Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa
Abstract
:1. Introduction
1.1. RONS in Cellular Signaling
1.2. Regulation of Nrf2 as a Cellular Signal for Oxidative Stress
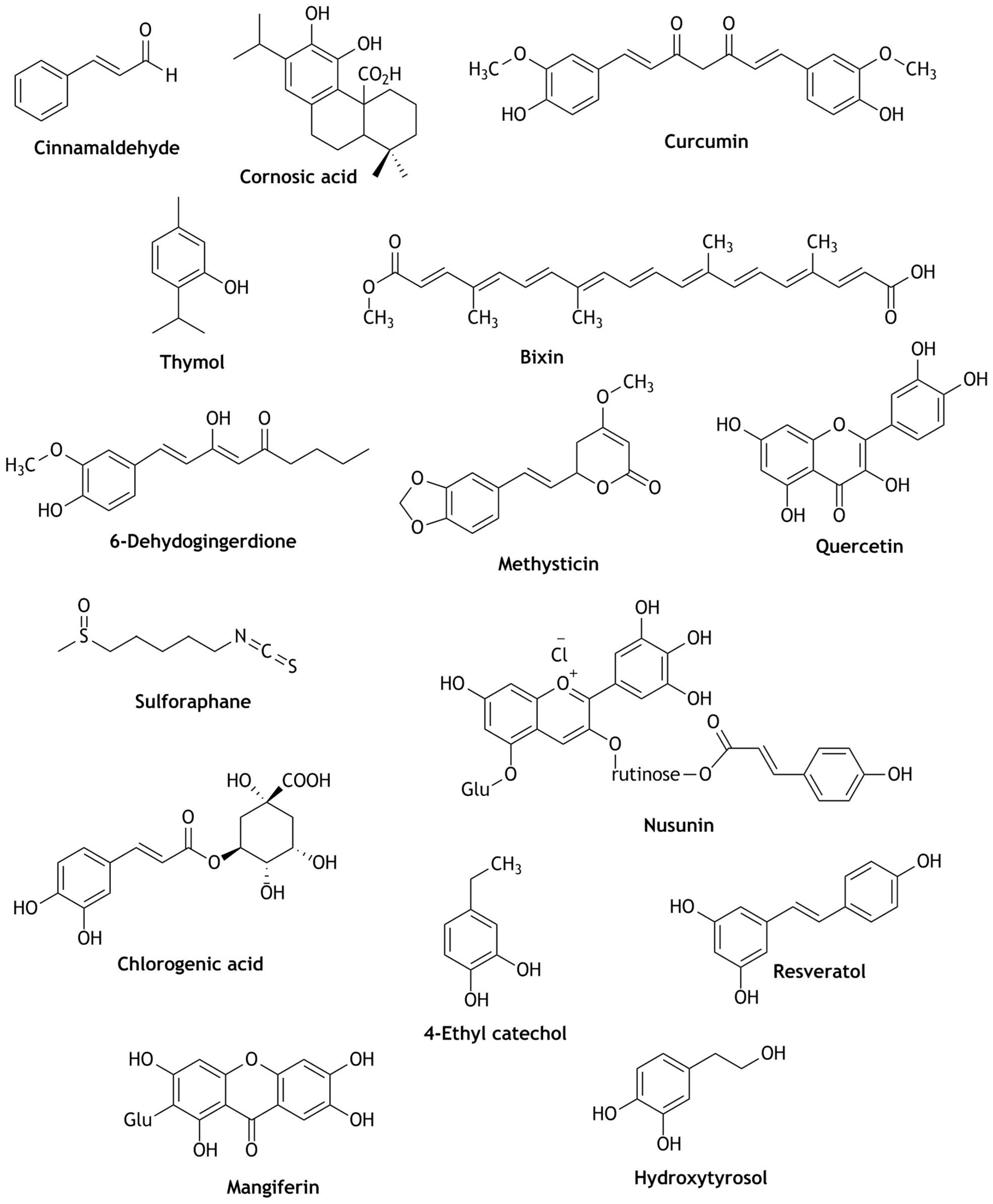
2. Nrf2 Modulation of Natural Compounds Present in Fruit Juices
3. Nrf2 Modulation of Natural Compounds Present in Wines
4. Nrf2 Modulation of Natural Compounds Present in Coffee and Cocoa
5. Nrf2 Inhibitors from Beverages
6. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durairajanayagam, D. Physiological Role of Reactive Oxygen Species in Male Reproduction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier Academic Press: New York, NY, USA, 2018; pp. 65–78. ISBN 9780128125014. [Google Scholar]
- Hardy, M.; Zielonka, J.; Karoui, H.; Sikora, A.; Michalski, R.; Podsiadły, R.; Lopez, M.; Vasquez-Vivar, J.; Kalyanaraman, B.; Ouari, O. Detection and Characterization of Reactive Oxygen and Nitrogen Species in Biological Systems by Monitoring Species-Specific Products. Antioxid. Redox Signal. 2018, 28, 1416–1432. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Current status review: Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989, 70, 737–757. [Google Scholar] [PubMed]
- Thuy, T.T.; Hai, H.; Kawada, N. Role of oxidative and nitrosative stress in hepatic fibrosis. In Liver Pathophysiology: Therapies and Antioxidants; Elsevier Academic Press: New York, NY, USA, 2017; pp. 213–224. [Google Scholar]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [Green Version]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Lyras, L.; Perry, R.H.; Perry, E.K.; Ince, P.G.; Jenner, A.; Jenner, P.; Halliwell, B. Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J. Neurochem. 1998, 71, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Thomas, S.R.; Keaney, J.F. Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic. Biol. Med. 2003, 35, 117–132. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive oxygen species: The good and the bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2018; pp. 7–20. ISBN 9781789231342. [Google Scholar]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–226. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Crestani, B.; Besnard, V.; Boczkowski, J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2011, 43, 1086–1089. [Google Scholar] [CrossRef]
- Da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadeja, R.N.; Upadhyay, K.K.; Devkar, R.V.; Khurana, S. Naturally occurring Nrf2 activators: Potential in treatment of liver injury. Oxid. Med. Cell. Longev. 2016, 2016, 3453926. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; McDonald, P.R.; Liu, J.; Klaassen, C.D. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med. 2014, 80, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Zhou, D.D.; Xie, T.; Malik, T.H.; Lu, C.B.; Li, H.J.; Wang, F.; Shu, C.; Liu, C.; Lu, C.W.; et al. Nrf2, a potential therapeutic target against oxidative stress in corneal diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2326178. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 2009, 77, 1273–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Tao, S.; De La Vega, M.R.; Jiang, T.; Wen, Q.; Park, S.L.; Zhang, D.D.; Wondrak, G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev. Res. 2015, 8, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Vemu, B.; Veenstra, J.; Petiwala, S.M.; Johnson, J.J. Carnosol, a dietary diterpene from rosemary (Rosmarinus officinalis) activates Nrf2 leading to sestrin 2 induction in colon cells. Integr. Mol. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Hou, G.; Wang, L.; Zuo, X.; Liu, Z. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb. Pathog. 2018, 116, 8–12. [Google Scholar] [CrossRef]
- De la Vega, M.R.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Targeting NRF2 for improved skin barrier function and photoprotection: Focus on the achiote-derived apocarotenoid bixin. Nutrients 2017, 9, 1371. [Google Scholar] [CrossRef] [Green Version]
- Schadich, E.; Hlaváč, J.; Volná, T.; Varanasi, L.; Hajdúch, M.; Džubák, P. Effects of ginger phenylpropanoids and quercetin on Nrf2-ARE pathway in human BJ fibroblasts and HaCaT keratinocytes. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.-J. Green pepper (Piper nigrum L.) extract suppresses oxidative stress and LPS-induced inflammation via regulation of JNK signaling pathways. Appl. Sci. 2020, 10, 2519. [Google Scholar] [CrossRef] [Green Version]
- Balstad, T.R.; Carlsen, H.; Myhrstad, M.C.W.; Kolberg, M.; Reiersen, H.; Gilen, L.; Ebihara, K.; Paur, I.; Blomhoff, R. Coffee, broccoli and spices are strong inducers of electrophile response element-dependent transcription in vitro and in vivo—Studies in electrophile response element transgenic mice. Mol. Nutr. Food Res. 2011, 55, 185–197. [Google Scholar] [CrossRef]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Noda, Y.; Kneyuki, T.; Igarashi, K.; Mori, A.; Packer, L. Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology 2000, 148, 119–123. [Google Scholar] [CrossRef]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by olive oil and wine polyphenols and neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senger, D.R.; Cao, S. Diabetic wound healing and activation of Nrf2 by herbal medicine. J. Nat. Sci. 2016, 2, e247. [Google Scholar] [PubMed]
- Kim, J.; Oh, J.; Averilla, J.N.; Kim, H.J.; Kim, J.S.; Kim, J.S. Grape peel extract and resveratrol inhibit wrinkle formation in mice model through activation of Nrf2/HO-1 signaling pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sport. Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [Green Version]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Copetti, C.; Franco, F.W.; Machado, E.D.; Soquetta, M.B.; Quatrin, A.; Ramos, V.D.; Moreira, J.C.; Emanuelli, T.; Sautter, C.K.; Penna, N.G. Acute consumption of bordo grape juice and wine improves serum antioxidant status in healthy individuals and inhibits reactive oxygen species production in human neuron-like cells. J. Nutr. Metab. 2018, 2018, 4384012. [Google Scholar] [CrossRef] [Green Version]
- Heron, M. National Vital Statistics Reports Volume 68, Number 6, June 24, 2019, Deaths: Leading Causes for 2017; U.S. Department of Health & Human Services: Hyattsville, MD, USA, 2019.
- Yuan, L.; Liu, J.; Zhen, J.; Xu, Y.; Chen, S.; Halm-Lutterodt, N.V.; Xiao, R. Vegetable and fruit juice enhances antioxidant capacity and regulates antioxidant gene expression in rat liver, brain and colon. Genet. Mol. Biol. 2017, 40, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.R.B.; Cadavid, C.O.M.; Carmona, L.; Peña, L.; De Paula, O.R. Pasteurized orange juice rich in carotenoids protects caenorhabditis elegans against oxidative stress and β-amyloid toxicity through direct and indirect mechanisms. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, H.; Latief, U.; Ahmad, R. Pomegranate action in curbing the incidence of liver injury triggered by Diethylnitrosamine by declining oxidative stress via Nrf2 and NFκB regulation. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, S.S.; Yin, P.; Li, D.Y.; Mei, C.; Yu, X.H.; Shi, Y.R.; Jiang, L.S.; Liu, F.H. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperth. 2016, 32, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Tang, J.S.; Vissers, M.C.M.; Anderson, R.F.; Sreebhavan, S.; Bozonet, S.M.; Scheepens, A.; Melton, L.D. Bioavailable blueberry-derived phenolic acids at physiological concentrations enhance Nrf2-regulated antioxidant responses in human vascular endothelial cells. Mol. Nutr. Food Res. 2018, 62, 1–34. [Google Scholar] [CrossRef]
- Soyalan, B.; Minn, J.; Schmitz, H.J.; Schrenk, D.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Apple juice intervention modulates expression of ARE-dependent genes in rat colon and liver. Eur. J. Nutr. 2011, 50, 135–143. [Google Scholar] [CrossRef]
- McClatchey, W. From Polynesian healers to health food stores: Changing perspectives of Morinda citrifolia (Rubiaceae). Integr. Cancer Ther. 2003, 1, 110–120. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Chen, Y.; Liang, H.; Cheng, C.; He, Q. Neuroprotective effects of chloroform and aqueous fractions of noni juice against t-butyl hydroperoxide-induced oxidative damage in SH-SY5Y cells. Food Nutr. Res. 2018, 62, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Manavalan, A.; Qader, M.; Wu, X.; Cao, S. Nrf2 activation by Morinda citrifolia L. (Noni) fruit juices. World J. Tradit. Chinese Med. 2020. In press. [Google Scholar]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; D’Erme, M.; et al. Neuroprotective effect of brassica oleracea sprouts crude juice in a cellular model of alzheimer’s disease. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawal, A.O.; Lawal, A.F.; Ologundudu, A.; Adeniran, O.Y.; Omonkhua, A.; Obi, F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J. Toxicol. Sci. 2011, 36, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajka-Kuźniak, V.; Szaefer, H.; Bartoszek, A.; Baer-Dubowska, W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: Comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br. J. Nutr. 2011, 105, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Li, X.; Li, G.; Dai, B.; Tan, W. Actinidia chinensis Planch. Improves the indices of antioxidant and anti-inflammation status of type 2 diabetes mellitus by activating keap1 and Nrf2 via the upregulation of MicroRNA-424. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs, P. Inventing Wine: A New History of One of the World’s Most Ancient Pleasures, 1st ed.; W.W. Nortan & Company, Inc.: New York, NY, USA, 2012; ISBN 9780393239645. [Google Scholar]
- Klatsky, A.L. Alcohol and cardiovascular diseases. Expert Rev. Cardiovasc. Ther. 2009, 7, 499–506. [Google Scholar] [CrossRef]
- Del Pino-García, R.; González-SanJosé, M.L.; Rivero-Pérez, M.D.; García-Lomillo, J.; Muñiz, P. The effects of heat treatment on the phenolic composition and antioxidant capacity of red wine pomace seasonings. Food Chem. 2017, 221, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and after fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Nile, S.H.; Kim, S.H.; Ko, E.Y.; Park, S.W. Polyphenolic contents and antioxidant properties of different grape (V. vinifera, V. labrusca, and V. hybrid) cultivars. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, E.; Casagrande, K.; Cardoso, P.Z.; da Silveira Pasa, M.; Ciotta, M.N.; Brighenti, A.F. Total polyphenols contents in different grapevine varieties in highlands of southern brazil. BIO Web Conf. 2017, 9, 01024. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in Rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A.; Barnes, K.F.; Bhatia, D.; Darvesh, A.S.; Carroll, R.T. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev. Res. 2010, 3, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurusamy, N.; Ray, D.; Lekli, I.; Das, D.K. Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J. Cell. Mol. Med. 2010, 14, 2235–2239. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xu, X.; Tao, Z.; Wang, X.J.; Pan, Y. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater effectiveness of ε-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yue, Y.; Li, J.; Li, Z.; Li, X.; Niu, Y.; Xiang, J.; Ding, H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol. Nutr. Food Res. 2015, 59, 1930–1941. [Google Scholar] [CrossRef]
- Bahia, P.K.; Rattray, M.; Williams, R.J. Dietary flavonoid (-)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J. Neurochem. 2008, 106, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Han, X.; Li, Q.; Wang, J. (−)-Epicatechin, a natural flavonoid compound, protects astrocytes against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways. Mol. Neurobiol. 2017, 54, 7898–7907. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Ramos, S.; Bravo, L.; Goya, L.; Martín, M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012, 51, 881–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, C.J.; Chen, M.J.; Yeh, C.T.; Yen, G.C. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. New Biotechnol. 2011, 28, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Gao, X.; Li, X.; Zhang, X.; Ma, R.; Jia, Y.; Li, D.; Wang, D.; Xu, F. Involvement of estrogen receptor-α in the activation of Nrf2-antioxidative signaling pathways by silibinin in pancreatic β-cells. Biomol. Ther. 2020, 28, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Byun, S.J.; Pae, H.O. Involvement of heme oxygenase-1 expression in neuroprotection by piceatannol, a natural analog and a metabolite of resveratrol, against glutamate-mediated oxidative injury in HT22 neuronal cells. Amino Acids 2013, 45, 393–401. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ijomone, O.M.; Sanni, O.; Aschner, M.; Islam, M.S. Type 2 diabetes induced oxidative brain injury involves altered cerebellar neuronal integrity and elemental distribution, and exacerbated Nrf2 expression: Therapeutic potential of raffia palm (Raphia hookeri) wine. Metab. Brain Dis. 2019, 34, 1385–1399. [Google Scholar] [CrossRef]
- Jeon, M.; Rahman, N.; Kim, Y.S. Cytoprotective effect of makgeolli lees on paraquat induced oxidative stress in A549 cells via activation of NRF2 and antioxidant genes. J. Microbiol. Biotechnol. 2015, 26, 277–286. [Google Scholar] [CrossRef]
- Zhai, X.; Chi, J.; Tang, W.; Ji, Z.; Zhao, F.; Jiang, C.; Lv, H.; Guo, H. Yellow wine polyphenolic compounds inhibit matrix metalloproteinase-2, -9 expression and improve atherosclerotic plaque in LDL-receptor–knockout mice. J. Pharmacol. Sci. 2014, 125, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, J.; Ni, T.; Lin, N.; Meng, L.; Gao, F.; Luo, H.; Liu, X.; Chi, J.; Guo, H. Yellow wine polyphenolic compounds prevents doxorubicin-induced cardiotoxicity through activation of the Nrf2 signalling pathway. J. Cell. Mol. Med. 2019, 23, 6034–6047. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.-S.; Xu, J.; Chen, M.; Wang, D.; Yang, Y.; Manavalan, A.; Wu, X.; Liu, Y.; Cao, S. Compound analysis of jing liqueur and Nrf2 activation by jing liqueur—One of the most popular beverages in China. Beverages 2019, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Lakenbrink, C.; Lapczynski, S.; Maiwald, B.; Engelhardt, U.H. Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages. J. Agric. Food Chem. 2000, 48, 2848–2852. [Google Scholar] [CrossRef]
- Ackar, D.; Valek Lendić, K.; Valek, M.; Šubarić, D.; Miličević, B.; Babić, J.; Nedić, I. cocoa polyphenols: Can we consider cocoa and chocolate as potential functional food? J. Chem. 2013, 2013, 289392. [Google Scholar] [CrossRef]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Amelioration of oxidative stress in Caco-2 cells treated with pro-inflammatory proteins by chlorogenic acid isomers via activation of the Nrf2-Keap1-ARE-signaling pathway. J. Agric. Food Chem. 2018, 66, 11008–11017. [Google Scholar] [CrossRef]
- Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J. Neurosci. 2010, 30, 5525–5532. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4328. [Google Scholar] [CrossRef] [Green Version]
- Higgins, L.G.; Cavin, C.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol. Appl. Pharmacol. 2008, 226, 328–337. [Google Scholar] [CrossRef]
- Cavin, C.; Holzhaeuser, D.; Scharf, G.; Constable, A.; Huber, W.W.; Schilter, B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002, 40, 1155–1163. [Google Scholar] [CrossRef]
- Hao, W.R.; Sung, L.C.; Chen, C.C.; Hong, H.J.; Liu, J.C.; Chen, J.J. Cafestol activates nuclear factor erythroid-2 related factor 2 and inhibits urotensin ii-induced cardiomyocyte hypertrophy. Am. J. Chin. Med. 2019, 47, 337–350. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Sung, L.C.; Haw, W.R.; Chen, C.C.; Huang, S.F.; Liu, J.C.; Cheng, T.H.; Chen, P.Y.; Loh, S.H.; Tsai, C.S. Cafestol, a coffee diterpene, inhibits urotensin II-induced interleukin-8 expression in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2018, 820, 106–112. [Google Scholar] [CrossRef]
- Fukuma, Y.; Sakai, E.; Nishishita, K.; Okamoto, K.; Tsukuba, T. Cafestol has a weaker inhibitory effect on osteoclastogenesis than kahweol and promotes osteoblast differentiation. BioFactors 2015, 41, 222–231. [Google Scholar] [CrossRef]
- Volz, N.; Boettler, U.; Winkler, S.; Teller, N.; Schwarz, C.; Bakuradze, T.; Eisenbrand, G.; Haupt, L.; Griffiths, L.R.; Stiebitz, H.; et al. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 9631–9641. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.; Zuo, J. Caffeic acid inhibits HCV replication via induction of IFNα antiviral response through p62-mediated Keap1/Nrf2 signaling pathway. Antivir. Res. 2018, 154, 166–173. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-κB and Nrf2 pathways. BioFactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Nilnumkhum, A.; Kanlaya, R.; Yoodee, S.; Thongboonkerd, V. Caffeine inhibits hypoxia-induced renal fibroblast activation by antioxidant mechanism. Cell Adhes. Migr. 2019, 13, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Rowley, T.J.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: Relevance of MAPKs. Mol. Nutr. Food Res. 2015, 59, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Wang, W.; Li, Q.; Han, X.; Xing, J.; Qi, C.; Lan, X.; Wan, J.; Potts, A.; Guan, F.; et al. Cerebroprotection of flavanol (-)-epicatechin after traumatic brain injury via Nrf2-dependent and-independent pathways. Free Radic. Biol. Med. 2016, 92, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Funakoshi-Tago, M.; Nonaka, Y.; Tago, K.; Takeda, M.; Ishihara, Y.; Sakai, A.; Matsutaka, M.; Kobata, K.; Tamura, H. Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Boettler, U.; Sommerfeld, K.; Volz, N.; Pahlke, G.; Teller, N.; Somoza, V.; Lang, R.; Hofmann, T.; Marko, D. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J. Nutr. Biochem. 2011, 22, 426–440. [Google Scholar] [CrossRef]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol. Cell. Biochem. 2019, 457. [Google Scholar] [CrossRef] [Green Version]
- Fürstenau, C.R.; de Souza, I.C.C.; de Oliveira, M.R. The effects of kahweol, a diterpene present in coffee, on the mitochondria of the human neuroblastoma SH-SY5Y cells exposed to hydrogen peroxide. Toxicol. Vitro 2019, 61. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-C.; Wu, S.-C.; Xu, K.-D.; Liao, B.-C.; Wu, J.-F.; Cheng, A.-S. Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation endproducts (AGEs) generation and anti-glycation. Molecules 2015, 20, 2786–2801. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef]
- Kim, W.D.; Kim, Y.W.; Cho, I.J.; Lee, C.H.; Kim, S.G. E-cadherin inhibits nuclear accumulation of Nrf2: Implications for chemoresistance of cancer cells. J. Cell Sci. 2012, 125, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D.; Chapman, E. The role of natural products in revealing NRF2 function. Nat. Prod. Rep. 2020, 37, 797–826. [Google Scholar] [CrossRef]
- Lin, H.; Qiao, Y.; Yang, H.; Nan, Q.; Qu, W.; Feng, F.; Liu, W.; Chen, Y.; Sun, H. Small molecular Nrf2 inhibitors as chemosensitizers for cancer therapy. Future Med. Chem. 2020, 12, 243–267. [Google Scholar] [CrossRef]
- Tarumoto, T.; Nagai, T.; Ohmine, K.; Miyoshi, T.; Nakamura, M.; Kondo, T.; Mitsugi, K.; Nakano, S.; Muroi, K.; Komatsu, N.; et al. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp. Hematol. 2004, 32, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.M.; Ke, Z.P.; Wang, J.N.; Yang, J.Y.; Chen, S.Y.; Chen, H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis 2013, 34, 1806–1814. [Google Scholar] [CrossRef]
- Kweon, M.H.; Adhami, V.M.; Lee, J.S.; Mukhtar, H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef] [Green Version]
- Xiu, J.W.; Hayes, J.D.; Henderson, C.J.; Wolf, C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 19589–19594. [Google Scholar] [CrossRef] [Green Version]
- Debelo, H.; Novotny, J.A.; Ferruzzi, M.G. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Wang, H.; Fan, L.; Wu, X.; Xin, A.; Ren, H.; Wang, X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011, 50, 1599–1609. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.L.; Schreiber, S.; Schäfer, H. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef] [PubMed]







| Reactive Oxygen Species (ROS) | Reactive Nitrogen Species (RNS) |
|---|---|
| Superoxide anions (O2•−) | Nitric oxide (NO•) |
| Hydroxyl radical (•OH) | Nitrogen dioxide (NO2•) |
| Peroxyl radical (RO2•) | Peroxy-nitrite (ONOO−) |
| Alkoxyl radical (RO•) | Nitroxyl anion (NO−) |
| Hydroperoxyl radical (HO2•) | Nitrous oxide (N2O) |
| Hydrogen peroxide (H2O2) | Nitrogen oxides (NO2, N2O4) |
| Hypochlorous acid (HOCl) | Nitrous acid (HNO2) |
| Hypobromus acid (HOBr) | Peroxynitrous acid (HNO3) |
| S-nitrosothiols (RSNO) | |
| Fe-dinitrosyl complexes |
| Sources | Bioactive Natural Compound/s | Study Observations | Therapeutic Indications | Reference |
|---|---|---|---|---|
| Apple juice | Chlorogenic acid, 4-coumaroylquinic acid, epicatechin, procyanidin B2 | In distal colon of mice GPX2, GSR, CAT and in liver GPX1 and NOQ-1 mRNA were significantly up-regulated. | Protection against ROS associated toxicity | [62] |
| Beetroot juices | Betanin | Activation and translocation of Nrf2 and significantly increased the expression of GSTP, GSTM, GSTT and NQO-1. | Cytoprotective, Anticarcinogenic Hepatoprotective | [69] |
| Blueberry juice | Chlorogenic acid, vanillic acid, syringic acid, trans-ferulic acid, = protocatechuic acid, p-coumaric acid | Activated Nrf2 and up-regulation of HO-1 and glutamate-cysteine ligase modifier subunit (GCLM) | Beneficial to endothelial cell activity and vascular function | [61] |
| Broccoli sprout crude juice | N/A | Protected against β-amyloid peptide –induced cytotoxicity and apoptosis; up-regulated the intracellular glutathione content and mRNA levels or activity of HO-1, thioredoxin, thioredoxin reductase (TrxR), and NAD(P)H:quinone (NQO-1) | Neuroprotective | [66] |
| Cabbage and sauerkraut juices | N/A | Sauerkraut juice significantly increased the activity of GST and NQO-1; translocation of Nrf2 and up-regulation of GST and NQO-1 by both juices. | Chemo-preventive | [68] |
| Fruit juice of Actinidia chinensis | N/A | Increased Keap1 and Nrf2 activity; up-regulation of SOD and GSH and down-regulation of ALT and AST diabetic patients | Antidiabetic Anti-inflammatory | [70] |
| Garlic juice | N/A | Reduces the ROS in presence of toxic heavy metal Cd; significantly induce the SOD and CAT activity; Nrf2 and NQO-1 expression was significantly increased; HO-1 expression not significant. | Prevents heavy metal (Cd) induced liver damages | [67] |
| Noni fruit juice | N/A | Aqueous and chloroform fractions protect cells from tert-Butyl hydroperoxide (TBHP)-induced cell damage; significantly decreases the TBHP cytotoxicity, ROS generation, mitochondrial membrane depolarization and apoptotic; nuclear accumulation of Nrf2 and HO-1, CAT, SOD-1. | Neuroprotective | [64] |
| 4-Methyl catechol, 4-Ethyl catechol, 4-Vinyl catechol, Scopoletin | Increased Nrf2 nuclear translocation, expression of HO-1, NQO-1 and glucose 6-phosphate dehydrogenase activity | Diabetic wound healing Cytotoxicity | [47,65] | |
| Orange juice | Lycopene, phytoene, all-E-β-carotene, and other carotenoids | Up-regulated the expression of GCS-1. GST-4, SOD-4, HSP-16.2 genes; significantly increased the ROS reductions, gene expression activation, oxidative stress resistance; induces GST-4::GFP expressions and increased SKN-1/Nrf2 transcription factor. | Neuroprotective and Suppress oxidative stress | [56] |
| Pomegranate juice | Anthocyanin and hydrolysable tannins | Decreased in SOD, GST, CAT and membrane-ATPases; significant increase in Nrf2 and NF-κB expression in nitrosodiethylamine (NDEA)-induced fibrotic rats. | Liver fibrosis Hepatoprotective | [58] |
| Pomegranate juice | Punicalagin | Increased Nrf2 translocation and up-regulation of HO-1; decreased the generation of RONS, NO; increased the production of SOD activity | Intestinal injuries | [59] |
| Source | Bioactive Natural Compound/s | Study Observations | Therapeutic Indications | Reference |
|---|---|---|---|---|
| Chinese Jing wine | Calycosin, ethyl ferulate and two cinnamic acid derivatives | Crude extract increased the Nrf2-ARE reporter activity by seven–eight fold; strongly activates the Nrf2-ARE reporter activity. | Cytotoxic | [97] |
| Makgeolli lees (ML) fermented raw rice wine | N/A | Decreased the production of intercellular ROS generated in 1,1′-dimethyl-4,4′-bipyridinium dichloride toxicity; up-regulation of expression and translocation of Nrf2 and ARE-GFP reporter activity; up-regulation the expression of NQO-1, HO-1, GPX, SOD, CAT, peroxiredoxin 3 and 4. | Suppress oxidative stress chemoprotective | [94] |
| Raffia Palm (Raphia hookeri) wine | N/A | Up-regulation of Nrf2 expression in diabetic brain cerebellar cortexes; significantly increased GSH, SOD and CAT activity. | Antidiabetic | [93] |
| Wine | Resveratrol | Significant decrease in hepatic lipid peroxidation, protein oxidation and expression of nitric oxide synthase and 3-nitrotyrosine. Dose of 100 or 300 mg/kg increased in Nrf2 mRNA expression in diethyl nitrosamine induced liver tumorigenesis | Chemo-preventive | [81] |
| Enhanced expression of Nrf2 and redox effector factor-1 (Ref-1) enhanced by resveratol; enhanced stem cell survival, proliferation and regeneration by expression of EGFP. | Improved cardiac function and regeneration | [82] | ||
| Dose of 20 mM protects against oxygen-glucose deprivation/reoxy-generation; activation of Nrf2 via upregulation of NQO-1 and HO-1; increase in SOD and GSH activity | Neuroprotective | [80] | ||
| Activation of Nrf2 via up-regulation of NQO-1, HO-1, GST-P1, GSH | Protect brain against cerebral ischemia, suppress oxidative and inflammatory stress | [77,78,79] | ||
| Parthenocissin A, quadrangularin A, pallidol (resveratrol dimer) | Selective singlet oxygen scavengers; deactivate hydroxyl or superoxide anions; pallidol activates ARE-dependent firefly luciferase reporter gene at 30 μM dose | Selective ROS scavengers | [83] | |
| ε-Viniferin (dehydro-dimer of resveratrol) | Inhibited platelet-derived growth factor-induced cell proliferation, migration, and ROS production, inducing nitric oxide (NO) generation; activation of Nrf2 and up-regulation of HO-1 | Prevent atherosclerosis and anti-proliferative | [84] | |
| Silibinin | Dose of 100 mg/kg induces the up-regulation of estrogen receptor-α (ERα), Nrf2 and HO-1 in pancreatic β-cells; increase insulin biosynthesis and secretion and decrease in ROS. | Antidiabetic | [91] | |
| Piceatannol | Dose of 10 μM activates the expression of HO-1 via Nrf2 activation; protected neuronal cells against glutamate-induced cell death by inducing HO-1 | Neuroprotective | [92] | |
| Catechin, epicatechin | Activation of Nrf2 and up-regulation of HO-1, GSH, NADPH, GST, NQO-1. | Cytoprotective, protect brain cells against cerebral ischemia. | [85,86,87] | |
| Quercetin | Activation of Nrf2 and translocation to the nucleus in mice model for traumatic brain injury; prevention of changes in cell morphology, apoptosis, activation of Nrf2 and increased expression of HO-1 and SOD in galactose induce mice neurotoxicity | Protection from traumatic brain injury, Neuroprotective | [83,88] | |
| Procyanidin B2 | Nuclear translocation of Nrf2 and up-regulation of expression of the glutathione S-transferase P1 (GSTP1), extracellular signal-regulated protein kinases (ERKs) and the p38 mitogen-activated protein kinase (MAPK) | Prevention of oxidative stress related intestinal injury and gut pathologies. | [89] | |
| Quercetin, epicatechin, morin, rutin, luteolin, naringenin, naringin, hesperidin | Quercetin is the strongest antioxidant and induce the expression of Metallothioneins 1 and 2 (MT-1/2); induces the transcriptional MT via the activation of JNK, p38 and PI3K/Akt signaling pathways; increased nuclear translocation of Nrf2 and DNA-binding activity | Hepatoprotective | [90] | |
| Yellow rice wine | Polyphenols | Dose of 30 mg/kg of polyphenols improved doxorubicin (DOX)-induced cardiac dysfunctions; prevent mitochondria-mediated cardiac apoptosis; down-regulation of transforming growth factor beta 1 pathway by promoting Nrf2 translocation. | Cardioprotective | [96] |
| Sources | Bioactive Natural Compound/s | Study Observations | Therapeutic Indications | Reference |
|---|---|---|---|---|
| Cocoa: Cocoa Cocoa/tea | Catechin | Nuclear translocation of Nrf2, expression of Nrf2 target genes: nuclear respiratory factor 1 (Nrf1), GA binding protein transcription factor alpha subunit (GABPA), essential genes for increasing mitochondrial function, improve cellular redox state, mitochondrial respiration, stimulates glucose secretion and β-cell function | Antidiabetic | [116] |
| Epicatechin | Decreased ROS production; activated nuclear Nrf2 without up-regulation of GPx, SOD1, HO-1 and GR activity, promotes cell protection and survival; did not alter HO-1 expression, inhibition of activator protein 1 (AP-1) | Antidiabetic, prevention of oxidative stress, cerebro-protection (brain neuro protection), ischemic heart diseases, ischemic brain diseases (stroke) | [117,118,119] | |
| Procyanidin B2 | Nuclear translocation of Nrf2 and up-regulation of expression of the glutathione S-transferase P1 (GSTP1), extracellular signal-regulated protein kinases (ERKs) and the p38 mitogen-activated protein kinase (MAPK) activity. | Prevention of oxidative stress related intestinal injury and gut pathologies | [89] | |
| Coffee: Coffee/tea Coffee (raw and roasted) Coffee/regular coffee/decaffeinated coffee Coffee | Chlorogenic acid | Activation of Nrf2 and up-regulation of HO-1, NQO-1, and GCLC. Reduces liver abnormalities, and activation of NLR3 inflammasomes; activation of Nrf2 and up-regulation of HO-1, NQO-1, glutathione, thioredoxin reductase 1 (TrxR), and thioredoxin 1. | Hepatoprotection, Neuroprotective | [102,103] |
| Pyrocatechol | Activation of Nrf2, inhibition of NF-κB, up-regulation of HO-1 and NQO-1 genes | Anti-inflammatory | [120] | |
| 5-O-Caffeoylquinic acid N-methylpyridinium | Increased Nrf2 translocation and up-regulation of NQO-1 and glutathione-S-transferase-α1 (GSTA1) and increased GST activity | Chemo-preventive | [112,121] | |
| Cafesterol | Decreased production of ROS, translocation of Nrf2 and up-regulation of HO-1, inhibition of redox signaling and cell proliferation; Nrf2 activation, gsrD1-GFP reporter activation | Cardioprotective Cancer therapy, Anti-angiogenic Parkinson’s, Neuroprotection | [105,109,110] | |
| 3-CQA (caffeoylquinic acid), 4-CQA, 5-CQA, 3,4/3,5/4,5-diCQA | diCQA show greater free radical scavenging activity and Nrf2 activation; up-regulation of genes related to Nrf2 expression: GSH, HO-1, NQO-1 | Prevention of oxidative stress | [104,122] | |
| Cafesterol Kahweol | Up-regulation of NQO-1, GSTA1, UDP-glucuronosyl transferase 1A6 (UGT1A6) and the glutamate cysteine ligase catalytic (GCLC) activity and Nrf2 nuclear translocation; induction of enzymes: glutathione S-transferases, glucuronosyl S-transferases, increased expression of gamma-glutamyl cysteine synthetase and HO-1 | Chemo-preventive, Anti-carcinogenic, Bone diseases | [107,108,111] | |
| Kahweol | Reduced production of ROS, suppress the effects of H2O2 on oxidative phosphorylation, activation of caspase-3 protein, up-regulation of HO-1 and Nrf2 | Prevention of oxidative stress, Neuroprotection | [123,124] | |
| Caffeic acid | Induce HO-1 activity and Nrf2, modulate Keap1/Nrf2 interaction via increasing p62 expression. Up-regulation of Nrf2/electrophile responsive element (EpRE) | Antiviral, Anti-inflammatory, Antidiabetic | [113,114] | |
| Caffeine | Decreased intercellular ROS, catalase activity, up-regulation of nuclear Nrf2, | Prevention of oxidative stress | [115] | |
| Scopoletin | Activation of Nrf2, catalyzing methylglyoxal to lactic acid, inhibits glycation end products | Antidiabetic | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qader, M.; Xu, J.; Yang, Y.; Liu, Y.; Cao, S. Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa. Beverages 2020, 6, 68. https://doi.org/10.3390/beverages6040068
Qader M, Xu J, Yang Y, Liu Y, Cao S. Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa. Beverages. 2020; 6(4):68. https://doi.org/10.3390/beverages6040068
Chicago/Turabian StyleQader, Mallique, Jian Xu, Yuejun Yang, Yuancai Liu, and Shugeng Cao. 2020. "Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa" Beverages 6, no. 4: 68. https://doi.org/10.3390/beverages6040068
APA StyleQader, M., Xu, J., Yang, Y., Liu, Y., & Cao, S. (2020). Natural Nrf2 Activators from Juices, Wines, Coffee, and Cocoa. Beverages, 6(4), 68. https://doi.org/10.3390/beverages6040068






