Terpenoids and Their Biosynthesis in Cyanobacteria
Abstract
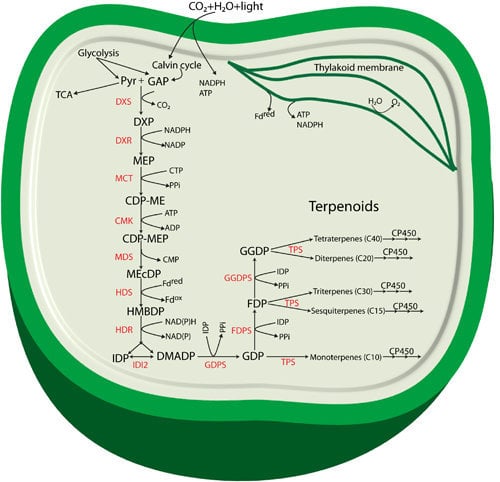
:1. Introduction
2. Specific Enzymes Involved in the MEP Pathway

3. Terpenoids Produced in Cyanobacteria
| Enzymes | Genes | Alternative Gene Symbols | Gene ID | Gene Size (bp) |
|---|---|---|---|---|
| 1-deoxy-d-xylulose 5-phosphate synthase (DXS) | dxs | - | sll1945 | 1923 |
| 1-deoxy-d-xylulose 5-phosphate reductoisomerase (DXR) | dxr | - | sll0019 | 1185 |
| 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase/CDP-ME synthase (MCT/CMS) | ispD | - | slr0951 | 693 |
| 4-(cytidine 5'-diphospho)-2-C-methyl-d-erythritol kinase (CMK) | ispE | - | sll0711 | 948 |
| 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase (MDS/MCS) | ispF | ygbB | slr1542 | 486 |
| 4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HDS) | ispG | gcpE | slr2136 | 1212 |
| 4-hydroxy-3-methylbut-2-enyl diphosphate reductase/IDP/DMADP synthase (HDR/IDS) | ispH | lytB | slr0348 | 1221 |
| Isopentenyl diphosphate isomerase (IDI/IDI-2) | idi | fni | sll1556 | 1050 |
| Geranyl diphosphate synthase (GDPS)/Farnesyl diphosphate synthase (FDPS)/Geranylgeranyl diphosphate synthase (GGDPS) | crtE | - | slr0739 | 909 |
3.1. Hemiterpenes (C5)
Heterologous Expression of Hemiterpenes in Cyanobacteria
3.2. Monoterpenes (C10)
Heterologous Expression of Monoterpenes in Cyanobacteria
| Host Strain | Terpenoids Produced | Gene and Source | Promoter | Maximum Amount Produced | Reference |
|---|---|---|---|---|---|
| Synechocystis | Isoprene | Synechocystis codon optimized isoprene synthase (IspS) from Pueraria montana (kudzu) | psbA2 | 50 μg g−1 DCW | [70] |
| Synechocystis | Isoprene | Synechocystis codon optimized isoprene synthase (IspS) from Pueraria montana (kudzu) | psbA2 | 150 μg L −1 | [71] |
| Synechocystis | Isoprene | MVA pathway genes encoding the enzymes, Hmg-CoA synthase (HmgS) and Hmg-CoA reductase (HmgR) from Enterococcus faecalis; | psbA2 | 250 μg g−1 DCW | [72] |
| Synechocystis | Limonene | Synechocystis codon optimized limonene synthase (LimS) from Schizonepeta tenuifolia, and overexpression with native genes dxs, crtE and idi | Ptrc | 41 µg L −1 day−1, 56 µg L −1 day−1 | [93] |
| Synechococcus sp. PCC 7002 | Limonene | Synechococcus codon optimized L-limonene synthase from Mentha spicata | cpcBA (cpc) | 4 mg L−1 | [95] |
| Anabaena sp. PCC 7120 | Limonene | Limonene synthase (LimS) from Picea sitchensis (Sitka spruce), Synthetic DXP operon with dxs from E. coli; IDI from Haematococcus pluvialis; and GDPS from Mycoplasma tuberculosis for co-expression with LimS | dual promoter Pnir/PpsbA1 | 3.6 ± 0.5 μg L−1 OD−1 h−1 | [94] |
| Synechocystis | β-phellandrene | Synechocystis codon-optimized β-phellandrene synthase (β-PHLS) gene from Lavandula angustifolia (lavender) | psbA2 | 1.0 μg L−1 h−1 | [96] |
| Synechocystis | β-phellandrene | Synechocystis codon-optimized β-phellandrene synthase (β-PHLS) gene from Lavandula angustifolia (lavender) | psbA2, psbA2 (no AT -box), ptrc-T7-g10 and cpc | 253.8 ± 54.8 μg g−1 DCW | [97] |
| Anabaena sp. PCC 7120 | Linalool | Linalool synthase (LinS) from Picea abies (Norway Spruce) | [100], Patent | ||
| Synechocystis | β-caryophyllene | β-caryophyllene synthase gene (QHS1) from Artemisia annua | psbA2 | 3.7 μg g−1 DCW week−1 | [101] |
| Anabaena sp. PCC 7120 | Farnesene | Anabaena codon-optimized farnesene synthesize (FaS) gene from Picea abies (Norway Spruce) | dual promoter Pnir/PpsbA1 | 305.4 ± 17.7 μg·L−1 | [102] |
| Synechococcus sp. PCC 7002 | Bisabolene | Synechococcus codon optimized (E)-a-bisabolene synthase from Abies grandis (Grand Fir) | cpcBA (cpc) | 0.6 mg L−1 | [95] |
3.3. Sesquiterpenes (C15)
Heterologous Expression of Sesquiterpenes in Cyanobacteria
3.4. Diterpenes (C20)
3.5. Triterpenes (C30)
3.6. Tetraterpenes (C40)
4. Strategies to Enhance Terpenoid Production
5. Future Perspective
Acknowledgments
Conflicts of Interest
References
- Mazid, M.; Khan, T.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Van Agtmael, M.A.; Eggelte, T.A.; van Boxtel, C.J. Artemisinin drugs in the treatment of malaria: From medicinal herb to registered medication. Trends Pharmacol. Sci. 1999, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Slichenmyer, W.J.; von Hoff, D.D. Taxol: A new and effective anti-cancer drug. Anticancer. Drugs 1991, 2, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Haridas, V.; Higuchi, M.; Jayatilake, G.S.; Bailey, D.; Mujoo, K.; Blake, M.E.; Arntzen, C.J.; Gutterman, J.U. Avicins: Triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc. Natl. Acad. Sci. USA 2001, 98, 5821–5826. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscler. Rep. 2009, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Song, H.; Liu, H.-W.; Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013, 17, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [PubMed]
- Rohmer, M.; Seemann, M.; Horbach, S.; Bringer-Meyer, S.; Sahm, H. Glyceraldehyde 3-Phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J. Am. Chem. Soc. 1996, 118, 2564–2566. [Google Scholar] [CrossRef]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl. Chem. 2003, 75, 375–387. [Google Scholar] [CrossRef]
- Lois, L.M.; Campos, N.; Putra, S.R.; Danielsen, K.; Rohmer, M.; Boronat, A. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid thiamin, and pyridoxol biosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 2105–2110. [Google Scholar] [CrossRef]
- Miller, B.; Heuser, T.; Zimmer, W. A Synechococcus leopoliensis SAUG 1402–1 operon harboring the 1-deoxyxylulose 5-phosphate synthase gene and two additional open reading frames is functionally involved in the dimethylallyl diphosphate synthesis. FEBS Lett. 1999, 460, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, E.; Salmi, M.; León, P. Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J. Exp. Bot. 2009, 60, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Proteau, P.J. Characterization of native and histidine-tagged deoxyxylulose 5-phosphate reductoisomerase from the cyanobacterium Synechocystis sp. PCC6803. Biochim. Biophys. Acta 2003, 1652, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Thérisod, M.; Fischer, J.-C.; Estramareix, B. The origin of the carbon chain in the thiazole moiety of thiamine in Escherichia coli: Incorporation of deuterated 1-deoxy-d-threo-2-pentulose. Biochem. Biophys. Res. Commun. 1981, 98, 374–379. [Google Scholar] [CrossRef]
- Hill, R.E.; Himmeldirk, K.; Kennedy, I.A.; Pauloski, R.M.; Sayer, B.G.; Wolf, E.; Spenser, I.D. The biogenetic anatomy of vitamin B6: A 13C-NMR investigation of the biosynthesis of pyridoxol in Escherichia coli. J. Biol. Chem. 1996, 271, 30426–30435. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seemann, M.; Tse Sum Bui, B.; Wolff, M.; Miginiac-Maslow, M.; Rohmer, M. Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: Direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett. 2006, 580, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hase, T. Cyanobacterial non-mevalonate pathway: (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase interacts with ferredoxin in Thermosynechococcus elongatus BP-1. J. Biol. Chem. 2005, 280, 20672–20679. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X., Jr.; Lafond, T.P.; Gantt, E. Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J. Bacteriol. 2000, 182, 5841–5848. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, K.; Estevez, Y.; Deffieux, A.; Peruch, F. Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie 2012, 94, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Sacchettini, J.C.; Poulter, C.D. Creating isoprenoid diversity. Science 1997, 277, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Ramak, P.; Osaloo, S.K.; Sharifi, M.; Ebrahimzadeh, H.; Behmanesh, M. Biosynthesis, regulation and properties of plant monoterpenoids. J. Med. Plant Res. 2014, 8, 983–991. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.S.; Chen, F.; Stewart, C.N. Ecological functions of terpenoids in changing climates. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. [Google Scholar]
- Davies, F.K.; Jinkerson, R.E.; Posewitz, M.C. Toward a photosynthetic microbial platform for terpenoid engineering. Photosynth. Res. 2014. [Google Scholar] [CrossRef]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1. [Google Scholar] [CrossRef]
- Agger, S.A.; Lopez-Gallego, F.; Hoye, T.R.; Schmidt-Dannert, C. Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC 7120. J. Bacteriol. 2008, 190, 6084–6096. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.O.; Pandhal, J.; Wright, P.C. Exploiting cyanobacterial P450 pathways. Curr. Opin. Microbiol. 2010, 13, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-J.; Cheng, Q.-Q.; Su, P.; Chen, X.; Wang, X.-J.; Gao, W.; Huang, L.-Q. Research progress relating to the role of cytochrome P450 in the biosynthesis of terpenoids in medicinal plants. Appl. Microbiol. Biotechnol. 2014, 98, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. Hopanoids. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–142. [Google Scholar]
- Zhao, L.; Chang, W.-C.; Xiao, Y.; Liu, H.-W.; Liu, P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Rodrı́guez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Ginger, M.L.; McFadden, G.I.; Michels, P.A.M. Rewiring and regulation of cross-compartmentalized metabolism in protists. Philos. Trans. R. Soc. 2010, 365, 831–845. [Google Scholar] [CrossRef]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W.; Packer, M.B. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tabata, S. Complete genome structure of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1997, 38, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.; Schwender, J.; Müller, C.; Lichtenthaler, H.K.; Rohmer, M. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC 6714. Biochem. J. 1998, 333, 381–388. [Google Scholar] [PubMed]
- Ershov, Y.; Gantt, R.R.; Cunningham, F.X., Jr.; Gantt, E. Isopentenyl diphosphate isomerase deficiency in Synechocystis sp. strain PCC 6803. FEBS Lett. 2000, 473, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Poliquin, K.; Ershov, Y.V.; Cunningham, F.X.J.; Woreta, T.T.; Gantt, R.R.; Gantt, E. Inactivation of sll1556 in Synechocystis Strain PCC 6803 impairs isoprenoid biosynthesis from pentose phosphate cycle substrates in vitro. J. Bacteriol. 2004, 186, 4685–4693. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, K.; Kuzuyama, T.; Takagi, M.; Hayakawa, Y.; Seto, H. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc. Natl. Acad. Sci. USA 2001, 98, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Barkley, S.J.; Desai, S.B.; Poulter, C.D. Type II isopentenyl diphosphate isomerase from Synechocystis sp. strain PCC 6803. J. Bacteriol. 2004, 186, 8156–8158. [Google Scholar] [CrossRef] [PubMed]
- Barkley, S.J.; Cornish, R.M.; Poulter, C.D. Identification of an Archaeal type II isopentenyl diphosphate isomerase in Methanothermobacter thermautotrophicus. J. Bacteriol. 2004, 186, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Poliquin, K.; Cunningham, F.X., Jr.; MacDonald, I.; Gantt, R.R.; Gantt, E. Impaired isoprenoid biosynthesis: A competitive disadvantage under light stress in Synechocystis PCC 6803. In Photosynthesis. Energy from the Sun: 14th International Congress on Photosynthesis; Allen, J.F., Gantt, E., Golbeck, J., Osmond, B., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 763–766. [Google Scholar]
- Ershov, Y.V.; Gantt, R.R.; Cunningham, F.X., Jr.; Gantt, E. Isoprenoid biosynthesis in Synechocystis sp. strain PCC 6803 is stimulated by compounds of the pentose phosphate cycle but not by pyruvate or deoxyxylulose-5-phosphate. J. Bacteriol. 2002, 184, 5045–5051. [Google Scholar] [CrossRef] [PubMed]
- Poliquin, K.; Cunningham, F.X., Jr.; Gantt, R.R.; Gantt, E. Interaction of isoprenoid pathway enzymes and indirect stimulation of isoprenoid biosynthesis by pentose phosphate cyacle substrates in Synechocystis PCC 6803. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer: New York, NY, USA, 2013; pp. 51–63. [Google Scholar]
- Winter, J.M.; Tang, Y. Synthetic biological approaches to natural product biosynthesis. Curr. Opin. Biotechnol. 2012, 23, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Tilley, E.; Hunter, I.S.; Legisa, M.; Glieder, A. Engineering primary metabolic pathways of industrial micro-organisms. J. Biotechnol. 2007, 129, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.A.; Siewers, V.; Nielsen, J. Advanced biofuel production by the yeast Saccharomyces cerevisiae. Curr. Opin. Chem. Biol. 2013, 17, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, Y.; Grace, S.; He, Q. Functional expression of an Arabidopsis p450 enzyme, p-coumarate-3-hydroxylase, in the cyanobacterium Synechocystis PCC 6803 for the biosynthesis of caffeic acid. J. Appl. Phycol. 2014, 26, 219–226. [Google Scholar] [CrossRef]
- Lassen, L.M.; Nielsen, A.Z.; Olsen, C.E.; Bialek, W.; Jensen, K.; Møller, B.L.; Jensen, P.E. Anchoring a plant cytochrome P450 via PsaM to the thylakoids in Synechococcus sp. PCC 7002: Evidence for light-driven biosynthesis. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E.; Lassen, M.M.; Gnanasekaran, T.; Nielsen, A.Z.; Møller, B.L. Using synthetic biology to retarget biosynthetic pathways to the chloroplast for direct access to the products of photosynthesis. Available online: http://www.isb.vt.edu/news/2013/Jul/JLGNM.pdf (accessed on 15 January 2015).
- Zhao, Y.; Yang, J.; Qin, B.; Li, Y.; Sun, Y.; Su, S.; Xian, M. Biosynthesis of isoprene in Escherichia coli via methylerythritol phosphate (MEP) pathway. Appl. Microbiol. Biotechnol. 2011, 90, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Singsaas, E.L.; Lerdau, M.; Winter, K.; Sharkey, T.D. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997, 115, 1413–1420. [Google Scholar] [PubMed]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene emission from plants: Why and how. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosynthesis-to-fuels: From sunlight to hydrogen, isoprene, and botryococcene production. Energy Environ. Sci. 2012, 5, 5531–5539. [Google Scholar] [CrossRef]
- Shaw, S.L.; Chisholm, S.W.; Prinn, R.G. Isoprene production by Prochlorococcus, a marine cyanobacterium, and other phytoplankton. Mar. Chem. 2003, 80, 227–245. [Google Scholar] [CrossRef]
- Shaw, S.L.; Gantt, B.; Meskhidze, N. Production and emissions of marine isoprene and monoterpenes: A Review. Adv. Meteorol. 2010, 2010. [Google Scholar] [CrossRef]
- Bonsang, B.; Gros, V.; Peeken, I.; Yassaa, N.; Bluhm, K.; Zöllner, E.; Sarda-Esteve, R.; Williams, J. Isoprene emission from phytoplankton monocultures: The relationship with chlorophyll-a, cell volume and carbon content. Environ. Chem. 2010, 7, 554–563. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bentley, F.K.; Melis, A. Diffusion-based process for carbon dioxide uptake and isoprene emission in gaseous/aqueous two-phase photobioreactors by photosynthetic microorganisms. Biotechnol. Bioeng. 2012, 109, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Bentley, F.K.; Zurbriggen, A.; Melis, A. Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol. Plant 2014, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Banthorpe, D.V.; Charlwood, B.V.; Francis, M.J.O. The biosynthesis of monoterpenes. Chem. Rev. 1972, 72, 115–155. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Mirata, M.A.; Holtmann, D.; Schrader, J. Biooxidation of monoterpenes with bacterial monooxygenases. Process Biochem. 2011, 46, 1885–1899. [Google Scholar] [CrossRef]
- Yassaa, N.; Peeken, I.; Zöllner, E.; Bluhm, K.; Arnold, S.; Spracklen, D.; Williams, J. Evidence for marine production of monoterpenes. Environ. Chem. 2008, 5, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Giri, A.; Dhingra, V.; Giri, C.C.; Singh, A.; Ward, O.P.; Narasu, M.L. Biotransformations using plant cells, organ cultures and enzyme systems: Current trends and future prospects. Biotechnol. Adv. 2001, 19, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Saidfar, G.; Mahmudi, S. Biotransformation of aromatic aldehydes by cell cultures of Peganum harmala L. and Silybum marianum (L.) Gaertn. Iran. J. Pharm. Res. 2004, 2, 127–130. [Google Scholar]
- Simeo, Y.; Sinisterra, J.V. Biotransformation of terpenoids: A green alternative for producing molecules with pharmacological activity. Mini. Rev. Org. Chem. 2009, 6, 128–134. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Fotooh-Abadi, E.; Ghasemi, Y. Biotransformation of monoterpenes by immobilized microalgae. J. Appl. Phycol. 2011, 23, 975–981. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Hamada, H.; Kaji, M.; Hirata, T. Asymmetric reduction of enones with Synechococcus sp. PCC 7942. Tetrahedron Asymmetry 2004, 15, 1677–1679. [Google Scholar] [CrossRef]
- Hamada, H.; Kondo, Y.; Ishihara, K.; Nakajima, N.; Hamada, H.; Kurihara, R.; Hirata, T. Stereoselective biotransformation of limonene and limonene oxide by cyanobacterium, Synechococcus sp. PCC 7942. J. Biosci. Bioeng. 2003, 96, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Utsukihara, T.; Chai, W.; Kato, N.; Nakamura, K.; Horiuchi, C.A. Reduction of (+)- and (−)-camphorquinones by cyanobacteria. J. Mol. Catal. B Enzym. 2004, 31, 19–24. [Google Scholar] [CrossRef]
- Balcerzak, L.; Lipok, J.; Strub, D.; Lochyński, S. Biotransformations of monoterpenes by photoautotrophic micro-organisms. J. Appl. Microbiol. 2014, 117, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G.; Hwang, C.J.; Krasner, S.W.; McGuire, M.J. Geosmin and 2-methylisoborneol from cyanobacteria in three water supply systems. Appl. Envir. Microbiol. 1982, 43, 708–714. [Google Scholar]
- Komatsu, M.; Tsuda, M.; Omura, S.; Oikawa, H.; Ikeda, H. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc. Natl. Acad. Sci. USA 2008, 105, 7422–7427. [Google Scholar] [CrossRef] [PubMed]
- Giglio, S.; Chou, W.K.W.; Ikeda, H.; Cane, D.E.; Monis, P.T. Biosynthesis of 2-methylisoborneol in cyanobacteria. Environ. Sci. Technol. 2011, 45, 992–998. [Google Scholar] [PubMed]
- Wang, Z.; Xu, Y.; Shao, J.; Wang, J.; Li, R. Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: Isolation, characterization, and expression in response to light. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.-C.; Lin, T.-F.; Yang, F.-C.; Liu, C.-L. Seasonal change and correlation with environmental parameters for 2-MIB in Feng-Shen Reservoir, Taiwan. Environ. Monit. Assess. 2008, 145, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hobson, P.; An, W.; Burch, M.D.; House, J.; Yang, M. Earthy odor compounds production and loss in three cyanobacterial cultures. Water Res. 2012, 46, 5165–5173. [Google Scholar] [CrossRef] [PubMed]
- Zimba, P.V.; Dionigi, C.P.; Millie, D.F. Evaluating the relationship between photopigment synthesis and 2-methylisoborneol accumulation in cyanobacteria. J. Phycol. 1999, 35, 1422–1429. [Google Scholar] [CrossRef]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium Pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Duetz, W.A.; Bouwmeester, H.; van Beilen, J.B.; Witholt, B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl. Microbiol. Biotechnol. 2003, 61, 269–277. [Google Scholar] [PubMed]
- Kiyota, H.; Okuda, Y.; Ito, M.; Hirai, M.Y.; Ikeuchi, M. Engineering of cyanobacteria for the photosynthetic production of limonene from CO2. J. Biotechnol. 2014, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.; Gu, L.; Zhou, R. Engineering cyanobacteria for the production of a cyclic hydrocarbon fuel from CO2 and H2O. Green Chem. 2014, 16, 3175–3185. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2. [Google Scholar] [CrossRef]
- Bentley, F.K.; García-Cerdán, J.G.; Chen, H.-C.; Melis, A. Paradigm of monoterpene (β-phellandrene) hydrocarbons production via photosynthesis in cyanobacteria. BioEnergy Res. 2013, 6, 917–929. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 2014, 240, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.-I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Cseke, L.; Dudareva, N.; Pichersky, E. Structure and evolution of linalool synthase. Mol. Biol. Evol. 1998, 15, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Gibbons, W. Genetically Engineered Cyanobacteria. U.S. Patent. 1 November 2012. Available online: http://images2.freshpatents.com/pdf/US20120276637A1.pdf (accessed on 15 January 2015).
- Reinsvold, R.E.; Jinkerson, R.E.; Radakovits, R.; Posewitz, M.C.; Basu, C. The production of the sesquiterpene β-caryophyllene in a transgenic strain of the cyanobacterium Synechocystis. J. Plant Physiol. 2011, 168, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.; Gu, L.; Gibbons, W.; Zhou, R. Genetically engineering cyanobacteria to convert CO2, water, and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (Essential oils). In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008. [Google Scholar]
- Giglio, S.; Jiang, J.; Saint, C.P.; Cane, D.; Monis, P.T. Isolation and characterization of the genes associated with geosmin production in cyanobacteria. Environ. Sci. Technol. 2008, 42, 8027–8032. [Google Scholar] [CrossRef] [PubMed]
- Höckelmann, C.; Becher, P.G.; von Reuß, S.H.; Jüttner, F. Sesquiterpenes of the geosmin-producing cyanobacterium Calothrix PCC 7507 and their toxicity to invertebrates. Z. Naturforsch. C. 2009, 64, 49–55. [Google Scholar] [PubMed]
- Orav, A.; Stulova, I.; Kailas, T.; Müürisepp, M. Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J. Agric. Food Chem. 2004, 52, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A. Local anaesthetic activity of β-caryophyllene. Il Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.; Malingre, T.M.; Batterman, S.; Bos, R. Mono- and sesqui-terpene hydrocarbons of the essential oil of Cannabis sativa. Phytochem. Rep. 1975, 14, 814–815. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Prinsep, M.R.; Thomson, R.A.; West, M.L.; Wylie, B.L. Tolypodiol, an antiinflammatory diterpenoid from the cyanobacterium Tolypothrix nodosa. J. Nat. Prod. 1996, 59, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Orjala, J.; Sticher, O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 1999, 62, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Heilmann, J.; Sticher, O. New antibacterial metabolites from the cyanobacterium Nostoc commune (EAWAG 122b). J. Nat. Prod. 2000, 63, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Pérez Gutiérrez, R.M.; Martínez Flores, A.; Vargas Solís, R.; Carmona Jimenez, J. Two new antibacterial norabietane diterpenoids from cyanobacteria, Microcoleous lacustris. J. Nat. Med. 2008, 62, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Shpilyov, A.V.; Zinchenko, V.V.; Shestakov, S.V.; Grimm, B.; Lokstein, H. Inactivation of the geranylgeranyl reductase (ChlP) gene in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2005, 1706, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-R.; Lin, Y.-K.; Fang, J.-Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B. Squalene emulsions for parenteral vaccine and drug delivery. Molecules 2009, 14, 3286–3312. [Google Scholar] [CrossRef] [PubMed]
- Günes, F.E. Medical use of squalene as a natural antioxidant. MÜSBED 2013, 3, 220–228. [Google Scholar]
- Lee, S.; Poulter, C.D. Cloning, solubilization, and characterization of squalene synthase from Thermosynechococcus elongatus BP-1. J. Bacteriol. 2008, 190, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Englund, E.; Pattanaik, B.; Ubhayasekera, S.J.K.; Stensjö, K.; Bergquist, J.; Lindberg, P. Production of squalene in Synechocystis sp. PCC 6803. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Siedenburg, G.; Jendrossek, D. Squalene-hopene cyclases. Appl. Environ. Microbiol. 2011, 77, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Daum, G. Squalene—Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Belin, G.K. Investigation of hopanoid biomarkers in lake sediments by GC-MS and RP-HPLC-APCI-MS. E-J. Chem. 2009, 6, 77–88. [Google Scholar] [CrossRef]
- Kannenberg, E.L.; Poralla, K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 1999, 86, 168–176. [Google Scholar] [CrossRef]
- Malott, R.J.; Steen-Kinnaird, B.R.; Lee, T.D.; Speert, D.P. Identification of hopanoid biosynthesis genes involved in polymyxin resistance in Burkholderia multivorans. Antimicrob. Agents Chemother. 2012, 56, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.M.; Harriott, O.T.; Moreau, R.A.; Osman, S.F.; Benson, D.R.; Jones, A.D. Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. USA 1993, 90, 6091–6094. [Google Scholar] [CrossRef] [PubMed]
- Welander, P.V.; Hunter, R.C.; Zhang, L.; Sessions, A.L.; Summons, R.E.; Newman, D.K. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 2009, 191, 6145–6156. [Google Scholar] [CrossRef] [PubMed]
- Schmerk, C.L.; Bernards, M.A.; Valvano, M.A. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J. Bacteriol. 2011, 193, 6712–6723. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.A.; Neuss, B.; Sahm, H. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J. Bacteriol. 1991, 173, 5592–5595. [Google Scholar] [PubMed]
- Horbach, S.; Neuss, B.; Sahm, H. Effect of azasqualene on hopanoid biosynthesis and ethanol tolerance of Zymomonas mobilis. FEMS Microbiol. Lett. 1991, 79, 347–350. [Google Scholar] [CrossRef]
- Welander, P.V.; Doughty, D.M.; Wu, C.-H.; Mehay, S.; Summons, R.E.; Newman, D.K. Identification and characterization of Rhodopseudomonas palustris TIE-1 hopanoid biosynthesis mutants. Geobiology 2012, 10, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Schmerk, C.L.; Welander, P.V.; Hamad, M.A.; Bain, K.L.; Bernards, M.A.; Summons, R.E.; Valvano, M.A. Elucidation of the Burkholderia cenocepacia hopanoid biosynthesis pathway uncovers functions for conserved proteins in hopanoid-producing bacteria. Environ. Microbiol. 2014. [Google Scholar] [CrossRef]
- Jürgens, U.J.; Simonin, P.; Rohmer, M. Localization and distribution of hopanoids in membrane systems of the cyanobacterium Synechocystis PCC 6714. FEMS Microbiol. Lett. 1992, 92, 285–288. [Google Scholar] [CrossRef]
- Talbot, H.M.; Summons, R.E.; Jahnke, L.L.; Cockell, C.S.; Rohmer, M.; Farrimond, P. Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org. Geochem. 2008, 39, 232–263. [Google Scholar] [CrossRef]
- Doughty, D.M.; Hunter, R.C.; Summons, R.E.; Newman, D.K. 2-Methylhopanoids are maximally produced in akinetes of Nostoc punctiforme: Geobiological implications. Geobiology 2009, 7, 524–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughty, D.M.; Dieterle, M.; Sessions, A.L.; Fischer, W.W.; Newman, D.K. Probing the subcellular localization of hopanoid lipids in bacteria using NanoSIMS. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Umeno, D.; Tobias, A.V.; Arnold, F.H. Evolution of the C30 carotenoid synthase CrtM for function in a C40 pathway. J. Bacteriol. 2002, 184, 6690–6699. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Sozer, O.; Komenda, J.; Ughy, B.; Domonkos, I.; Laczkó-Dobos, H.; Malec, P.; Gombos, Z.; Kis, M. Involvement of carotenoids in the synthesis and assembly of protein subunits of photosynthetic reaction centers of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2010, 51, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yoon, S.-H.; Lee, S.-H.; Kim, J.-Y.; Oh, D.-K.; Kim, S.-W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.-H.; Kim, S.-W. Synthetic biology and metabolic engineering for marine carotenoids: New opportunities and future prospects. Mar. Drugs 2014, 12, 4810–4832. [Google Scholar] [CrossRef] [PubMed]
- Estévez, J.M.; Cantero, A.; Reindl, A.; Reichler, S.; León, P. 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biol. Chem. 2001, 276, 22901–22909. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Kawano, Y.; Hotta, S.; Sekine, M.; Watanabe, T.; Ihara, M. Prerequisite for highly efficient isoprenoid production by cyanobacteria discovered through the over-expression of 1-deoxy-d-xylulose 5-phosphate synthase and carbon allocation analysis. J. Biosci. Bioeng. 2014, 118, 20–28. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattanaik, B.; Lindberg, P. Terpenoids and Their Biosynthesis in Cyanobacteria. Life 2015, 5, 269-293. https://doi.org/10.3390/life5010269
Pattanaik B, Lindberg P. Terpenoids and Their Biosynthesis in Cyanobacteria. Life. 2015; 5(1):269-293. https://doi.org/10.3390/life5010269
Chicago/Turabian StylePattanaik, Bagmi, and Pia Lindberg. 2015. "Terpenoids and Their Biosynthesis in Cyanobacteria" Life 5, no. 1: 269-293. https://doi.org/10.3390/life5010269
APA StylePattanaik, B., & Lindberg, P. (2015). Terpenoids and Their Biosynthesis in Cyanobacteria. Life, 5(1), 269-293. https://doi.org/10.3390/life5010269