.; Pavlov, G.M.; Schubert, U.S. Characterization of poly(methyl methacrylate) nanoparticles prepared by nanoprecipitation using analytical ultracentrifugation, dynamic light scattering, and scanning electron microscopy. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3924–3931. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef] [Green Version]
- Swarnavalli, G.C.J.; Kannappan, V.; Roopsingh, D.; Joseph, V. Effect of solvent on the size and properties of polymeric nanoparticles of poly(epsilon-caprolactam). J. Mol. Liq. 2017, 236, 61–67. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, H.S.; Choung, I.Y.; Cho, K.I.; Ahn, Y.; Choi, E.J. The effect of type of organic phase solvents on the particle size of poly(d,l-lactide-co-glycolide) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Haque, S.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M.; Whittaker, M.R. Suggested Procedures for the Reproducible Synthesis of Poly(d,l-lactideco-glycolide) Nanoparticles Using the Emulsification Solvent Diffusion Platform. Curr. Nanosci. 2018, 14, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Sahana, D.; Mittal, G.; Bhardwaj, V.; Kumar, M.R. PLGA Nanoparticles for Oral Delivery of Hydrophobic Drugs: Influence of Organic Solvent on Nanoparticle Formation and Release Behavior In Vitro and In Vivo Using Estradiol as a Model Drug. J. Pharm. Sci. 2008, 97, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- ECHA, Acetonitrile. Available online: https://echa.europa.eu/brief-profile/-/briefprofile/100.000.760 (accessed on 2 November 2020).
- ECHA, Tetrahydrofuran. Available online: https://echa.europa.eu/brief-profile/-/briefprofile/100.003.389 (accessed on 2 November 2020).
- ECHA, Ethyl Acetate. Available online: https://echa.europa.eu/brief-profile/-/briefprofile/100.005.001 (accessed on 2 November 2020).
- Carr, A.C.; Piunova, V.A.; Maarof, H.; Rice, J.E.; Swope, W.C. Influence of Solvent on the Drug-Loading Process of Amphiphilic Nanogel Star Polymers. J. Phys. Chem. B 2018, 122, 5356–5367. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, I.; Edirisinghe, M. Microfluidic preparation of polymer nanospheres. J. Nanopart. Res. 2014, 16, 2626. [Google Scholar] [CrossRef] [Green Version]
- Dhakar, R.C.; Maurya, S.D.; Sagar, B.P.S.; Bhagat, S.; Prajapati, S.K.; Jain, C.P. Variables influencing the drug entrapment efficiency of microspheres: A pharmaceutical review. Der Pharm. Lett. 2010, 2, 102–116. [Google Scholar]
- Fu, X.; Ping, Q.; Gao, Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J. Microencapsul. 2005, 22, 57–66. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Madlova, M.; Jones, S.A.; Zwerschke, I.; Ma, Y.; Hider, R.C.; Forbes, B. Poly(vinyl alcohol) nanoparticle stability in biological media and uptake in respiratory epithelial cell layers in vitro. Eur. J. Pharm. Biopharm. 2009, 72, 438–443. [Google Scholar] [CrossRef]
- Faraji, M.; Poursalehi, R.; Aliofkhazraei, M. The Effect of Surfactant on Colloidal Stability, Oxidation and Optical Properties of Aluminum Nanoparticles Prepared via Dc Arc Discharge in Water. Procedia Mater. Sci. 2015, 11, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Pancani, E.; Menendez-Miranda, M.; Pastor, A.; Brisset, F.; Bernet-Camard, M.-F.; Desmaele, D.; Gref, R. Nanoparticles with high payloads of pipemidic acid, a poorly soluble crystalline drug: Drug-initiated polymerization and self-assembly approach. Acta Pharm. Sin. B 2018, 8, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Sokolsky-Papkov, M.; Kabanov, A.V. Synthesis of Well-Defined Gold Nanoparticles Using Pluronic: The Role of Radicals and Surfactants in Nanoparticles Formation. Polymers 2019, 11, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]





| Formulation Parameter | DLS/ELS Measurement | Drug Content | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P# | Solvent | BRP-187 % (w/w) | cPLGA [mg mL−1] | cPVA [%] | a QW/QPS [mL min−1] | b Zavg [nm] | PDI | c ζ [mV] | d LC [%] | d EE [%] | |
| P1 | Ac | 0 | 5 | 0.3 | 0.4:0.1 | 194 | 0.11 | −32.80 | - | - | |
| P2 | Ac | 0 | 5 | 0.3 | 2.0:0.5 | 175 | 0.05 | −30.00 | - | - | |
| P3 | Ac | 0 | 5 | 0.3 | 8.0:2.0 | 119 | 0.18 | −30.20 | - | - | |
| P4 | Ac | 3 | 5 | 0.3 | 0.4:0.1 | 212 | 0.09 | −30.90 | 0.69 | 23.03 | |
| P5 | Ac | 3 | 5 | 0.3 | 2.0:0.5 | 172 | 0.05 | −30.37 | 0.42 | 14.10 | |
| P6 | Ac | 3 | 5 | 0.3 | 4.0:0.5 | 137 | 0.07 | −23.70 | 0.33 | 10.91 | |
| P7 | Ac | 3 | 5 | 0.3 | 6.0:0.5 | 141 | 0.10 | −27.70 | 0.59 | 19.57 | |
| P8 | Ac | 3 | 5 | 0.3 | 8.0:0.5 | 138 | 0.16 | −27.20 | 0.89 | 29.58 | |
| P9 | Ac | 3 | 5 | 0.3 | 8.0:2.0 | 121 | 0.12 | −33.25 | 0.59 | 19.80 | |
| P10 | e Ac:THF | 3 | 5 | 0.3 | 2.0:0.5 | 194 | 0.10 | −34.40 | 0.89 | 29.82 | |
| P11 | f Ac:EtOAc | 3 | 5 | 0.3 | 2.0:0.5 | 246 | 0.09 | −34.10 | 0.45 | 15.10 | |
| P12 | ACN | 3 | 5 | 0.3 | 2.0:0.5 | 196 | 0.10 | −29.10 | 0.59 | 19.51 | |
| P13 | Ac | 3 | 15 | 0.3 | 2.0:0.5 | 211 | 0.14 | −34.12 | 2.29 | 76.35 | |
| P14 | Ac | 3 | 25 | 0.3 | 2.0:0.5 | 233 | 0.17 | −32.42 | 3.25 | 108.31 | |
| P15 | Ac | 5 | 15 | 0.3 | 2.0:0.5 | 214 | 0.18 | −29.17 | 3.80 * | * | |
| P16 | Ac | 10 | 15 | 0.3 | 2.0:0.5 | 222 | 0.17 | −29.02 | 7.29 * | * | |
| P17 | e Ac:THF | 5 | 15 | 0.3 | 2.0:0.5 | 220 | 0.13 | −27.00 | 3.92 * | * | |
| P18 | e Ac:THF | 10 | 15 | 0.3 | 2.0:0.5 | 237 | 0.20 | −31.67 | 6.86 * | * | |
| P19 | Ac | 10 | 15 | 1.0 | 2.0:0.5 | 259 | 0.18 | −29.95 | 7.00 * | * | |
| P20 | Ac | 10 | 15 | 3.0 | 2.0:0.5 | 245 | 0.12 | −29.03 | 3.43 | 34.33 | |
| P# | Solvent | BRP-187 % (w/w) | cPLGA [mg mL−1] | cNP [mg mL−1] | a LC [%] | a EE [%] |
|---|---|---|---|---|---|---|
| P15 | Ac | 5 | 15 | 8.20 | 3.80 | * |
| fP15_f | 0.62 | 2.23 | 44.60 | |||
| cP15_c | 3.13 | 1.43 | 28.57 | |||
| P16 | Ac | 10 | 15 | 8.38 | 7.29 | * |
| fP16_f | 1.08 | 6.91 | 69.14 | |||
| cP16_c | 3.19 | 7.18 | 71.79 | |||
| P17 | b Ac/THF | 5 | 15 | 5.00 | 3.92 | * |
| fP17_f | 1.97 | 2.75 | 54.96 | |||
| cP17_c | 2.30 | 1.33 | 26.61 | |||
| P18 | b Ac/THF | 10 | 15 | 5.60 | 6.86 | * |
| fP18_f | 2.30 | 6.61 | 66.12 | |||
| cP18_c | 2.42 | 3.95 | 39.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behnke, M.; Vollrath, A.; Klepsch, L.; Beringer-Siemers, B.; Stumpf, S.; A. Czaplewska, J.; Hoeppener, S.; Werz, O.; S. Schubert, U. Optimized Encapsulation of the FLAP/PGES-1 Inhibitor BRP-187 in PVA-Stabilized PLGA Nanoparticles Using Microfluidics. Polymers 2020, 12, 2751. https://doi.org/10.3390/polym12112751
Behnke M, Vollrath A, Klepsch L, Beringer-Siemers B, Stumpf S, A. Czaplewska J, Hoeppener S, Werz O, S. Schubert U. Optimized Encapsulation of the FLAP/PGES-1 Inhibitor BRP-187 in PVA-Stabilized PLGA Nanoparticles Using Microfluidics. Polymers. 2020; 12(11):2751. https://doi.org/10.3390/polym12112751
Chicago/Turabian StyleBehnke, Mira, Antje Vollrath, Lea Klepsch, Baerbel Beringer-Siemers, Steffi Stumpf, Justyna A. Czaplewska, Stephanie Hoeppener, Oliver Werz, and Ulrich S. Schubert. 2020. "Optimized Encapsulation of the FLAP/PGES-1 Inhibitor BRP-187 in PVA-Stabilized PLGA Nanoparticles Using Microfluidics" Polymers 12, no. 11: 2751. https://doi.org/10.3390/polym12112751
APA StyleBehnke, M., Vollrath, A., Klepsch, L., Beringer-Siemers, B., Stumpf, S., A. Czaplewska, J., Hoeppener, S., Werz, O., & S. Schubert, U. (2020). Optimized Encapsulation of the FLAP/PGES-1 Inhibitor BRP-187 in PVA-Stabilized PLGA Nanoparticles Using Microfluidics. Polymers, 12(11), 2751. https://doi.org/10.3390/polym12112751