Soft Drink Consumption in Young Mexican Adults Is Associated with Higher Total Body Fat Percentage in Men but Not in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Data Collection
2.3.1. Instructional Session
2.3.2. Anthropometric Data
2.3.3. SDsIQ
2.4. Statistical Methods
3. Results
3.1. Subjects
3.2. SD Intake
3.3. Anthropometric and Metabolic Characteristics
3.4. Correlation between CSD Consumption and the Anthropometric and Metabolic Variables
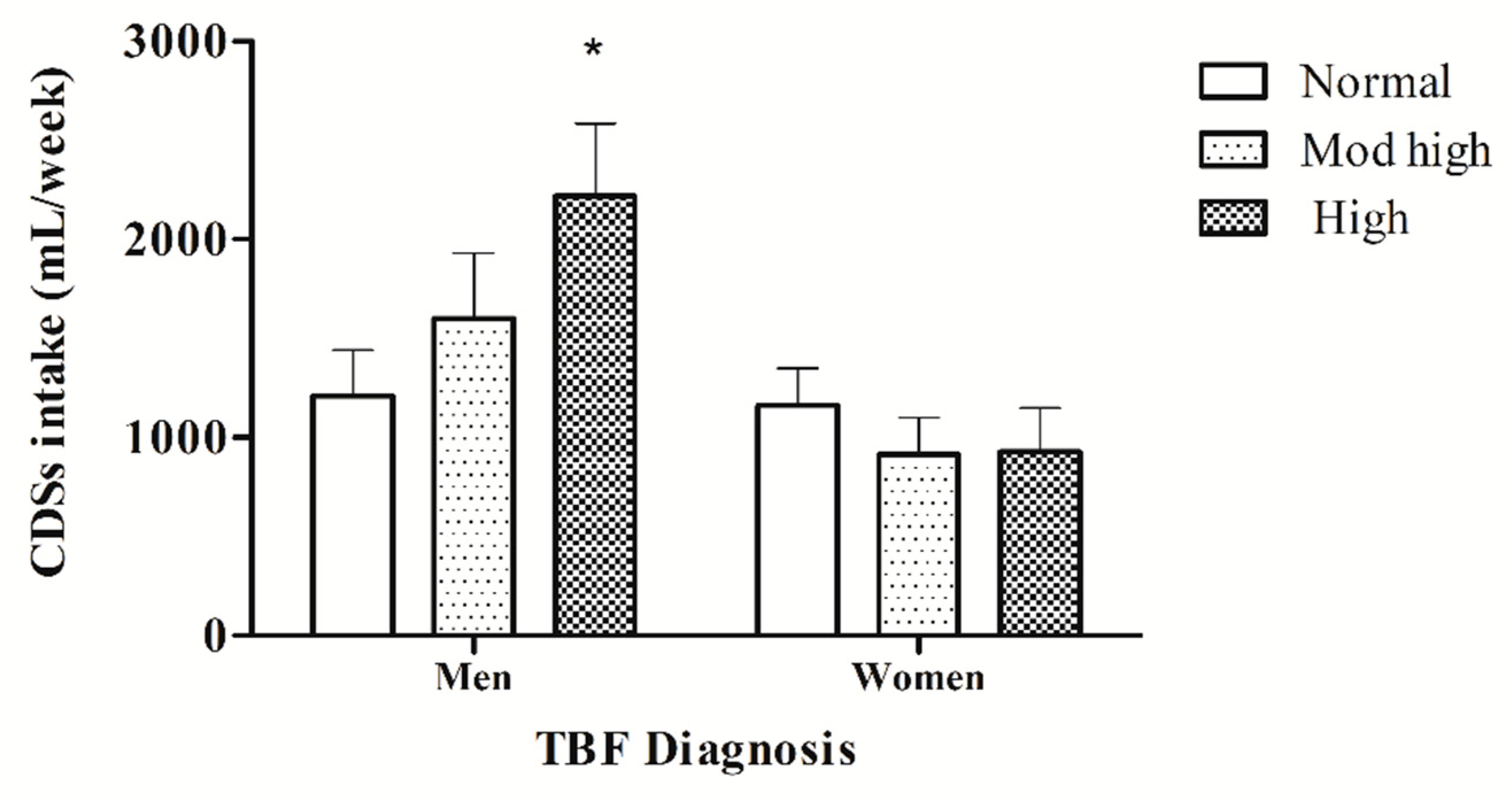
3.5. Comparison of CSD Consumption Based on TBF% Diagnosis
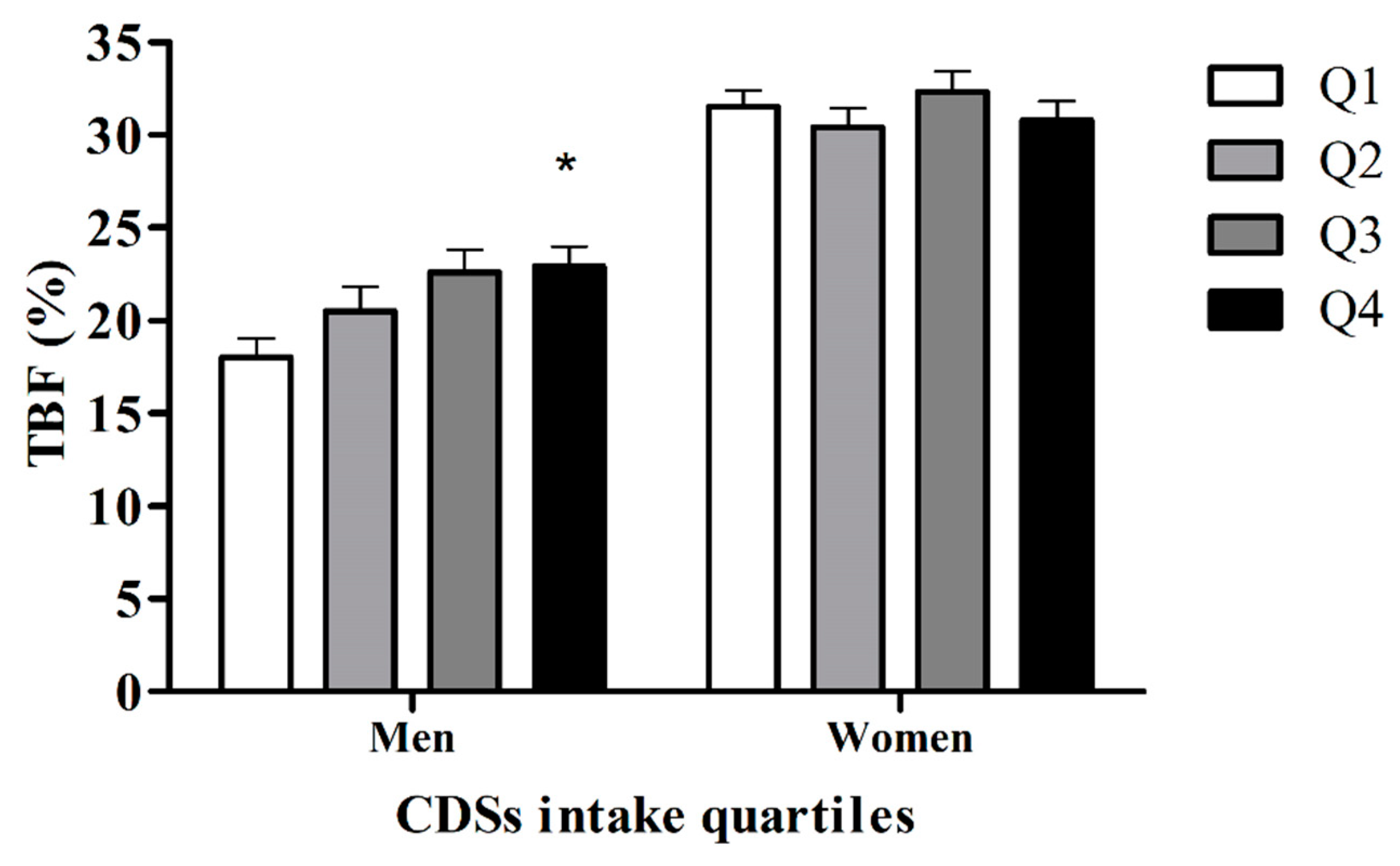
3.6. Comparing TBF% between the Quartiles of the Amount/Week CSD Consumption
3.7. Prediction Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jovic, D.; Dimkovic, N.; Rakocevic, I.; Boricic, K.; Atanasijevic, D.; Vasic, M. Prevalence and factors associated with self-reported kidney disease among Serbian adults: Results of 2013 National Health Survey. PLoS ONE 2018, 13, e0203620. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.; Biryukov, S.; Abbafati, C.; Ferede, S.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Shamah, T.; Cuevas, L.; Rivera, J.; Hernández, M. Encuesta Nacional de Salud y Nutrición 2016; Instituto Nacional de Salud Pública: Cuernavaca, México, 2016; Available online: http://ensanut.insp.mx (accessed on 15 March 2020).
- Bhardwaj, B.; O’Keefe, E.L.; O’Keefe, J.H. Death by Carbs: Added Sugars and Refined Carbohydrates Cause Diabetes and Cardiovascular Disease in Asian Indians. Missouri Med. 2016, 113, 395. [Google Scholar] [PubMed]
- Bradley, P. Refined carbohydrates, phenotypic plasticity and the obesity epidemic. Med. Hypotheses 2019, 131, 109317. [Google Scholar] [CrossRef]
- Wang, Y.C.; Bleich, S.N.; Gortmaker, S.L. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008, 121, e1604–e1614. [Google Scholar] [CrossRef]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review–. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Young, L.R.; Nestle, M. The contribution of expanding portion sizes to the US obesity epidemic. Am. J. Public Health 2002, 92, 246–249. [Google Scholar] [CrossRef]
- Ervin, R.B.; Wang, C.Y.; Wright, J.D.; Kennedy-Stephenson, J. Dietary intake of selected minerals for the United States population: 1999-2000. Energy 2004, 1, 6. [Google Scholar]
- Teff, K.L.; Elliott, S.S.; Tschöp, M.; Kieffer, T.; Rader, D.; Heiman, M.; Townsend, R.; Keim, N.; D’Allesio, D.; Havel, P. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef]
- Weigle, D.S.; Cummings, D.E.; Newby, P.D.; Breen, P.; Scott, F.; Matthys, C.; Callahan, H.; Purnell, J. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J. Clin. Endocrinol. Metab. 2003, 88, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.; Bremer, A.; Graham, J.; Hatcher, B.; Cox, C.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.; Nunez, M.; Chen, G.; Keim, N.; Havel, P. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015, 101, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.A.; Van Buren, J.M.; Warren, J.J.; Cavanaugh, J.E.; Levy, S.M. Beverage consumption patterns at age 13 to 17 years are associated with weight, height, and body mass index at age 17 years. J. Acad Nutr. Diet 2017, 117, 698–706. [Google Scholar] [CrossRef]
- Akgün, S.; Ertel, N.H. Plasma glucose and insulin after fructose an high-fructose corn syrup meals in subjects with non-insulin-dependent diabetes mellitus. Diabetes Care 1981, 4, 464–467. [Google Scholar] [CrossRef]
- Hung, C.T. Effects of high-fructose (90%) corn syrup on plasma glucose, insulin, and C-peptide in non-insulin-dependent diabetes mellitus and normal subjects. Taiwan Yi Xue Hui Za Zhi. J Formos. Med. Assoc. 1989, 88, 883–885. [Google Scholar]
- Chang, K.T.; Lampe, J.W.; Schwarz, Y.; Breymeyer, K.; Noar, K.; Song, X.; Nuehouser, M. Low glycemic load experimental diet more satiating than high glycemic load diet. Nutr. Cancer 2012, 64, 666–673. [Google Scholar] [CrossRef]
- Ma, J.; Jacques, P.F.; Meigs, J.B.; Fox, C.; Roger, G.; Smith, C.; Mckeown, N. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J. Nutr. 2016, 146, 2544–2550. [Google Scholar] [CrossRef]
- Basu, S.; McKee, M.; Galea, G.; Stuckler, D. Relationship of soft drink consumption to global overweight, obesity, and diabetes: A cross-national analysis of 75 countries. Am. J. Public Health 2013, 103, 2071–2077. [Google Scholar] [CrossRef]
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A. The possible role of sugar-sweetened beverages in obesity etiology: A review of the evidence. Intl. J. Obes. 2006, 30, S28. [Google Scholar] [CrossRef]
- Benton, D. Can artificial sweeteners help control body weight and prevent obesity? Nutr. Res. Rev. 2005, 18, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.; Michos, E.; Jacobs, E. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care 2009, 32, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.; Fox, C.; Meigs, J.; Vasan, N. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Stichelen, O.V.; Rother, K.I.; Hanover, J.A. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front. Microbiol. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.E.; Han, W.; Schwalm, N.D.; Raghavan, V.; Barry, N.; Godman, A.; Grosman, E. Dietary sugar silences a colonization factor in a mammalian gut symbiont. Proc. Natl. Acad Sci. USA 2019, 116, 233–238. [Google Scholar] [CrossRef]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Wang, Q.P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of sweeteners on the gut microbiota: A review of experimental studies and clinical trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, H.; Chen, P.; Xie, H.; Tao, Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 2019, 4, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Wright, M.L.; Anil Kumar, N.V.; Qawasmeh, A.; Hassan, S.; Mocan, A.; Haddad, P. Significance of microbiota in obesity and metabolic diseases and the modulatory potential by medicinal plant and food ingredients. Front. Pharmacol. 2017, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Durán, S.; Record, J.; Encina, C.; Salazar, J.; Cordon, K.; Cereceda, M.; Espinoza, S. Consumo de edulcorantes no nutritivos en bebidas carbonatadas en estudiantes universitarios de países de Latinoamerica. Nutr. Hosp. 2015, 31, 95965. [Google Scholar] [CrossRef]
- World Health Organization. Guide for Physical Measurements. Training Guide and Practical Instructions. 2006. Available online: http://www.who.int/chp/steps (accessed on 20 March 2020).
- McCarthy, H.D.; Cole, T.J.; Fry, T. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.; Murgatroy, P.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, R.T.; Ansari, W.; Maxwell, A.E. Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutr. J. 2009, 8, 31. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Vitolins, M.Z.; Carmichael, S.L.; Hemphill, S.; Tsaroucha, G.; Rushing, J.; Levin, S. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiologic study. Ann. Epidemiol. 1999, 9, 314–324. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of metabolomics in improving assessment of dietary intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.; Engell, R.; Ezzati, M. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: A systematic assessment of beverage intake in 187 countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Li, Q.; Dang, S.; Yan, H. Equation-derived body fat percentage indicates metabolic abnormalities among normal-weight adults in a rural Chinese population. Am. J. Hum. Biol. 2017, 29, e22964. [Google Scholar] [CrossRef] [PubMed]
- Tayefi, M.; Tayefi, B.; Darroudi, S.; Mohammadi-Bajgiran, M.; Mouhebat, I.M.; Heidari-Bakavoli, A.; Ghayour-Mobarthan, M. There is an association between body fat percentage and metabolic abnormality in normal weight subjects: Iranian large population. Transl. Metab. Syndr. Res. 2019, 2, 11–16. [Google Scholar] [CrossRef]
- Silver, A.J.; Guillen, C.P.; Kahl, M.J.; Morley, J.E. Effect of aging on body fat. J. Am. Geriatr. Soc. 1993, 41, 211–213. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Gallagher, D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 2010, 26, 152–155. [Google Scholar] [CrossRef]
- Westerbacka, J.; Corner, A.; Tiikkainen, M.; Tamminen, M.; Vehkavaara, S.; Häkkinen, A.M.; Yki-Järvinen, H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia 2004, 47, 1360–1369. [Google Scholar] [CrossRef]
- Björntorp, P. Abdominal fat distribution and the metabolic syndrome. J. Cardiovasc. Pharmacol. 1992, 20, S26–S28. [Google Scholar] [CrossRef]
- Bouchard, C.; Despres, J.P.; Mauriège, P. Genetic and nongenetic determinants of regional fat distribution. Endocr. Rev. 1993, 14, 72–93. [Google Scholar] [CrossRef]
- Després, J.P. The insulin resistance dyslipidemic syndrome of visceral obesity: Effect on patients’ risk. Obes. Res. 1998, 6, 8S–17S. [Google Scholar] [CrossRef]
- Björntorp, P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 1997, 13, 795–803. [Google Scholar] [CrossRef]
- Ford, E.S. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care 2005, 28, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; López, M. Central regulation of energy metabolism by estrogens. Mol. Metab. 2018, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Rebuffé-Scrive, M.; Andersson, B.; Olbe, L.; Björntorp, P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism 1989, 38, 453–458. [Google Scholar] [CrossRef]
- Shi, H.; Seeley, R.J.; Clegg, D.J. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 2009, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R.A. Clinical review on triglycerides. Eur. Heart J. 2020, 41, 99c–109c. [Google Scholar] [CrossRef]
- Assadi, S.N. What are the effects of psychological stress and physical work on blood lipid profiles? Medicine 2017, 96, e6816. [Google Scholar] [CrossRef]
- Estima, C.C.; Bruening, M.; Hannan, P.J.; Alvarenga, M.; Leal, G.; Phillipi SNeumark, D. A cross-cultural comparison of eating behaviors and home food environmental factors in adolescents from São Paulo (Brazil) and Saint Paul–Minneapolis (US). J. Nutr. Educ. Behav. 2014, 46, 370–375. [Google Scholar] [CrossRef]
- Westenhoefer, J.; von Katzler, R.; Jensen, H.J.; Zyriax, B.; Jagemann, B.; Harth, V.; Oldenburg, M. Cultural differences in food and shape related attitudes and eating behavior are associated with differences of Body Mass Index in the same food environment: Cross-sectional results from the Seafarer Nutrition Study of Kiribati and European seafarers on merchant ships. BMC Obes. 2018, 5, 1. [Google Scholar]
- Hallam, J.; Boswell, G.R.; DeVito, E.E.; Kober, H. Focus: sex and gender health: gender-related differences in food craving and obesity. Yale J. Biol. Med. 2016, 82, 161–173. [Google Scholar]
- De Castro, J.M.; Kreitzman, S.M. A microregulatory analysis of spontaneous human feeding patterns. Physiol. Behav. 1985, 35, 329–335. [Google Scholar] [CrossRef]
- Zandian, M.; Ioakimidis, I.; Bergh, C.; Leon, M.; Södersten, P. A sex difference in the response to fasting. Physiol. Behav. 2011, 103, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Visser, M.; Sepulveda, D.; Pierson, R.; Hrris, T.; Heimsfield, S. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am. J. Epidemiol. 1996, 143, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Parnell, W.; Wilson, N.; Alexander, D.; Wholers, M.; Williden, M.; Mann, J.; Gray, A. Exploring the relationship between sugars and obesity. Public Health Nutr. 2008, 11, 860–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, S.E.; Liu, M.; Palaniappan, L.P.; Wang, E.J.; Wong, N.D. Gender and ethnic differences in the prevalence of type 2 diabetes among Asian subgroups in California. J. Dia Comp. 2013, 27, 229–235. [Google Scholar] [CrossRef]
- Bleich, S.N.; Wang, Y.C.; Wang, Y.; Gortmaker, S.L. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am. J. Clin. Nutr. 2009, 89, 372–381. [Google Scholar] [CrossRef]
- Drewnowski, A.; Kurth, C.; Holden-Wiltse, J.; Saari, J. Food preferences in human obesity: Carbohydrates versus fats. Appetite 1992, 18, 207–221. [Google Scholar] [CrossRef]
- Macdiarmid, J.I.; Vail, A.; Cade, J.E.; Blundell, J.E. The sugar–fat relationship revisited: Differences in consumption between men and women of varying BMI. Int. J. Obes. 1998, 22, 1053–1061. [Google Scholar] [CrossRef]
- McEwen-Wurst, C.; Ruggieri, M.; Allison, K.C. Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann. N. Y. Acad Sci. 2018, 1411, 96–105. [Google Scholar] [CrossRef]
- Arnold, A.P. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009, 55, 570–578. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- Tonn-Eisinger, K.R.; Larson, E.B.; Boulware, M.I.; Thomas, M.J.; Mermelstein, P.G. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 2018, 133, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.S.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food reward system: Current perspectives and future research needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Gentry, R.T.; Wade, G.N. Androgenic control of food intake and body weight in male rats. J. Comp. Physiol. Psychol. 1976, 90, 18. [Google Scholar] [CrossRef]
- Bian, C.; Bai, B.; Gao, Q.; Li, S.; Zhao, Y. 17β-Estradiol Regulates Glucose Metabolism and Insulin Secretion in Rat Islet β Cells Through GPER and Akt/mTOR/GLUT2 Pathway. Front. Endocrinol. 2019, 10, 531. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Shen, Z.; Liu, W. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef]
- Turano, A.; Osborne, B.F.; Schwarz, J.M. Sexual Differentiation and Sex Differences in Neural Development; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–42. [Google Scholar]
- Klump, K.L.; Gobrogge, K.L.; Perkins, P.S.; Thorne, D.; Sisk, C.; Breedlove, S. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol. Med. 2006, 36, 539–546. [Google Scholar] [CrossRef]
- Diaz, N.; Ribas, L.; Piferrer, F. Effects of changes in food supply at the time of sex differentiation on the gonadal transcriptome of juvenile fish. Implications for natural and farmed populations. PLoS ONE 2014, 9, e111304. [Google Scholar] [CrossRef]
- Hu, T.Y.; Chen, Y.C.; Lin, P.; Shinh, C.; Bai, C.; Yuan, K.; Chang, J. Testosterone-associated dietary pattern predicts low testosterone levels and hypogonadism. Nutrients 2018, 10, 1786. [Google Scholar] [CrossRef]
- Thunders, M.; Mangai, S.; Cooper, R. Nutrigenetics, Nutrigenomics, and the Future of Dietary Advice. Food Nutr. Sci. 2013, 4, 999–1003. [Google Scholar] [CrossRef]
- Corcuff, J.B.; Merched, A.J. Nutrigenomics and Nutrigenetics: The Basis of Molecular Nutrition. In Molecular Basis of Nutrition and Agin; Acad Press: Amsterdam, The Netherlands, 2016; pp. 21–29. [Google Scholar] [CrossRef]
- Martin, B.; Pearson, M.; Brenneman, R. Gonadal transcriptome alterations in response to dietary energy intake: Sensing the reproductive environment. PLoS ONE 2009, 4, e4146. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Nugent, B.M. Epigenetic contributions to hormonally-mediated sexual differentiation of the brain. J. Neuroendocrinol. 2013, 25, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. The psychophysics of taste. Am. J. Clin. Nutr. 1978, 31, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, P.M.; Monroe, S. Taste responses in the parabrachial pons of ovariectomized rats. Brain Res. Bull. 1990, 25, 741–748. [Google Scholar] [CrossRef]
- Verhagen, J.V.; Giza, B.K.; Scott, T.R. Effect of amiloride on gustatory responses in the ventroposteromedial nucleus of the thalamus in rats. J. Neurophysiol. 2005, 93, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Building a neuroscience of pleasure and well-being. Psychol. Well-Being 2011, 1, 1–3. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; Stehberg, J. The Amygdala: A Discrete Multitasking Manager. In The Insular Cortex and the Amygdala: Shared Functions and Interactions, 1st ed.; Web of Science: Boston, MA, USA, 2012. [Google Scholar]
- Wang, L.; Gillis-Smith, S.; Peng, Y.; Zhang, J.; Chen, X.; Salzman, C.; Zuker, C. The coding of valence and identity in the mammalian taste system. Nature 2018, 558, 127. [Google Scholar] [CrossRef]
- Bermúdez-Rattoni, F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004, 5, 209–217. [Google Scholar] [CrossRef]
- Desgranges, B.; Ramirez-Amaya, V.; Ricaño-Cornejo, I.; Lévy, F.; Ferreira, G. Flavor preference learning increases olfactory and gustatory convergence onto single neurons in the basolateral amygdala but not in the insular cortex in rats. PLoS ONE 2010, 5, e10097. [Google Scholar] [CrossRef]
- Guzmán-Ramos, K.; Bermúdez-Rattoni, F. Interplay of amygdala and insular cortex during and after associative taste aversion memory formation. Rev. Neurosci. 2012, 23, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Baumann, M.H. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann. N. Y. Acad Sci. 2006, 1074, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Liu, Y.; Shriner, R.; Gold, M. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr. Pharm. Des. 2011, 17, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Miyazaki, K.W.; Doya, K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J. Neurosci. 2011, 31, 469–479. [Google Scholar] [CrossRef]
- Hayes, D.J.; Greenshaw, A.J. 5-HT receptors and reward-related behaviour: A review. Neurosci. Biobehav. Rev. 2011, 35, 1419–1449. [Google Scholar] [CrossRef]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef]
- Morin, J.P.; Rodríguez-Durán, L.F.; Guzmán-Ramos, K.; Perez-Cruz, C.; Ferreira, G.; Diaz-Cintra, S.; Pacheco-López, G. Palatable hyper-caloric foods impact on neuronal plasticity. Front. Behav. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef]
- Galipeau, D.; Verma, S.; McNeill, J.H. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2478–H2484. [Google Scholar] [CrossRef]
- Ko, E.A.; Kim, H.R.; Kim, Y.B.; Kim, H.S.; Lee, S.H. Effect of High Fructose Corn Syrup (HFCS) Intake on the Female Reproductive Organs and Lipid Accumulation in Adult Rats. Dev. Reproduct. 2017, 21, 151. [Google Scholar] [CrossRef]
- Sánchez-Pimienta, T.G.; Batis, C.; Lutter, C.K.; Rivera, J.A. Sugar-sweetened beverages are the main sources of added sugar intake in the Mexican population. J. Nutr. 2016, 146, 1888S–1896S. [Google Scholar] [CrossRef]
| Men (n = 158) | Women (n = 238) | ||
|---|---|---|---|
| SD Flavor | mL/Week a | mL/Week a | p-Value b |
| Cola | 755.4 ± 1149.0 | 535.2 ± 1234.4 | 0.077 |
| Apple | 171.0 ± 441.6 | 143.3 ± 524.3 | 0.587 |
| Orange | 205.4 ± 667.6 | 101.4 ± 299.5 | 0.038 |
| Lemon-Lime | 244.8 ± 619.2 | 127.5 ± 333.2 | 0.015 |
| Grapefruit | 68.5 ± 156.9 | 70.8 ± 192.5 | 0.903 |
| Grape | 52.5 ± 185.7 | 24.1 ± 69.8 | 0.033 |
| CSD Intake | 1489.9 ± 1909 | 999.5 ± 1672.6 | 0.007 |
| NCSD Intake | 53.2 ± 436.4 | 104.5 ± 631.1 | 0.257 |
| Total SD Intake | 1543.1 ± 1829 | 1104.1 ± 1847.8 | 0.021 |
| Variable | Men (n = 158) a | Women (n = 238) a | p-Value b |
|---|---|---|---|
| Weight (kg) | 70.55 ± 12.80 | 59.71 ± 12.31 | 0.000 |
| Height (cm) | 171.35 ± 6.32 | 158.89 ± 8.24 | 0.000 |
| BMI (kg/m2) | 23.96 ± 4.14 | 23.49 ± 4.17 | 0.309 |
| Waist (cm) | 84.43 ± 10.54 | 77.92 ± 10.33 | 0.000 |
| Hip (cm) | 97.52 ± 7.50 | 97.88 ± 9.24 | 0.689 |
| WHR | 0.80 ± 0.22 | 0.75 ± 0.18 | 0.009 |
| WHTR | 0.46 ± 0.13 | 0.46 ± 0.13 | 0.918 |
| TBF (%) | 21.35 ± 7.37 | 31.13 ± 7.57 | 0.000 |
| VF (%) | 2.75 ± 1.92 | 2.01 ± 1.82 | 0.000 |
| Glucose (mg/dL) | 84.98 ± 8.96 | 82.05 ± 6.64 | 0.000 |
| TG (mg/dL) | 97.10 ± 69.04 | 80.20 ± 42.72 | 0.004 |
| TC (mg/dL) | 165.91 ± 33.85 | 166.03 ± 29.40 | 0.971 |
| HDL (mg/dL) | 49.53 ± 15.28 | 53.62 ± 19.57 | 0.032 |
| VLDL (mg/dL) | 19.12 ± 13.88 | 15.90 ± 8.64 | 0.006 |
| LDL (mg/dL) | 94.74 ± 29.78 | 95.74 ± 30.13 | 0.752 |
| Variable | Men (n = 158) | Women (n = 238) |
|---|---|---|
| Weight (kg) | 0.130 | −0.076 |
| Height (cm) | −0.153 | −0.084 |
| BMI (kg/m2) | 0.248 ** | −0.051 |
| Waist (cm) | 0.243 ** | −0.053 |
| Hip (cm) | 0.251 ** | −0.074 |
| WHR | 0.177 * | 0.030 |
| WHTR | 0.298 ** | −0.048 |
| TBF (%) | 0.288 ** | −0.023 |
| VF (%) | 0.261 ** | 0.029 |
| Glucose (mg/dL) | 0.189 * | 0.108 |
| TG (mg/dL) | 0.028 | 0.147 * |
| TC (mg/dL) | 0.072 | −0.011 |
| HDL (mg/dL) | −0.075 | −0.041 |
| VLDL (mg/dL) | 0.047 | 0.154 * |
| LDL (mg/dL) | 0.147 | 0.014 |
| Males (n = 158) | Females (n = 238) | |||
|---|---|---|---|---|
| Variable | Β ± SE | p-Value | Β ± SE | p-Value |
| Weight (kg) | 0.122 ± 0.001 | 0.59 | −0.004 ± 0.001 | 0.953 |
| BMI (kg/m2) | 0.167 ± 0.000 | 0.053 | 0.000 ± 0.000 | 0.998 |
| Waist (cm) | 0.156 ± 0.000 | 0.061 | 0.002 ± 0.000 | 0.982 |
| Hip (cm) | 0.14 ± 0.000 | 0.091 | −0.013 ± 0.000 | 0.847 |
| TBF (%) | 0.199 ± 0.000 | 0.021 | 0.018 ± 0.000 | 0.667 |
| VF (%) | 0.14 ± 0.000 | 0.106 | 0.029 ± 0.000 | 0.669 |
| Glucose (mg/dL) | 0.115 ± 0.000 | 0.166 | 0.074 ± 0.000 | 0.265 |
| TG (mg/dL) | −0.014 ± 0.003 | 0.87 | 0.034 ± 0.002 | 0.611 |
| TC (mg/dL) | 0.065 ± 0.001 | 0.434 | 0.095 ± 0.001 | 0.155 |
| HDL (mg/dL) | −0.081 ± 0.000 | 0.33 | −0.041 ± 0.001 | 0.538 |
| VLDL (mg/dL) | 0.4 ± 0.001 | 0.963 | 0.04 ± 0.000 | 0.549 |
| LDL (mg/dL) | 0.169 ± 0.001 | 0.04 | 0.12 ± 0.001 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Ramírez, C.; Ramírez-Amaya, V.; Olalde-Mendoza, L.; Palacios-Delgado, J.; Anaya-Loyola, M.A. Soft Drink Consumption in Young Mexican Adults Is Associated with Higher Total Body Fat Percentage in Men but Not in Women. Foods 2020, 9, 1760. https://doi.org/10.3390/foods9121760
Campos-Ramírez C, Ramírez-Amaya V, Olalde-Mendoza L, Palacios-Delgado J, Anaya-Loyola MA. Soft Drink Consumption in Young Mexican Adults Is Associated with Higher Total Body Fat Percentage in Men but Not in Women. Foods. 2020; 9(12):1760. https://doi.org/10.3390/foods9121760
Chicago/Turabian StyleCampos-Ramírez, Cesar, Víctor Ramírez-Amaya, Liliana Olalde-Mendoza, Jorge Palacios-Delgado, and Miriam Aracely Anaya-Loyola. 2020. "Soft Drink Consumption in Young Mexican Adults Is Associated with Higher Total Body Fat Percentage in Men but Not in Women" Foods 9, no. 12: 1760. https://doi.org/10.3390/foods9121760
APA StyleCampos-Ramírez, C., Ramírez-Amaya, V., Olalde-Mendoza, L., Palacios-Delgado, J., & Anaya-Loyola, M. A. (2020). Soft Drink Consumption in Young Mexican Adults Is Associated with Higher Total Body Fat Percentage in Men but Not in Women. Foods, 9(12), 1760. https://doi.org/10.3390/foods9121760





