Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle
Abstract
:1. Introduction
2. Exercise and Aging
2.1. Exercise and Metabolism
2.1.1. Resting Metabolism
2.1.2. Exercise Metabolism
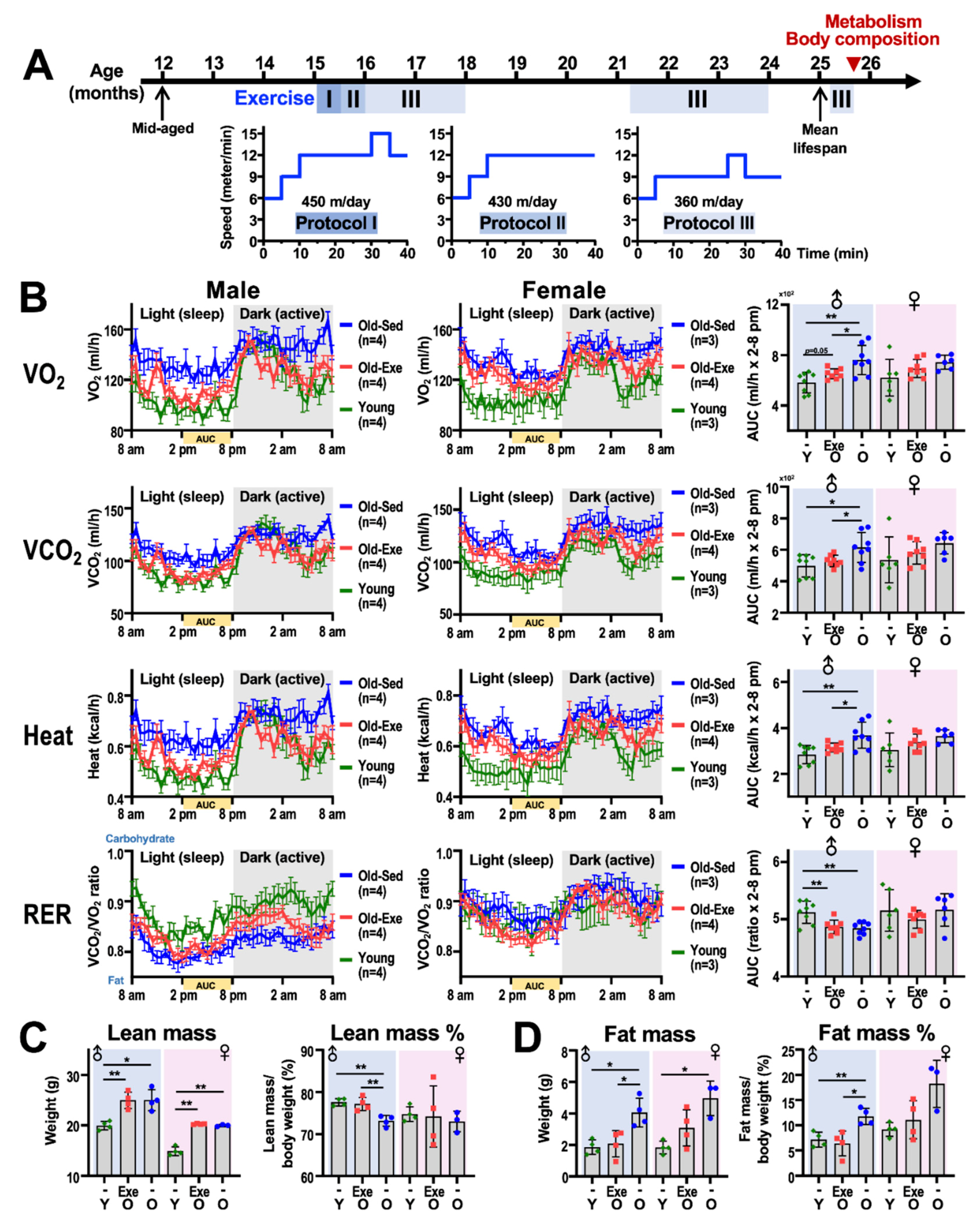
2.1.3. Impact of Middle-to-Old Age Exercise on Metabolism
2.2. Exercise and Body Composition
2.2.1. Body Composition Measurement Techniques
2.2.2. Body Composition and Aging
3. Exercise and Sexual Dimorphism
4. Pro-Longevity Genes
Long-Lived Mouse Models
5. Cisd2 in Aging
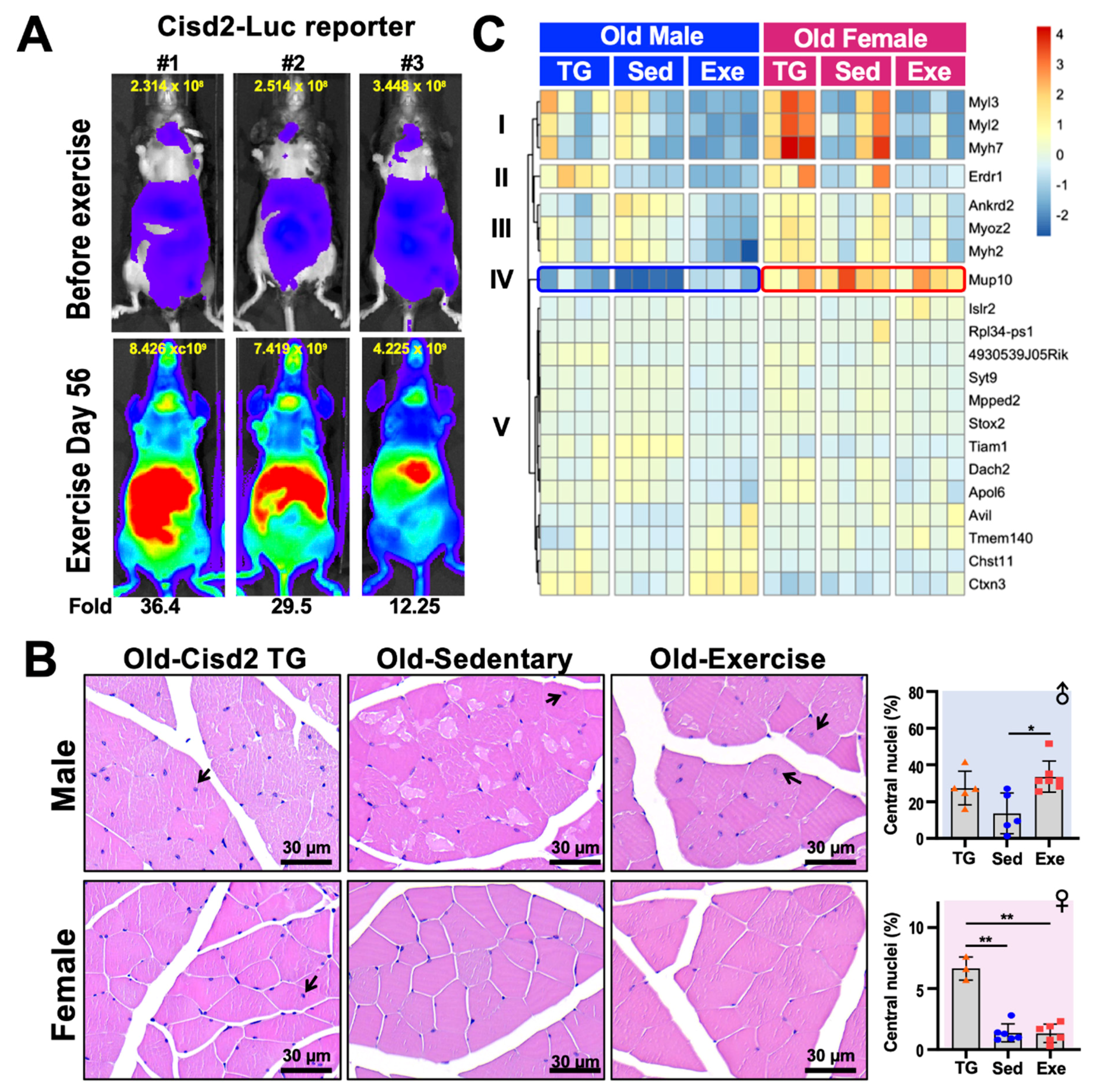
6. Cisd2 and Exercise
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC5 | Adenylyl cyclase type 5 |
| Ankrd2 | ankyrin repeat domain 2 (stretch responsive muscle) |
| ATG5 | Autophagy related 5 |
| Avil | Advillin |
| Bcl2 | B cell leukemia/lymphoma 2 |
| Chst11 | Carbohydrate sulfotransferase 11 |
| Cisd1 | CDGSH iron sulfur domain 1 |
| Cisd2 | CDGSH iron sulfur domain 2 |
| Ctxn3 | Cortexin 3 |
| DE | Differentially expressed |
| DEXA | Dual energy X-ray absorptiometry |
| EE | Energy expenditure |
| ER | Endoplasmic reticulum |
| FFA | Free fatty acid |
| Fgf21 | Fibroblast growth factor 21 |
| FRD | False discovery rate |
| GH | Growth hormone |
| GHR/BP | Growth hormone receptor |
| Gdf15 | Growth differentiation factor 15 |
| HGPS | Hutchinson-Gilford progeria syndrome |
| IGF-1 | Insulin-like growth factor-1 |
| Irs1 | Insulin receptor substrate 1 |
| Irs2 | Insulin receptor substrate 2 |
| Islr2 | immunoglobulin superfamily containing leucine-rich repeat 2 |
| LFC | Log2 fold change |
| MAM | Mitochondria-associated membrane |
| MCAT | Mitochondrially-targeted catalase |
| MIF | Macrophage migration inhibitory factor |
| MRI | Magnetic resonance imaging |
| Mup10 | Major urinary protein 10 |
| Myh7 | Myosin, heavy polypeptide 7, cardiac muscle, beta |
| Myl2 | Myosin, light polypeptide 2, regulatory, cardiac, slow |
| Myl3 | Myosin, light polypeptide 3 |
| Myoz2 | Myozenin 2 |
| Ox2r | Orexin type 2 receptor |
| Pgc-1a | Peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| Pou1f1 | POU domain, class 1, transcription factor 1 |
| PKA | Protein kinase A |
| Prop1 | Paired like homeodomain factor 1 |
| Pten | Phosphatase and tensin homolog |
| REE | Resting energy expenditure |
| RER | Respiratory exchange ratio |
| ROS | Reactive oxygen species |
| Rpl34-ps1 | Ribosomal protein L34, pseudogene 1 |
| S6K1 | Ribosomal protein S6 kinase 1 |
| TEE | Total energy expenditure |
| Tmem40 | Transmembrane protein 40 |
| Ucp1 | Uncoupling protein 1 |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasty, P.; Campisi, J.; Hoeijmakers, J.; Van Steeg, H.; Vijg, J. Aging and Genome Maintenance: Lessons from the Mouse? Science 2003, 299, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B.; et al. Phenotype and Course of Hutchinson-Gilford Progeria Syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnowski, A.; Ong, P.F.; Wong, E.S.M.; Lim, J.S.Y.; Mutalif, R.A.; Navasankari, R.; Dutta, B.; Yang, H.; Liow, Y.Y.; Sze, S.K.; et al. Progerin reduces LAP2α-telomere association in Hutchinson-Gilford progeria. eLife 2015, 4, e07759. [Google Scholar] [CrossRef]
- Wood, A.M.; Danielsen, J.M.R.; Lucas, C.A.; Rice, E.L.; Scalzo, D.; Shimi, T.; Goldman, R.D.; Smith, E.D.; Le Beau, M.M.; Kosak, S.T. TRF2 and lamin A/C interact to facilitate the functional organization of chromosome ends. Nat. Commun. 2014, 5, 5467. [Google Scholar] [CrossRef] [Green Version]
- Benson, E.K.; Lee, S.W.; Aaronson, S.A. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 2010, 123, 2605–2612. [Google Scholar] [CrossRef] [Green Version]
- Thoppil, H.; Riabowol, K.T. Senolytics: A Translational Bridge between Cellular Senescence and Organismal Aging. Front. Cell Dev. Biol. 2019, 7, 367. [Google Scholar] [CrossRef]
- Hillson, O.; Gonzalez, S.; Rallis, C. Prospects of Pharmacological Interventions to Organismal Aging. Biomol. Concepts 2018, 9, 200–215. [Google Scholar] [CrossRef]
- Pollock, R.D.; O’Brien, K.A.; Daniels, L.J.; Nielsen, K.B.; Rowlerson, A.; Duggal, N.A.; Lazarus, N.R.; Lord, J.M.; Philp, A.; Harridge, S.D.R. Properties of the vastus lateralis muscle in relation to age and physiological function in master cyclists aged 55–79 years. Aging Cell 2018, 17, e12735. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. (Lausanne) 2018, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E. Treatment of sarcopenia: The road to the future. J. Cachexia Sarcopenia Muscle 2018, 9, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Measuring Energy Metabolism in the Mouse—Theoretical, Practical, and Analytical Considerations. Front. Physiol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Carneiro, I.P.; Elliott, S.A.; Siervo, M.; Padwal, R.; Bertoli, S.; Battezzati, A.; Prado, C.M. Is Obesity Associated with Altered Energy Expenditure? Adv. Nutr. 2016, 7, 476–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimburger, D.C.; Ard, J.D. Handbook of Clinical Nutrition; Mosby: Philadelphia, PA, USA, 2006. [Google Scholar]
- Amatruda, J.M.; Statt, M.C.; Welle, S.L. Total and resting energy expenditure in obese women reduced to ideal body weight. J. Clin. Investig. 1993, 92, 1236–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostendorf, D.; Melanson, E.L.; Caldwell, A.E.; Creasy, S.A.; Pan, Z.; MacLean, P.S.; Wyatt, H.R.; Hill, J.O.; Catenacci, V.A. No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. Am. J. Clin. Nutr. 2018, 108, 658–666. [Google Scholar] [CrossRef]
- Foster, G.D.; McGuckin, B.G. Estimating Resting Energy Expenditure in Obesity. Obes. Res. 2001, 9 (Suppl. 5), 367S–372S. [Google Scholar] [CrossRef]
- Montero, D.; Madsen, K.; Meinild-Lundby, A.-K.; Edin, F.; Lundby, C. Sexual dimorphism of substrate utilization: Differences in skeletal muscle mitochondrial volume density and function. Exp. Physiol. 2018, 103, 851–859. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Seidenberg, P.H.; Beutler, A.I. The Sports Medicine Resource Manual; Saunders/Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Sahlin, K. Muscle Energetics during Explosive Activities and Potential Effects of Nutrition and Training. Sports Med. 2014, 44 (Suppl. 2), S167–S173. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-S.; Patki, G.; Das-Panja, K.; Le, W.-D.; Ahmad, S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 2011, 33, 1264–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohia-Nwoko, O.; Montazari, S.; Lau, Y.-S.; Eriksen, J.L. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol. Neurodegener. 2014, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, K.J. Human Body Composition: In Vivo Methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Henderson, G.C. Sexual Dimorphism in the Effects of Exercise on Metabolism of Lipids to Support Resting Metabolism. Front. Endocrinol. (Lausanne) 2014, 5, 162. [Google Scholar] [CrossRef] [Green Version]
- Hedrington, M.S.; Davis, S.N. Sexual Dimorphism in Glucose and Lipid Metabolism during Fasting, Hypoglycemia, and Exercise. Front. Endocrinol. (Lausanne) 2015, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Brief Review: Ergospirometry in Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2018, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, A.S., Jr.; Speck, A.E.; Amaral, I.M.; Canas, P.M.; Cunha, R.A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci. Rep. 2018, 8, 10742. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Fattor, J.A.; Horning, M.A.; Faghihnia, N.; Johnson, M.L.; Mau, T.L.; Luke-Zeitoun, M.; Brooks, G.A. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 2007, 584, 963–981. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Alderman, B.L. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J. Appl. Physiol. 2014, 116, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown-Borg, H.M.; Borg, K.E.; Meliska, C.J.; Bartke, A. Dwarf mice and the ageing process. Nature 1996, 384, 33. [Google Scholar] [CrossRef] [PubMed]
- Flurkey, K.; Papaconstantinou, J.; Harrison, D.E. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech. Ageing Dev. 2002, 123, 121–130. [Google Scholar] [CrossRef]
- Flurkey, K.; Papaconstantinou, J.; Miller, R.A.; Harrison, D.E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA 2001, 98, 6736–6741. [Google Scholar] [CrossRef] [Green Version]
- Coschigano, K.T.; Clemmons, D.; Bellush, L.L.; Kopchick, J.J. Assessment of Growth Parameters and Life Span of GHR/BP Gene-Disrupted Mice. Endocrinology 2000, 141, 2608–2613. [Google Scholar] [CrossRef]
- Bokov, A.; Garg, N.; Ikeno, Y.; Thakur, S.; Musi, N.; DeFronzo, R.A.; Zhang, N.; Erickson, R.C.; Gelfond, J.; Hubbard, G.B.; et al. Does Reduced IGF-1R Signaling in Igf1r+/− Mice Alter Aging? PLoS ONE 2011, 6, e26891. [Google Scholar] [CrossRef]
- Holzenberger, M.; Dupont, J.; Ducos, B.; Leneuve, P.; Géloën, A.; Even, P.C.; Cervera, P.; Le Bouc, Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003, 421, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gontier, G.; Chaker, Z.; Lacube, P.; Dupont, J.; Holzenberger, M. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell 2014, 13, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, J.; Sjögren, K.; Fäldt, J.; Andersson, N.; Isaksson, O.; Jansson, J.-O.; Ohlsson, C. Liver-Derived IGF-I Regulates Mean Life Span in Mice. PLoS ONE 2011, 6, e22640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selman, C.; Lingard, S.; Choudhury, A.I.; Batterham, R.L.; Claret, M.; Clements, M.; Ramadani, F.; Okkenhaug, K.; Schuster, E.; Blanc, E.; et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008, 22, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, A.; Wartschow, L.M.; White, M.F. Brain IRS2 Signaling Coordinates Life Span and Nutrient Homeostasis. Science 2007, 317, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Blüher, M.; Kahn, B.B.; Kahn, C.R. Extended Longevity in Mice Lacking the Insulin Receptor in Adipose Tissue. Science 2003, 299, 572–574. [Google Scholar] [CrossRef] [Green Version]
- Selman, C.; Tullet, J.M.A.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Berglund, E.D.; Coate, K.C.; He, T.T.; Katafuchi, T.; Xiao, G.; Potthoff, M.J.; Wei, W.; Wan, Y.; et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 2012, 1, e00065. [Google Scholar] [CrossRef]
- Wang, X.; Chrysovergis, K.; Kosak, J.; Kissling, G.; Streicker, M.; Moser, G.; Li, R.; Eling, T.E. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging (Albany N. Y.) 2014, 6, 690–704. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.M.; Wilkinson, J.E.; Miller, R.A. Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J. 2010, 24, 2436–2442. [Google Scholar] [CrossRef] [Green Version]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, J.F.; Strong, R.; Bokov, A.; Diaz, V.; Ward, W. Probing the Relationship between Insulin Sensitivity and Longevity Using Genetically Modified Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1332–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, A.C.; Bernal-Mizrachi, C.; Chinault, S.L.; Feng, C.; Schneider, J.G.; Coleman, T.; Malone, J.P.; Townsend, R.R.; Chakravarthy, M.V.; Semenkovich, C.F. Respiratory Uncoupling in Skeletal Muscle Delays Death and Diminishes Age-Related Disease. Cell Metab. 2007, 6, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enns, L.C.; Morton, J.F.; Treuting, P.R.; Emond, M.J.; Wolf, N.S.; McKnight, G.S.; Rabinovitch, P.S.; Ladiges, W. Disruption of Protein Kinase A in Mice Enhances Healthy Aging. PLoS ONE 2009, 4, e5963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Molina, A.; Efeyan, A.; Lopez-Guadamillas, E.; Muñoz-Martin, M.; Gómez-López, G.; Cañamero, M.; Mulero, F.; Pastor, J.; Martinez, S.; Romanos, E.; et al. Pten Positively Regulates Brown Adipose Function, Energy Expenditure, and Longevity. Cell Metab. 2012, 15, 382–394. [Google Scholar] [CrossRef] [Green Version]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.-I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [Green Version]
- Pyo, J.-O.; Yoo, S.-M.; Ahn, H.-H.; Nah, J.; Hong, S.H.; Kam, T.-I.; Jung, S.; Jung, Y.-K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R.; Talbot, D.A.; Selman, C.; Snart, S.; McLaren, J.S.; Redman, P.; Krol, E.; Jackson, D.M.; Johnson, M.S.; Brand, M.D. Uncoupled and surviving: Individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004, 3, 87–95. [Google Scholar] [CrossRef]
- Weindruch, R.; Walford, R.L. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science 1982, 215, 1415–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.C.C.; Cerqueira, F.M.; Barbosa, L.F.; Medeiros, M.H.G.; Kowaltowski, A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 2008, 7, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron Transfer between Cytochrome c and p66Shc Generates Reactive Oxygen Species that Trigger Mitochondrial Apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef]
- Migliaccio, E.; Giorgio, M.; Mele, S.; Pelicci, G.; Reboldi, P.; Pandolfi, P.P.; Lanfrancone, L.; Pelicci, P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999, 402, 309–313. [Google Scholar] [CrossRef]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.; Ladiges, W.C.; Wolf, N.; Van Remmen, H.; et al. Extension of Murine Life Span by Overexpression of Catalase Targeted to Mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef] [Green Version]
- Doyle, T.B.; Muntean, B.S.; Ejendal, K.F.; Hayes, M.P.; Soto-Velasquez, M.; Martemyanov, K.A.; Dessauer, C.W.; Hu, C.-D.; Watts, V.J. Identification of Novel Adenylyl Cyclase 5 (AC5) Signaling Networks in D1 and D2 Medium Spiny Neurons using Bimolecular Fluorescence Complementation Screening. Cells 2019, 8, 1468. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Vatner, D.E.; O’Connor, J.P.; Ivessa, A.; Ge, H.; Chen, W.; Hirotani, S.; Ishikawa, Y.; Sadoshima, J.; Vatner, S.F. Type 5 Adenylyl Cyclase Disruption Increases Longevity and Protects Against Stress. Cell 2007, 130, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Fernández, Á.F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; He, C.; Ting, T.; Liu, Y.; Chiang, W.-C.; et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 558, 136–140. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kao, C.-H.; Wang, C.-H.; Wu, C.-Y.; Tsai, C.-Y.; Liu, F.-C.; Yang, C.-W.; Wei, Y.-H.; Hsu, M.-T.; Tsai, S.-F.; et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009, 23, 1183–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-Y.; Chen, Y.-F.; Wang, C.-H.; Kao, C.-H.; Zhuang, H.-W.; Chen, C.-C.; Chen, L.-K.; Kirby, R.; Wei, Y.-H.; Tsai, S.-F.; et al. A persistent level of Cisd2 extends healthy lifespan and delays aging in mice. Hum. Mol. Genet. 2012, 21, 3956–3968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.-H.; Shen, Z.-Q.; Hsiung, S.-Y.; Wu, P.-C.; Teng, Y.-C.; Chou, Y.-J.; Fang, S.-W.; Chen, C.-F.; Yan, Y.-T.; Kao, L.-S.; et al. Cisd2 is essential to delaying cardiac aging and to maintaining heart functions. PLoS Biol. 2019, 17, e3000508. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-Q.; Chen, Y.-F.; Chen, J.-R.; Jou, Y.-S.; Wu, P.-C.; Kao, C.-H.; Wang, C.-H.; Huang, Y.-L.; Chen, C.-F.; Huang, T.-S.; et al. CISD2 Haploinsufficiency Disrupts Calcium Homeostasis, Causes Nonalcoholic Fatty Liver Disease, and Promotes Hepatocellular Carcinoma. Cell Rep. 2017, 21, 2198–2211. [Google Scholar] [CrossRef] [Green Version]
- Yokokawa, T.; Kido, K.; Suga, T.; Sase, K.; Isaka, T.; Hayashi, T.; Fujita, S. Exercise training increases CISD family protein expression in murine skeletal muscle and white adipose tissue. Biochem. Biophys. Res. Commun. 2018, 506, 571–577. [Google Scholar] [CrossRef]
- Charles, J.P.; Cappellari, O.; Spence, A.J.; Hutchinson, J.R.; Wells, D.J. Musculoskeletal Geometry, Muscle Architecture and Functional Specialisations of the Mouse Hindlimb. PLoS ONE 2016, 11, e0147669. [Google Scholar] [CrossRef] [Green Version]
- Medler, S. Mixing it up: The biological significance of hybrid skeletal muscle fibers. J. Exp. Biol. 2019, 222, jeb200832. [Google Scholar] [CrossRef]
- Lin, I.-H.; Chang, J.-L.; Hua, K.; Huang, W.-C.; Hsu, M.-T.; Chen, Y.-F. Skeletal muscle in aged mice reveals extensive transformation of muscle gene expression. BMC Genet. 2018, 19, 55. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Julien, I.B.; Sephton, C.F.; Dutchak, P.A. Metabolic Networks Influencing Skeletal Muscle Fiber Composition. Front. Cell Dev. Biol. 2018, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Barrientos, T.; Shelton, J.M.; Frank, D.; Rütten, H.; Gehring, D.; Kuhn, C.; Lutz, M.; Rothermel, B.A.; Bassel-Duby, R.; et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat. Med. 2004, 10, 1336–1343. [Google Scholar] [CrossRef]
- Bean, C.; Salamon, M.; Raffaello, A.; Campanaro, S.; Pallavicini, A.; Lanfranchi, G. The Ankrd2, Cdkn1c and Calcyclin Genes are Under the Control of MyoD during Myogenic Differentiation. J. Mol. Biol. 2005, 349, 349–366. [Google Scholar] [CrossRef]
- Bean, C.; Verma, N.K.; Yamamoto, D.L.; Chemello, F.; Cenni, V.; Filomena, M.C.; Chen, J.; Bang, M.L.; Lanfranchi, G. Ankrd2 is a modulator of NF-κB-mediated inflammatory responses during muscle differentiation. Cell Death Dis. 2014, 5, e1002. [Google Scholar] [CrossRef]
- Belgrano, A.; Rakicevic, L.; Mittempergher, L.; Campanaro, S.; Martinelli, V.C.; Mouly, V.; Valle, G.; Kojic, S.; Faulkner, G. Multi-Tasking Role of the Mechanosensing Protein Ankrd2 in the Signaling Network of Striated Muscle. PLoS ONE 2011, 6, e25519. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Rui, L. Major Urinary Protein Regulation of Chemical Communication and Nutrient Metabolism. Vitam. Horm. 2010, 83, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Nadanaka, S.; Kinouchi, H.; Kitagawa, H. Histone deacetylase-mediated regulation of chondroitin 4-O-sulfotransferase-1 (Chst11) gene expression by Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2016, 480, 234–240. [Google Scholar] [CrossRef]
- Klüppel, M.; Wight, T.N.; Chan, C.; Hinek, A.; Wrana, J.L. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 2005, 132, 3989–4003. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.-C.; Wang, J.-Y.; Chi, Y.-H.; Tsai, T.-F. Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 9059. https://doi.org/10.3390/ijms21239059
Teng Y-C, Wang J-Y, Chi Y-H, Tsai T-F. Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle. International Journal of Molecular Sciences. 2020; 21(23):9059. https://doi.org/10.3390/ijms21239059
Chicago/Turabian StyleTeng, Yuan-Chi, Jing-Ya Wang, Ya-Hui Chi, and Ting-Fen Tsai. 2020. "Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle" International Journal of Molecular Sciences 21, no. 23: 9059. https://doi.org/10.3390/ijms21239059
APA StyleTeng, Y.-C., Wang, J.-Y., Chi, Y.-H., & Tsai, T.-F. (2020). Exercise and the Cisd2 Prolongevity Gene: Two Promising Strategies to Delay the Aging of Skeletal Muscle. International Journal of Molecular Sciences, 21(23), 9059. https://doi.org/10.3390/ijms21239059




