Development of Hydrophobic, Anticorrosive, Nanocomposite Polymeric Coatings from Canola Oil: A Sustainable Resource
Abstract
:1. Introduction
2. Materials and Methods
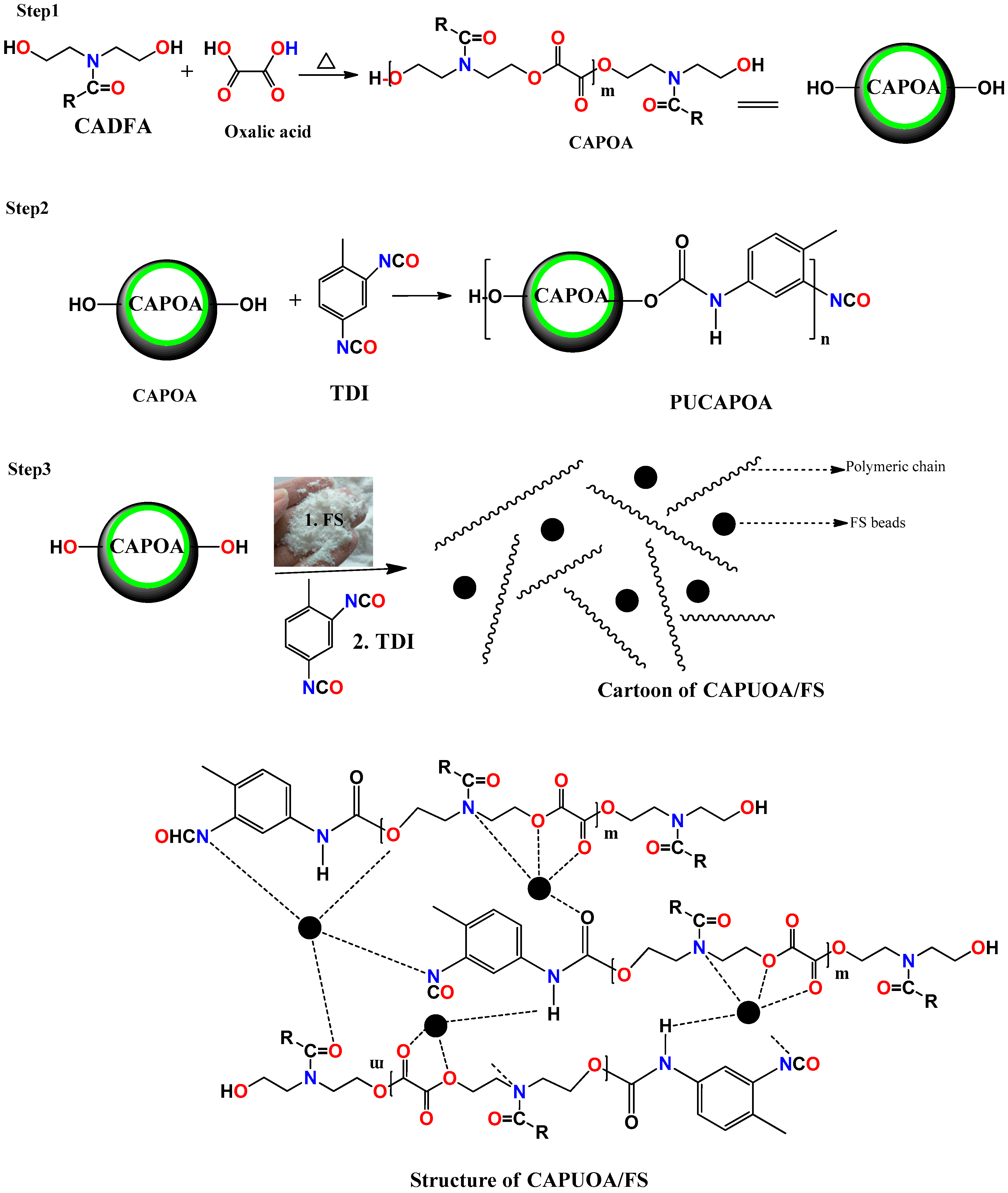
2.1. Synthesis of Canola Diol Fatty Amide (CADFA)
2.2. Synthesis of CA Oil Based Poly (Oxalate-Amide) (CAPOA) (Step 1)
2.3. Synthesis of CA Oil Based Poly(Urethane- Oxalate-Amide) (CAPUOA) (Step 2)
2.4. Preparation of Poly(Urethane-Oxalate-Amide)/Fumed Silica Nanocomposite(Step 3)
2.5. Preparation of CAPUOA/FS Nanocomposite Coating
3. Results and Discussion
3.1. Solubility and Physico-Chemical Properties
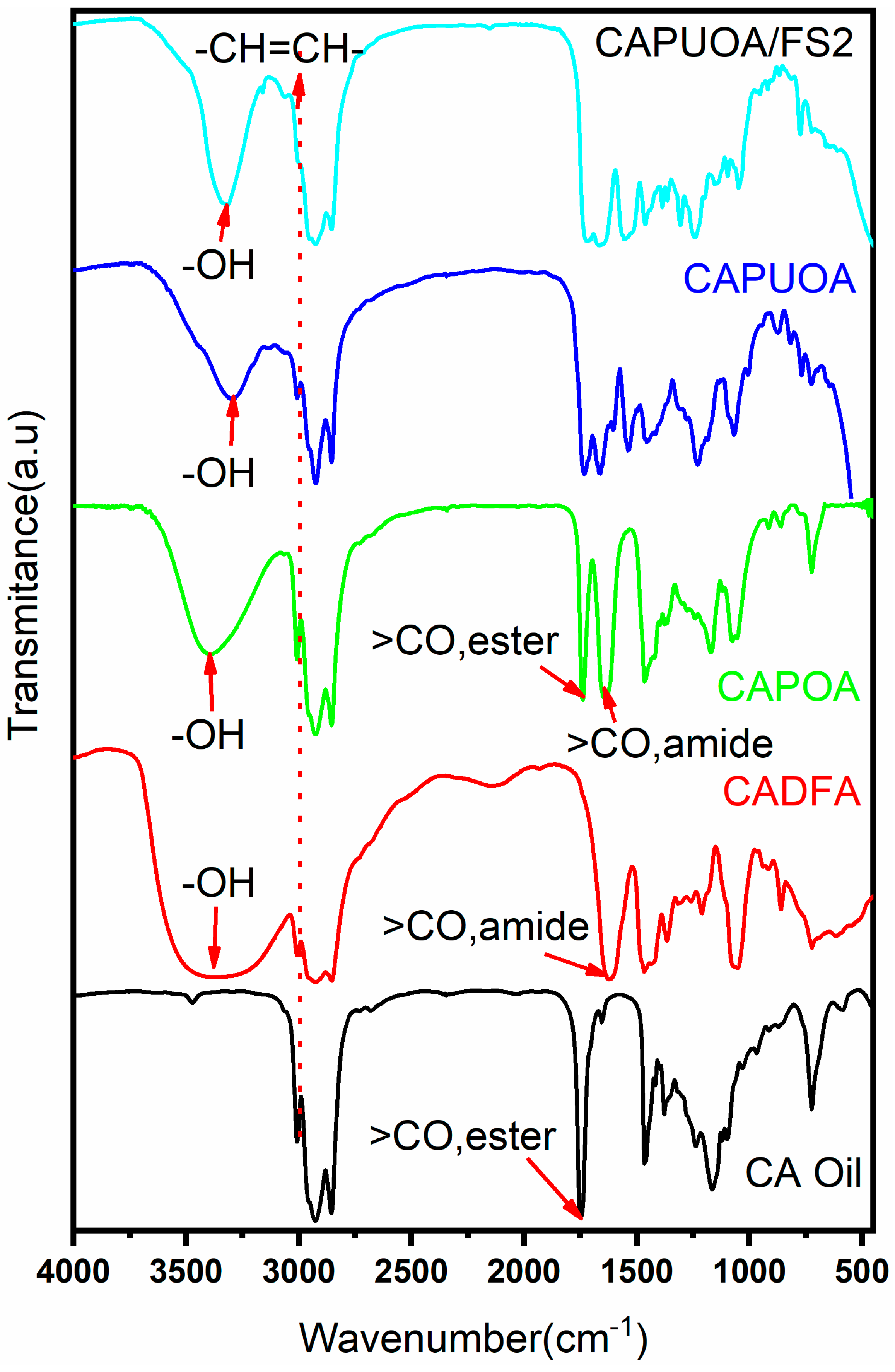
3.2. Spectral Analysis
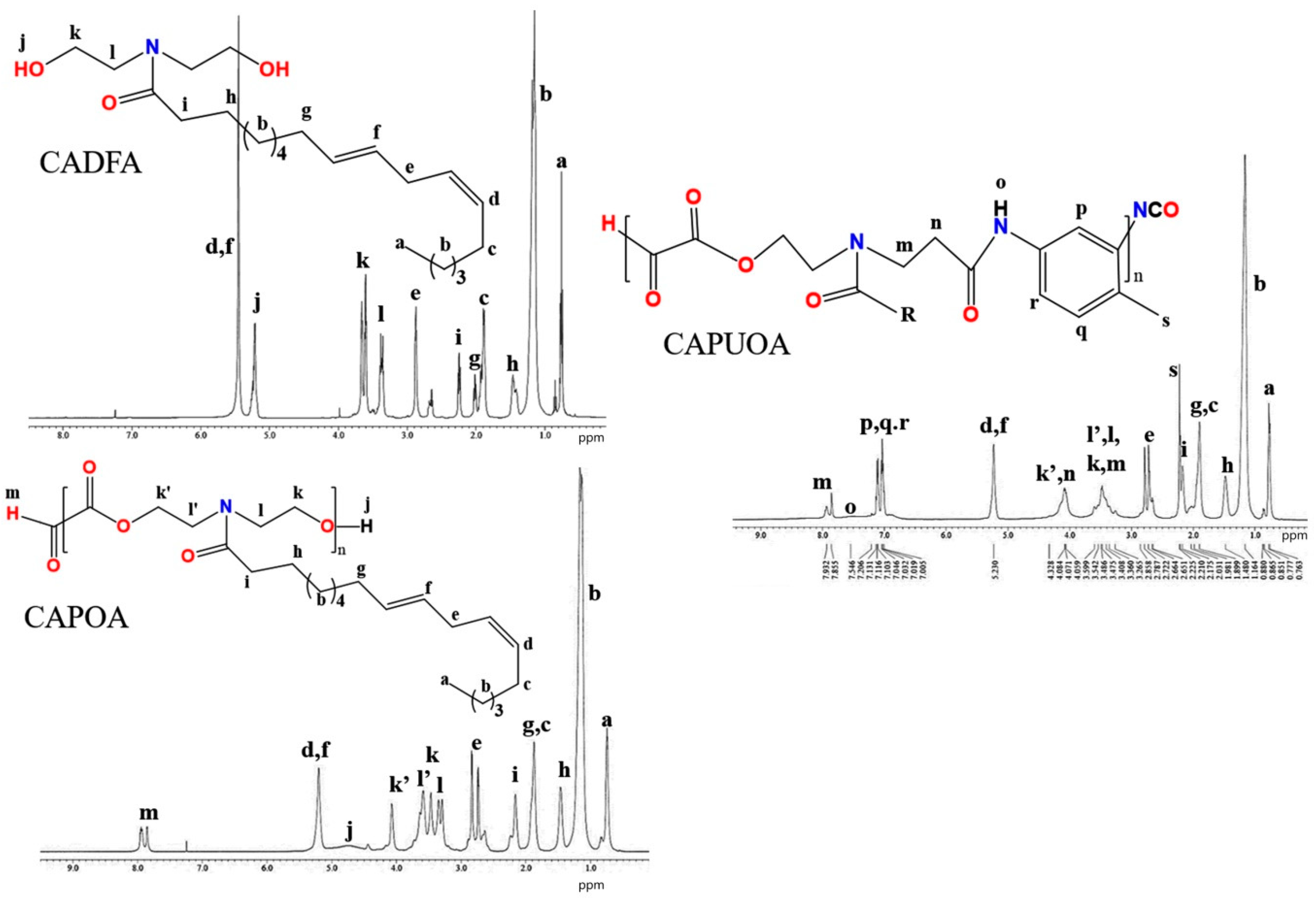
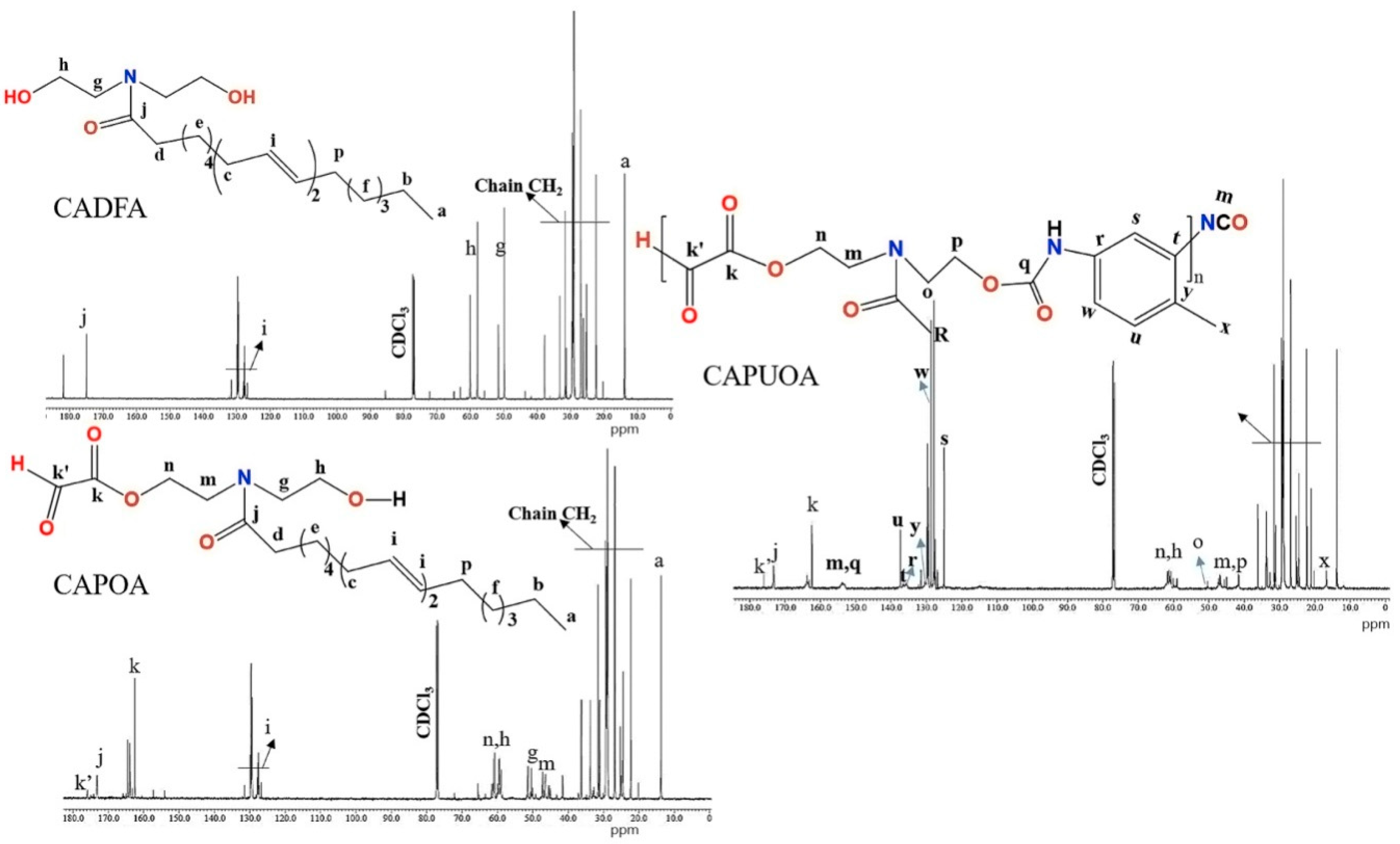
3.3. NMR Spectra
3.4. XRD Analysis
3.5. Surface Wettability
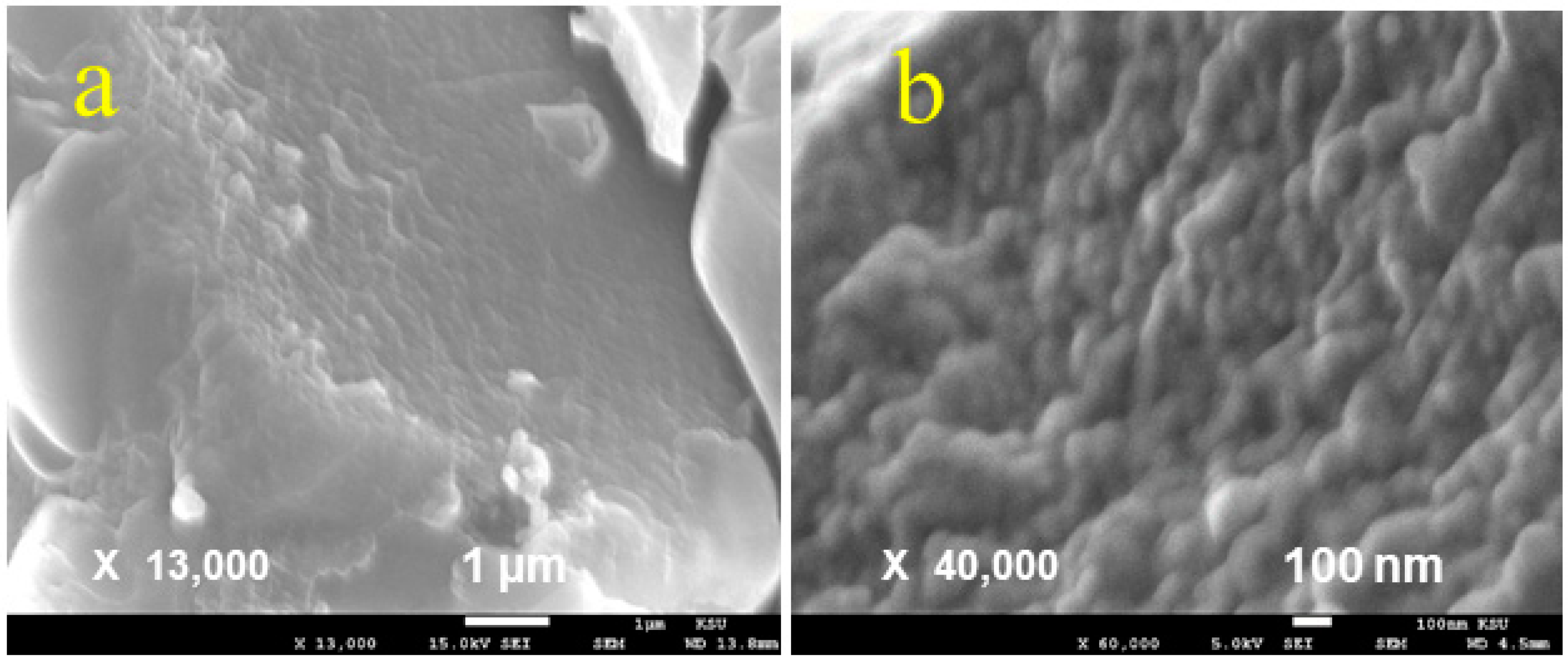
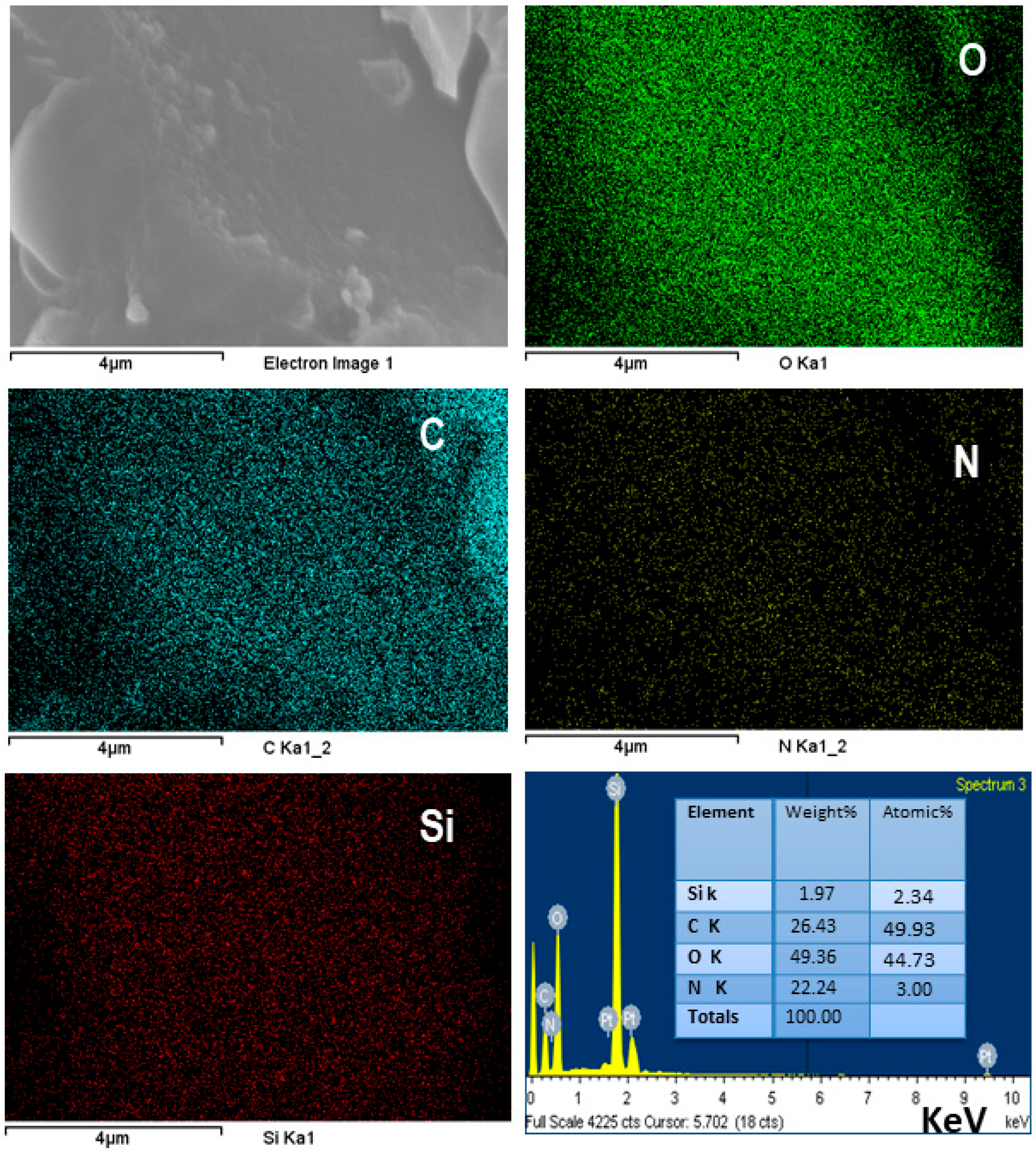
3.6. SEM and EDX Analysis
3.7. Physico-Mechanical Characterization
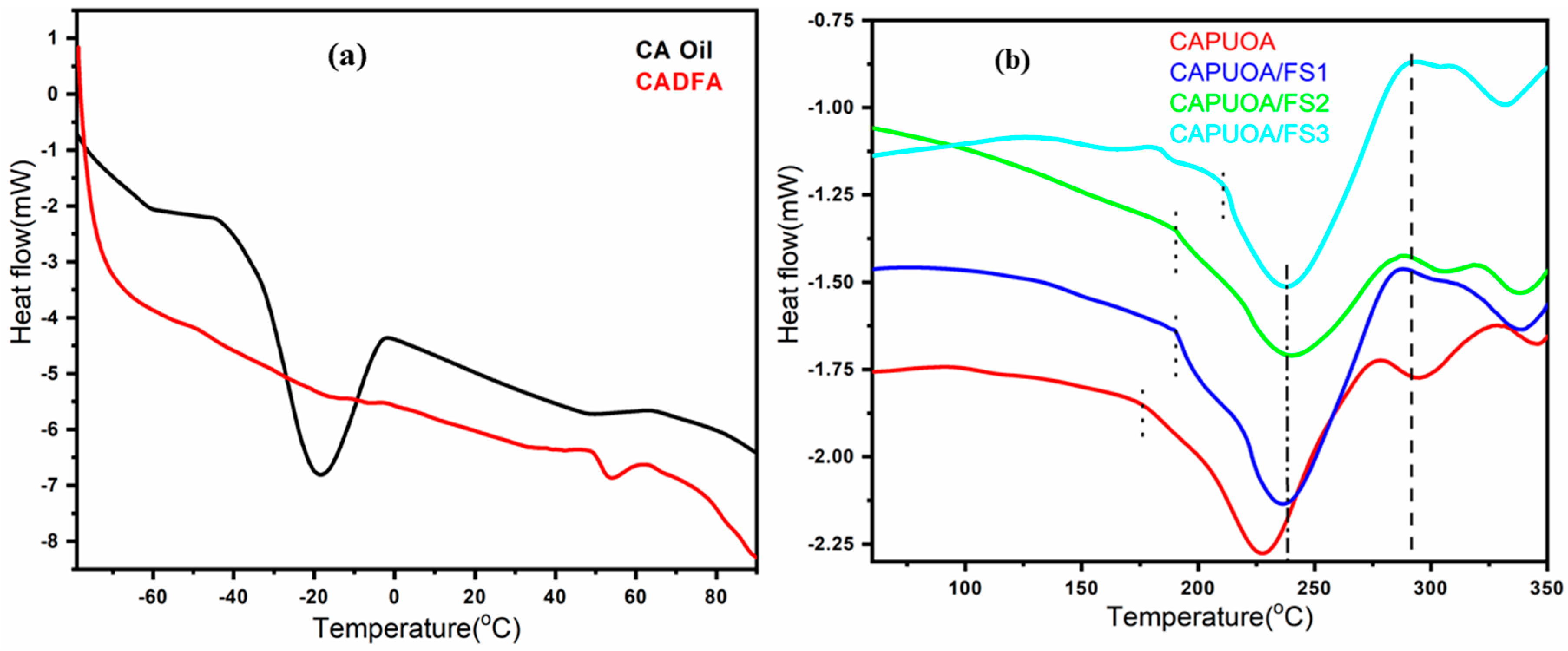
3.8. Thermal Analysis
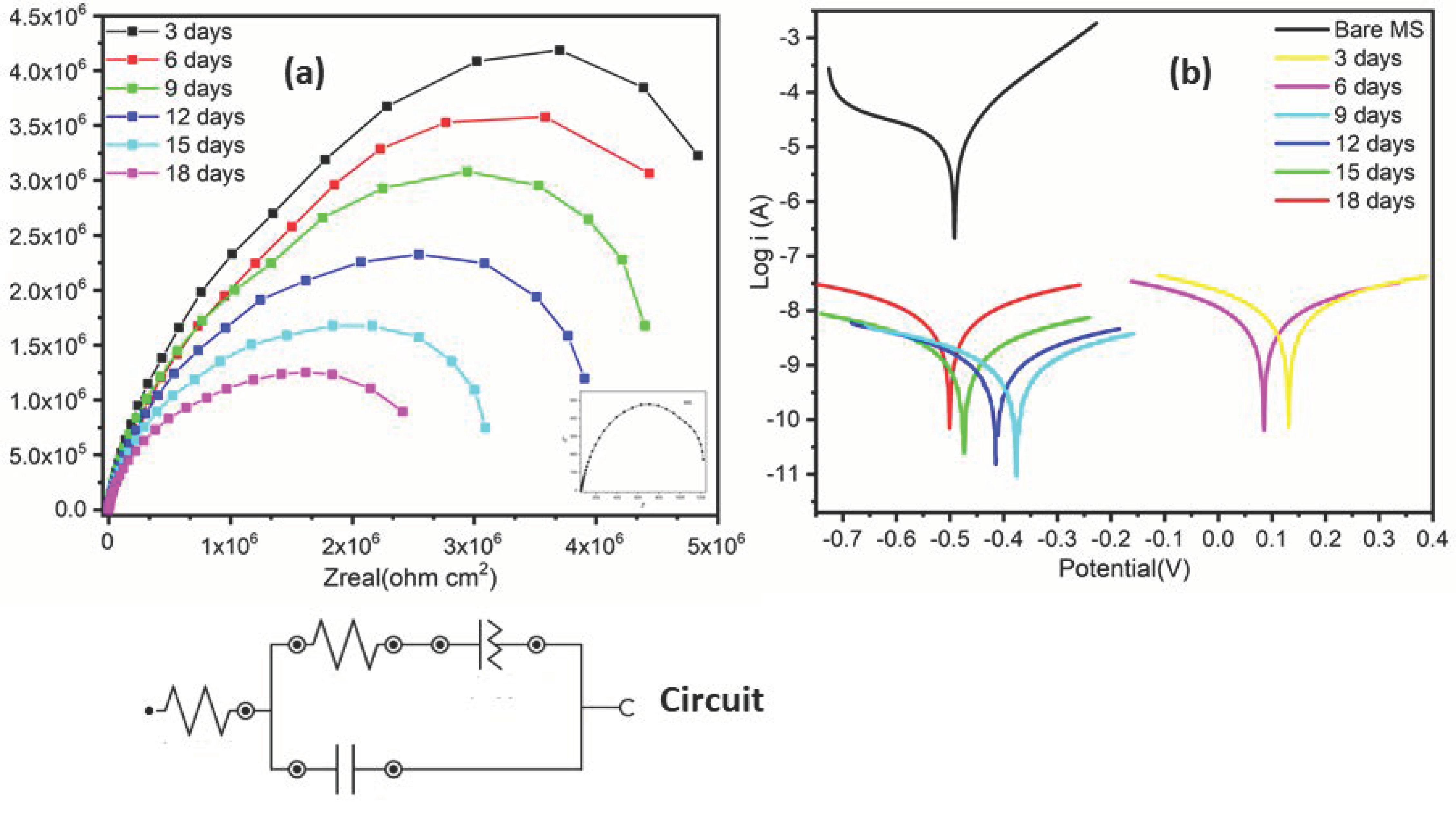
3.9. Corrosion Study
3.9.1. Potentiodynamic Polarization
3.9.2. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 9016–9028. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Adekunle, K.F. A Review of Vegetable oil-based polymers: Synthesis and applications. Open J. Polym. Chem. 2015, 5, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Zafar, F.; Sharmin, E.; Garg, N.; Kashif, M. Synthesis and characterization of corrosion protective polyurethanefattyamide / silica hybrid coating material. Prog. Org. Coat. 2012, 73, 112–117. [Google Scholar] [CrossRef]
- Akram, D.; Ahmad, S.; Sharmin, E.; Ahmad, S. Silica reinforced organic-inorganic hybrid polyurethane nanocomposites from sustainable resource. Macromol. Chem. Phys. 2010, 211, 412–419. [Google Scholar] [CrossRef]
- Heidarian, M.; Shishesaz, M.R.; Kassiriha, S.M.; Nematollahi, M. Characterization of structure and corrosion resistivity of polyurethane/organoclay nanocomposite coatings prepared through an ultrasonication assisted process. Pro. Org. Coat. 2010, 68, 180–188. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; Elmarakbi, A.; El-Saeed, A.M.; Selimd, M.M.; El-Safty, S.A. Sunflower oil-based hyperbranched alkyd/spherical ZnO nanocomposite modeling for mechanical and anticorrosive applications. RSC Adv. 2017, 7, 21796–21808. [Google Scholar] [CrossRef] [Green Version]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; El-Sockaryb, M.A.; Elenienb, O.M.A.; EL-Saeed, A.M. Robust alkyd/exfoliated graphene oxide nanocomposite as a surface coating. Prog. Org. Coat. 2019, 126, 106–118. [Google Scholar] [CrossRef]
- More, P.; Mhaske, S.T. Anticorrosive coating of polyesteramide resin by functionalized ZnOAl2O3-Fly ash composite and functionalized multiwalled carbon nanotubes. Prog. Org. Coat. 2016, 99, 240–250. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crop. Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Andrzejewska, E. Photocurablepolymethacrylate-silica nanocomposites: Correlation between dispersion stability, curing kinetics, morphology and properties. J. Polym. Res. 2016, 23, 116. [Google Scholar] [CrossRef] [Green Version]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Protsaka, I.; Tertykha, V.; Pakhlova, E.; Derylo-Marczewskaca, A. Modification of fumed silica surface with mixtures of polyorganosiloxanes and dialkyl carbonates. Prog. Org. Coat. 2017, 106, 163–169. [Google Scholar] [CrossRef]
- Akram, D.; Sharmin, E.; Ahmad, S. Linseed polyurethane/ tetraethoxyorthosilane/ fumed silica hybrid nanocomposite coatings: Physico-mechanical and potentiodynamic polarization measurements studies. Prog. Org. Coat. 2014, 77, 957–964. [Google Scholar] [CrossRef]
- Alam, M. Corn oil based poly(urethane-ether-amide)/fumed silica nanocomposite coatings for anticorrosion application. Int. J. Polym. Anal. Charact. 2019, 24, 533–547. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Ahmad, N.; Sharmin, E.; Ahmed, M. Corn oil-derived poly (urethane-glutaric-esteramide)/fumed silica nanocomposite coatings for anticorrosive applications. J. Polym. Environ. 2020, 28, 1010–1020. [Google Scholar] [CrossRef]
- Narine, S.S.; Yue, J.; Kong, X. Production of polyols from canola oil and their chemical identification and physical properties. J. Am. Oil Chem. Soc. 2007, 84, 173–179. [Google Scholar] [CrossRef]
- Nimbalkar, R.V.; Athawale, V.D. Synthesis and Characterization of Canola Oil Alkyd Resins Based on Novel Acrylic Monomer (ATBS). J. Am. Oil Chem. Soc. 2010, 87, 947–954. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Ahmad, N.; Alam, M.A.; Sharmin, E. Jatropha seed oil derived poly(esteramideurethane)/fumed silica nanocomposite coatings for corrosion protection. Open Chem. 2019, 17, 206–219. [Google Scholar] [CrossRef]
- Wu, G.; He, X.; Xu, L.; Zhang, H.; Yan, Y. Synthesis and characterization of biobased polyurethane/SiO2 nanocomposites from natural Sapiumsebiferum oil. RSC Adv. 2015, 5, 27097–27106. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M. Corn oil based poly(ether amide urethane) coating material –Synthesis, characterization and coating properties. Ind. Crops Prod. 2014, 57, 17–28. [Google Scholar] [CrossRef]
- Zafar, F.; Sharmin, E.; Zafar, H.; Shah, M.Y.; Nishat, N.; Ahmad, N. Facile microwave-assisted preparation of waterborne polyesteramide/OMMT clay bio-nanocomposites for protective coatings. Ind. Crops Prod. 2015, 67, 484–491. [Google Scholar] [CrossRef]
- Zafar, F.; Zafar, H.; Sharmin, E. Vegetable oil based polyurethanamide/organo-montmorillonite bio-nanocomposite. J. Appl. Polym. Sci. 2014, 131, 40278. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Kulkarni, R.D. Synthesis of isostearic acid/dimer fatty acid-based polyesteramide polyol for the development of green polyurethane Coatings. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Hejazi, I.; Seyfi, J.; Hejazi, E.; Sadeghi, G.M.M.; Jafari, S.H.; Khonakdar, H.A. Investigating the role of surface micro/nano structure in cell adhesion behavior of superhydrophobic polypropylene/nanosilica surfaces. Colloids Surf. B 2015, 127, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Butruk, B.A.; Ziętek, P.A.; Ciach, T. Simple method of fabrication of hydrophobic coatings for polyurethanes. Cent. Eur. J. Chem. 2011, 9, 1039–1045. [Google Scholar] [CrossRef]
- Bakshi, M.I.; Ahmad, S. In-situ synthesis of synergistically active ceria doped polypyrrole oleo-polyesteramide hybrid nanocomposite coatings: Corrosion protection and flame retardancy behaviour. Prog. Org. Coat. 2020, 147, 105778. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Mechanically robust hydrophobic interpenetrating polymer network-based nanocomposite of hyperbranched polyurethane and polystyrene as an effective anticorrosive coating. New J. Chem. 2020, 44, 5980–5994. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Hou, B. Corrosion performance of epoxy coatings modified bynanoparticulate SiO2. Mater Corros. 2010, 61, 1–10. [Google Scholar]
- Bagherzadeh, M.; Haddadi, H.; Iranpour, M. Electrochemical evaluation and surface study of magnetite/PANI nanocomposite for carbon steel protection in 3.5% NaCl. Prog. Org. Coat. 2016, 101, 149–160. [Google Scholar] [CrossRef]
- Lacombre, C.V.; Bouvet, G.; Trinh, D.; Mallarino, S.; Touzain, S. Water uptake in free films and coatings using the brasher and kingsbury equation: A possible explanation of the different values obtained by electrochemical Impedance spectroscopy and gravimetry. Electrochim. Acta. 2017, 231, 162–170. [Google Scholar] [CrossRef]
- Sari, M.G.; Shamshiri, M.; Ramezanzadeh, B. Fabricating an epoxy composite coating with enhanced corrosion resistance through impregnation of functionalized graphene oxide-co-montmorillonite nanoplatelet. Corros. Sci. 2017, 129, 38–53. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Sabouri, M.; Shahrabi, T. Corrosion protection of mild steel by polypyrrole phosphate composite coating. Prog. Org. Coat. 2007, 60, 178–185. [Google Scholar] [CrossRef]


| System | Compositions | Properties of Coatings | References |
|---|---|---|---|
| Poly (urethane-oxalate-amide) nanocomposite | [Canola diol fatty amide + Oxalic acid + TDI]-FS | Anticorrosion (up to 18 days,3.5 wt.% NaCl), scratch hardness: 3.0 kg, Pencil hardness: 4H, Contact angle = 108°.Gloss:100, Thermal stability up to 200 °C. | Present work |
| Poly(diamino cyclohexane urethaneesteramide) nanocomposite | [Jatropha diol fattyamide + trans 1,2 diaminocyclo-hexane-N,N,N’,N’-tetraacetic acid + TDI]-FS | Anticorrosion (3.5 wt.% HCl, 3.5 wt.% NaCl, Tap water), Scratch hardness: 2.8 kg Pencil hardness: HB, Thermal stability up to 200 °C. | [20] |
| Poly(urethane-glutaric-esteramide)-nanocomposite | [Corn diol fattyamide + glutaricacid + TDI] + FS | Anticorrosion (up to 39 days, 3.5 wt.% NaCl), scratch hardness: 2.8 kg, pencil hardness:5H Contact angle:108°, Thermal stability up to 270 °C. | [17] |
| Polyurethane/tetraethoxyorthosilane(TEOS)/fumed silica hybrid nanocomposite | [linseed polyol + TDI]-TEOS + FS | Anticorrosion (3.5% NaOH and 3.5% HCl), antibacterial, scratch hardness: 5.5 kg, Thermal stability up to 270 °C. | [15] |
| Polyurethaneamide/silica | [Linseed diol fatty amide + TEOS + TDI] | Anticorrosion (HCl, NaOH), antibacterial, Thermally stable upto 240 °C. | [6] |
| Polyurethane/SiO2 nanocomposites | [Sapiumsebiferum oil Polyurethane3-aminopropyltriethoxysilane + IPDI] nano SiO2] | Tensile strength (12.4 MPa), glass transition temperature (69.9 °C), water and toluene resistance | [21] |
| Analysis | CAPUOA | CAPUOA/FS1 | CAPUOA/FS2 | CAPUOA/FS3 |
|---|---|---|---|---|
| Thickness (micron) | 196 | 210 | 230 | 235 |
| Scratch hardness (Kg) | 2.5 | 2.8 | 3.0 | 3.2 |
| Pencil hardness | HB | 3H | 4H | 5H |
| Cross hatch (100%) | Passes | Passes | Passes | Passes |
| Bending (1/8 inch) | Passes | Passes | Passes | Fail |
| Impact (100 lb/inch) | Passes | Passes | Passes | Fail |
| Gloss at 45° | 93 | 96 | 100 | 103 |
| Immersion Time (days) | Ba (V/dec) | Bc (V/dec) | Ecorr (V) | Icorr (A) | CR (mm/y) | LPR (Ω·cm2) |
|---|---|---|---|---|---|---|
| Bare CS (1 h) | 0.140 | 0.323 | −0.492 | 1.583 × 10−5 | 0.184 | 2683.9 |
| 3 | 0.380 | 0.351 | 0.131 | 1.019 × 10−9 | 1.184 × 10−5 | 7.702 × 107 |
| 6 | 0.357 | 0.367 | 0.084 | 1.180 × 10−9 | 1.372 × 10−5 | 6.770 × 107 |
| 9 | 0.311 | 0.430 | −0.376 | 1.848 × 10−9 | 2.138 × 10−5 | 4.315 × 107 |
| 12 | 0.343 | 0.397 | −0.414 | 4.004 × 10−9 | 2.148 × 10−5 | 1.138 × 107 |
| 15 | 0.345 | 0.392 | −0.474 | 7.600 × 10−9 | 8.831 × 10−5 | 1.036 × 107 |
| 18 | 0.358 | 0.376 | −0.500 | 9.818 × 10−9 | 1.409 × 10−5 | 8.079 × 106 |
| Immersion Time (Days) | Solution Resistance, Rs (Ω) | Charge Transfer Resistance, Rct (MΩ) | Coating Capacitance Cc (Pf) | CPE | |
|---|---|---|---|---|---|
| Y0 (nmho × sN) | n | ||||
| Bare CS (1 h) | 108 | 7.620 × 10−4 | 0.579 × 10−3 | 0.00165 | 0.80 |
| 3 | −745 | 7.143 | 124 | 6.61 | 0.92 |
| 6 | −769 | 4.432 | 132 | 14.8 | 0.83 |
| 9 | −824 | 4.721 | 121 | 1.11 | 0.80 |
| 12 | −754 | 4.176 | 122 | 14.5 | 0.97 |
| 15 | −690 | 3.028 | 134 | 62.1 | 0.95 |
| 18 | −537 | 2.086 | 197 | 24.3 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.; Alandis, N.M.; Ahmad, N.; Zafar, F.; Khan, A.; Alam, M.A. Development of Hydrophobic, Anticorrosive, Nanocomposite Polymeric Coatings from Canola Oil: A Sustainable Resource. Polymers 2020, 12, 2886. https://doi.org/10.3390/polym12122886
Alam M, Alandis NM, Ahmad N, Zafar F, Khan A, Alam MA. Development of Hydrophobic, Anticorrosive, Nanocomposite Polymeric Coatings from Canola Oil: A Sustainable Resource. Polymers. 2020; 12(12):2886. https://doi.org/10.3390/polym12122886
Chicago/Turabian StyleAlam, Manawwer, Naser M. Alandis, Naushad Ahmad, Fahmina Zafar, Aslam Khan, and Mohammad Asif Alam. 2020. "Development of Hydrophobic, Anticorrosive, Nanocomposite Polymeric Coatings from Canola Oil: A Sustainable Resource" Polymers 12, no. 12: 2886. https://doi.org/10.3390/polym12122886







