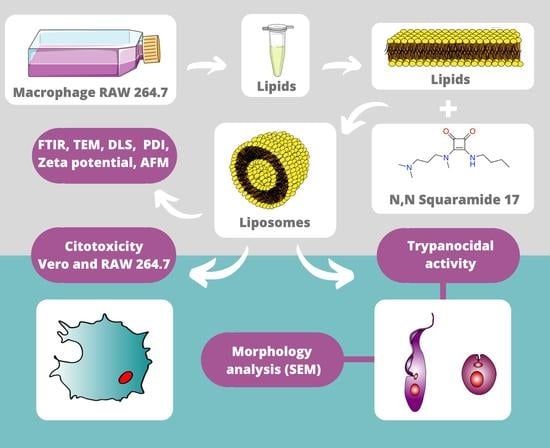
Liposomes Composed by Membrane Lipid Extracts from Macrophage Cell Line as a Delivery of the Trypanocidal N,N’-Squaramide 17 towards Trypanosoma cruzi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Parasites
2.2. Nanostructure Synthesis
2.2.1. Lipid Extraction from Macrophages RAW 264.7
2.2.2. Production of Nanostructured Systems by Extrusion Method
2.3. Entrapment Efficiency
2.4. Nanostructure Characterization
2.4.1. Infrared Vibrational Spectroscopy (IR) Analysis
2.4.2. Dynamic Light Scattering and Electrophoretic Mobility for Hydrodynamic Diameter and Zeta Potential Evaluation
2.4.3. Atomic Force Microscopy (AFM) Analysis
2.4.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Trypanocidal Activity and Cytotoxicity
2.5.1. Cytotoxicity Assays in VERO and Macrophage RAW 264.7 Cells
2.5.2. Trypanocidal Activity of Nanostructures
2.5.3. Ultrastructural Changes in Epimastigotes
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanostructured Systems
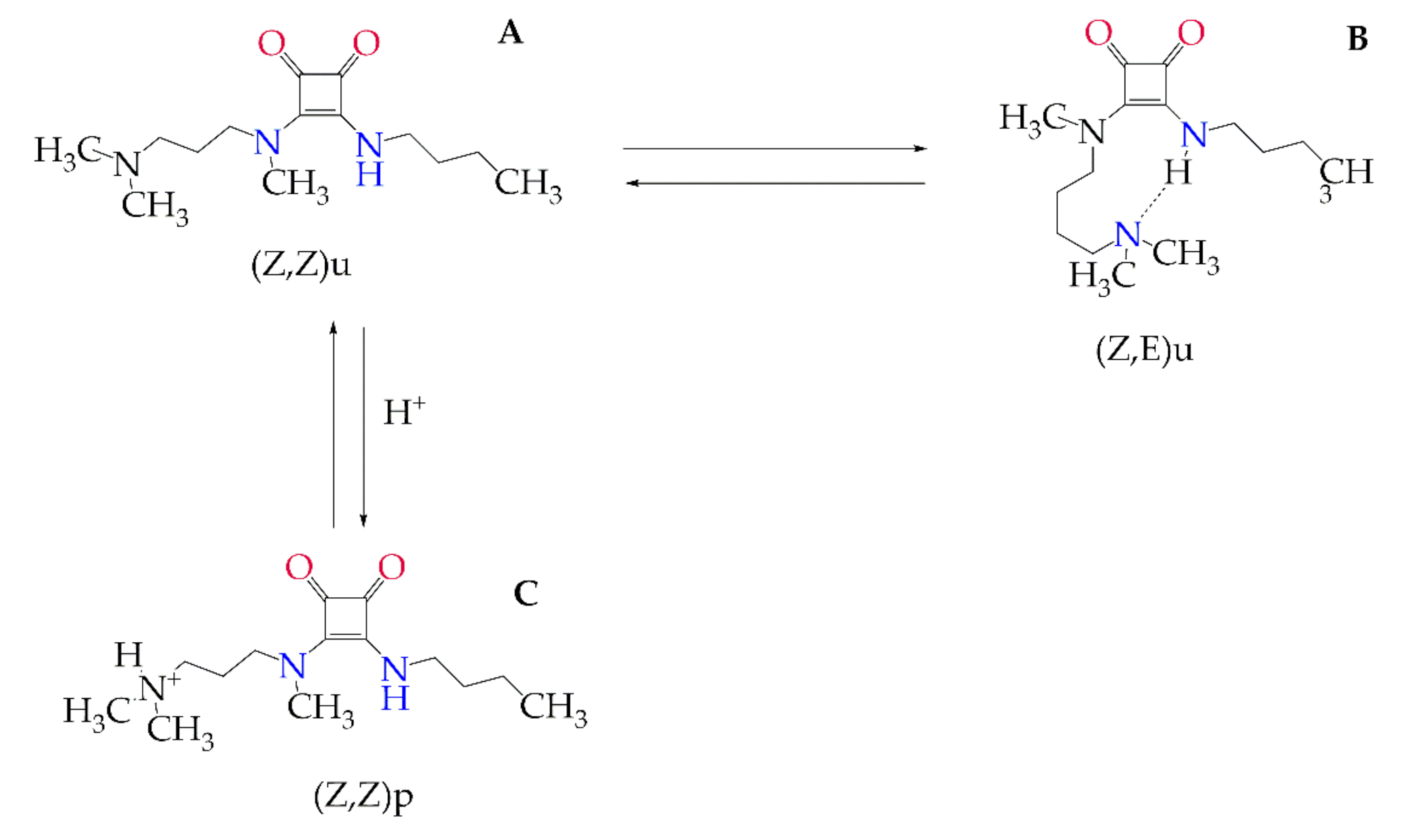
3.2. Analysis of Nanostructured Systems by Infrared Vibrational Spectroscopy (IR) and Entrapment Efficiency
3.3. Evaluation of the Stability of Nanostructures
3.4. Trypanocidal Activity and Cytotoxicity
3.5. Structural Changes in T. Cruzi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2017; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas heart disease: Pathophysiologic mechanisms, prognostic factors and risk stratification. Memórias Do Inst. Oswaldo Cruz 2009, 104, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Chatelain, E. Chagas disease research and development: Is there light at the end of the tunnel? Comput. Struct. Biotechnol. J. 2017, 15, 98–103. [Google Scholar] [CrossRef]
- Olmo, F.; Rotger, C.; Ramírez-Macías, I.; Martínez--Crespo, L.; Marín, C.; Carreras, L.; Urbanová, K.; Vega, M.; Chaves-Lemaur, G.; Sampedro, A.; et al. Synthesis and Biological Evaluation of N,N′-Squaramides with High in Vivo Efficacy and Low Toxicity: Toward a Low-Cost Drug against Chagas Disease. J. Med. Chem. 2014, 57, 987–999. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Sosnik, A.; Chiappetta, D.A.; Carcaboso, A.M. Drug delivery systems in HIV pharmacotherapy: What has been done and the challenges standing ahead. J. Control. Release 2009, 138, 2–15. [Google Scholar] [CrossRef]
- Romero, E.L.; Morilla, M.J. Nanotechnological approaches against Chagas disease. Adv. Drug Deliv. Rev. 2010, 62, 576–588. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J.Y. Trends and Developments in Liposome Drug Delivery Systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Alharbi, H.M.; Campbell, R.B. Nano-formulations composed of cell membrane-specific cellular lipid extracts derived from target cells: Physicochemical characterization and in vitro evaluation using cellular models of breast carcinoma. Aaps Open 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Ameri, A.; Moghimipour, E.; Ramezani, Z.; Kargar, M.; Hashemitabar, M.; Saremy, S.; Handali, S. Formulation of a New Generation of Liposomes from Bacterial and Archeal Lipids. Trop. J. Pharm. Res. 2016, 15, 215. [Google Scholar] [CrossRef] [Green Version]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Omaer, A.; AlQarni, A.; Randulfe, C.; Alharbi, H.; Campbell, R. Abstract 3881: Formulating a cell membrane lipid-extracted nanoliposome (CLENS) drug platform for the treatment of prostate cancer. Exp. Mol. Ther. 2018, 78, 3881. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [Green Version]
- Tempone, A.; Borborema, S.T.; De Andrade, H.; Gualda, N.D.A.; Yogi, Á.; Carvalho, C.S.; Bachiega, D.; Lupo, F.; Bonotto, S.; Fischer, D. Antiprotozoal activity of Brazilian plant extracts from isoquinoline alkaloid-producing families. Phytomedicine 2005, 12, 382–390. [Google Scholar] [CrossRef]
- Fernández-Presas, A.M.; Tato, P.; Becker, I.; Solano, S.; Kopitin, N.; Berzunza, M.; Willms, K.; Hernández-Ruiz, J.; Molinari, J.L. Specific antibodies induce apoptosis in Trypanosoma cruzi epimastigotes. Parasitol. Res. 2010, 106, 1327–1337. [Google Scholar] [CrossRef]
- Bonatto, C.C.; Silva, L.P.; Joanitti, G.A. Method for Obtaining Bioactive Molecules in Microstructured and Nanostructured Carrier Systems. WO/2016/119030A1, 4 August 2016. [Google Scholar]
- Tewes, F.; Munnier, E.; Antoon, B.; Okassa, L.N.; Cohen-Jonathan, S.; Marchais, H.; Douziech-Eyrolles, L.; Soucé, M.; Dubois, P.; Chourpa, I. Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur. J. Pharm. Biopharm. 2007, 66, 488–492. [Google Scholar] [CrossRef]
- Ximenis, M.; Bustelo, E.; Algarra, A.G.; Vega, M.; Rotger, C.; Basallote, M.G.; Costa, A. Kinetic Analysis and Mechanism of the Hydrolytic Degradation of Squaramides and Squaramic Acids. J. Org. Chem. 2017, 82, 2160–2170. [Google Scholar] [CrossRef]
- Kannan, V.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J. Liposome Res. 2014, 25, 270–278. [Google Scholar] [CrossRef]
- Lawson, G.; Ogwu, J.; Tanna, S. Quantitative screening of the pharmaceutical ingredient for the rapid identification of substandard and falsified medicines using reflectance infrared spectroscopy. PLoS ONE 2018, 13, e0202059. [Google Scholar] [CrossRef]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef]
- Azeredo, C.M.; Santos, T.G.; de Noronha Sales, B.H.; Soares, M.J. In vitro biological evaluation of eight different essential oils against Trypanosoma cruzi, with emphasis on Cinnamomum verum essential oil. Bmc Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pace, R.T.; Burg, K.J.L. Toxic effects of resazurin on cell cultures. Cytotechnology 2013, 67, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Rolón, M.; Vega, C.; Escario, J.A.; Gómez-Barrio, A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2006, 99, 103–107. [Google Scholar] [CrossRef]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, 59. [Google Scholar] [CrossRef] [Green Version]
- Ardani, H.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. In Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd. leaves using polyvinyl alcohol. In Proceedings of the IOP Conf. Ser. Mater. Sci. Eng,. International Symposium on Current Progress in Functional Materials, Bali, Indonesia, 26–27 July 2016; p. 012056. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Revised; Academic Press: London, UK, 2013; Volume 2, pp. 21–32. [Google Scholar]
- Butterworth, M.; Corradi, R.; Johal, J.; Lascelles, S.F.; Maeda, S.; Armes, S. Zeta Potential Measurements on Conducting Polymer-Inorganic Oxide Nanocomposite Particles. J. Colloid Interface Sci. 1995, 174, 510–517. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. Nanomater. Drug Deliv. Ther. 2019, 91–116. [Google Scholar] [CrossRef]
- Kirby, B.J.; Hasselbrink, E.F. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef]
- Shnoudeh, A.; Hamad, I.; Abdo, R.; Qadumii, L.; Jaber, A.; Surchi, H.; Alkelany, S. Synthesis, characterization, and applications of metal nanoparticles. In Biomaterials and Bionanotechnology; Academic Press: London, UK, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Capelletti, R.; Mora, C. Structural features and functional properties of water in model DMPC membranes: Thermally stimulated depolarization currents (TSDCs) and Fourier transform infrared (FTIR) studies. J. Phys. D Appl. Phys. 2013, 46, 485401. [Google Scholar] [CrossRef]
- Julieta, F.R.M.; Macarena, S.; Daniela, I.; Jimena, P.M.; Del Valle, A.S.; Silvia, C.N. Lipid-Polymer Membranes as Carriers for L-Tryptophan: Molecular and Metabolic Properties. Open J. Med. Chem. 2013, 3, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Nzai, J.M.; Proctor, A. Determination of phospholipids in vegetable oil by fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1998, 75, 1281–1289. [Google Scholar] [CrossRef]
- Pahari, A.; Chauhan, B. Engineering Chemistry; Laxmi Publications: New Delhi, India, 2006; pp. 95–97. [Google Scholar]
- Severcan, F.; Haris, P.I.; Aksoy, C.; Ozek, N.S. Progress in vibrational spectroscopy in diagnosis and screening. Biomed. Spectrosc. Imag. 2013, 2, 73–81. [Google Scholar] [CrossRef]
- Lopes, M.A.; Monteiro, F.J.; Santos, J.D.; Serro, A.P.; Saramago, B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 1999, 45, 370–375. [Google Scholar] [CrossRef]
- Yucel, C.; DeĞim, Z.; Yilmaz, S. Development of Cisplatin-loaded Liposome and Evaluation of Transport Properties Through Caco-2 Cell Line. Turk. J. Pharm. Sci. 2016, 13, 95–108. [Google Scholar] [CrossRef]
- González, P.; Marín, C.; Rodríguez-González, I.; Hitos, A.B.; Rosales, M.J.; Reina, M.; Díaz, J.I.; González-Coloma, A.; Sánchez-Moreno, M. In vitro activity of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents 2005, 25, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, A.; Villalonga-Planells, R.; Vega, M.; Ramis, G.; De Mattos, S.F.; Villalonga, P.; Costa, A.; Rotger, C. Cell Uptake and Localization Studies of Squaramide Based Fluorescent Probes. Bioconjugate Chem. 2014, 25, 1537–1546. [Google Scholar] [CrossRef]
- Rassi, A.; De Rezende, J.M. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Gupta, S.; Pal, A.; Vyas, S.P. Drug delivery strategies for therapy of visceral leishmaniasis. Expert Opin. Drug Deliv. 2010, 7, 371–402. [Google Scholar] [CrossRef]
- De Duve, C.; De Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Lysosomotropic agents. Biochem. Pharm. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Mao, Z.; Zhou, X.; Gao, C. Influence of structure and properties of colloidal biomaterials on cellular uptake and cell functions. Biomater. Sci. 2013, 1, 896–911. [Google Scholar] [CrossRef]
- Alcantara, C.D.L.; Vidal, J.C.; De Souza, W.; Cunha-E.-Silva, N.L. The cytostome–cytopharynx complex ofTrypanosoma cruziepimastigotes disassembles during cell division. J. Cell Sci. 2016, 130, 164–176. [Google Scholar] [CrossRef] [Green Version]
- De Souza, W. Structural organization of Trypanosoma cruzi. Memórias Do Inst. Oswaldo Cruz 2009, 104, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Anjos, D.O.; Alves, E.S.S.; Gonçalves, V.T.; Fontes, S.S.; Nogueira, M.L.; Suarez-Fontes, A.M.; Dacosta, J.B.N.; Rios-Santos, F.; Vannier-Santos, A.M.A.W.D.S.M.A. Effects of a novel β-lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porto-Carreiro, I.; Attias, M.; Miranda, K.; De Souza, W.; Cunha-e-Silva, N.J.E. Trypanosoma cruzi epimastigote endocytic pathway: Cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur. J. Cell Biol. 2000, 79, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Webster, P.; Russell, D.G. The flagellar pocket of trypanosomatids. Parasitol. Today 1993, 9, 201–206. [Google Scholar] [CrossRef]
- Tessarolo, L.D.; De Menezes, R.R.P.P.B.; Mello, C.P.; Lima, D.B.; Magalhães, E.P.; Bezerra, E.M.; Sales, F.A.M.; Neto, I.L.B.; Oliveira, M.D.F.; Dos Santos, R.P.; et al. Nanoencapsulation of benznidazole in calcium carbonate increases its selectivity to Trypanosoma cruzi. Parasitology 2018, 145, 1191–1198. [Google Scholar] [CrossRef]





| Nanostructure/Drugs | Hydrodynamic Diameter (nm) * | Polydispersity Index (PdI) * | Zeta Potential (mV) * |
|---|---|---|---|
| MLS | 196.2 ± 11.0 | 0.418 ± 0.086 | −61.43 ± 2.30 |
| MLV | 203.1 ± 8.5 | 0.428 ± 0.092 | −12.93 ± 1.21 |
| Nanostructure/Drugs | Epimastigote | Intracellular Amastigotes | VERO Cell | RAW 264.7 Macrophage | Epimastigote | Amastigotes in VERO Cell |
|---|---|---|---|---|---|---|
| IC50 (μM) a | CC50 (μM) b | SI c | ||||
| – | – | – | – | – | – | – |
| Z | 15.81 ± 4.63 | 4.76 ± 4.45 | 284.44 ± 1.25 | 554.80 ± 4.90 | 17.99 | 59.76 |
| S | 13.12 ± 5.12 | 51.18 ± 4.91 | 736.21 ± 1.23 | 1654.377 ± 5.20 | 56.11 | 14.38 |
| MLV | – | – | – | – | – | – |
| MLS | 15.85 ± 4.82 | 24.92 ± 4.80 | 1199.50 ± 1.22 | 1973.97 ± 5.98 | 75.68 | 48.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quijia, C.R.; Bonatto, C.C.; Silva, L.P.; Andrade, M.A.; Azevedo, C.S.; Lasse Silva, C.; Vega, M.; de Santana, J.M.; Bastos, I.M.D.; Carneiro, M.L.B. Liposomes Composed by Membrane Lipid Extracts from Macrophage Cell Line as a Delivery of the Trypanocidal N,N’-Squaramide 17 towards Trypanosoma cruzi. Materials 2020, 13, 5505. https://doi.org/10.3390/ma13235505
Quijia CR, Bonatto CC, Silva LP, Andrade MA, Azevedo CS, Lasse Silva C, Vega M, de Santana JM, Bastos IMD, Carneiro MLB. Liposomes Composed by Membrane Lipid Extracts from Macrophage Cell Line as a Delivery of the Trypanocidal N,N’-Squaramide 17 towards Trypanosoma cruzi. Materials. 2020; 13(23):5505. https://doi.org/10.3390/ma13235505
Chicago/Turabian StyleQuijia, Christian Rafael, Cínthia Caetano Bonatto, Luciano Paulino Silva, Milene Aparecida Andrade, Clenia Santos Azevedo, Camila Lasse Silva, Manel Vega, Jaime Martins de Santana, Izabela Marques Dourado Bastos, and Marcella Lemos Brettas Carneiro. 2020. "Liposomes Composed by Membrane Lipid Extracts from Macrophage Cell Line as a Delivery of the Trypanocidal N,N’-Squaramide 17 towards Trypanosoma cruzi" Materials 13, no. 23: 5505. https://doi.org/10.3390/ma13235505
APA StyleQuijia, C. R., Bonatto, C. C., Silva, L. P., Andrade, M. A., Azevedo, C. S., Lasse Silva, C., Vega, M., de Santana, J. M., Bastos, I. M. D., & Carneiro, M. L. B. (2020). Liposomes Composed by Membrane Lipid Extracts from Macrophage Cell Line as a Delivery of the Trypanocidal N,N’-Squaramide 17 towards Trypanosoma cruzi. Materials, 13(23), 5505. https://doi.org/10.3390/ma13235505