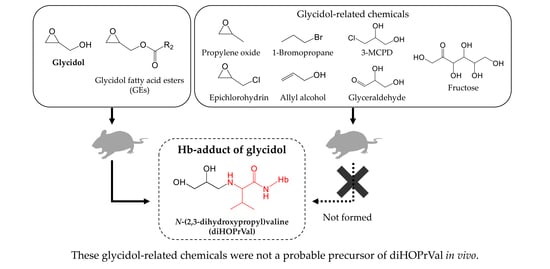
Does External Exposure of Glycidol-Related Chemicals Influence the Forming of the Hemoglobin Adduct, N-(2,3-dihydroxypropyl)valine, as a Biomarker of Internal Exposure to Glycidol?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Hemoglobin Adducts of Glycidol (diHOPrVal), Epichlorohydrin, and Glyceraldehyde
2.3. Animals and Glycidol-Related Chemicals
2.4. Determination of Hemoglobin Adduct by LC-MS/MS
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inagaki, R.; Ito, F.; Shimamura, Y.; Masuda, S. Effect of chloride on the formation of 3-monochloro-1, 2-propanediol fatty acid diesters and glycidol fatty acid esters in fish, meats and acylglycerols during heating. Food Addit. Contam. Part A 2019, 36, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, R.; Hirai, C.; Shimamura, Y.; Masuda, S. Formation of glycidol fatty acid esters in meat samples cooked by various methods. J. Food Process. Technol. 2016, 7, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, J. Determination of bound 2,3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. Eur. J. Lipid Sci. Technol. 2011, 113, 335–344. [Google Scholar] [CrossRef]
- MacMahon, S.; Begley, T.H.; Diachenko, G.W. Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit. Contam. Part A 2013, 30, 2081–2092. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Fromberg, A. Monochloropropanediol and glycidyl esters in infant formula and baby food products on the Danish market: Occurrence and preliminary risk assessment. Food Control 2020, 110, 106980. [Google Scholar] [CrossRef]
- Wöhrlin, F.; Fry, H.; Lahrssen-Wiederholt, M.; Preiß-Weigert, A. Occurrence of fatty acid esters of 3-MCPD, 2-MCPD and glycidol in infant formula. Food Addit. Contam. Part A 2015, 32, 1810–1822. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and carcinogenesis studies of glycidol. F344/N rats and B6C3F1 mice. Tech. Rep. Ser. 1990, 1990374, 1–229. [Google Scholar]
- Ramy, R.E.; Elhkim, M.O.; Lezmi, S.; Poul, J.M. Evaluation of the genotoxic potential of 3-monochloropropane-1,2-diol (3-MCPD) and its metabolites, glycidol and beta-chlorolactic acid, using the single cell gel/comet assay. Food Chem. Toxicol. 2007, 45, 41–48. [Google Scholar] [CrossRef]
- Aasa, J.; Vare, D.; Motwani, H.V.; Jenssen, D.; Törnqvist, M. Quantification of the mutagenic potency and repair of glycidol-induced DNA lesions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 805, 38–45. [Google Scholar] [CrossRef]
- Aasa, J.; Abramsson-Zetterberg, L.; Carlsson, H.; Törnqvist, M. The genotoxic potency of glycidol established from micronucleus frequency and hemoglobin adduct levels in mice. Food Chem. Toxicol. 2017, 100, 168–174. [Google Scholar] [CrossRef]
- Honda, H.; Onishi, M.; Fujii, K.; Ikeda, N.; Yamaguchi, T.; Fujimori, T.; Nishiyama, N.; Kasamatsu, T. Measurement of glycidol hemoglobin adducts in humans who ingest edible oil containing small amounts of glycidol fatty acid esters. Food Chem. Ttoxicol. 2011, 49, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Fujii, K.; Yamaguchi, T.; Ikeda, N.; Nishiyama, N.; Kasamatsu, T. Glycidol exposure evaluation of humans who have ingested diacylglycerol oil containing glycidol fatty acid esters using hemoglobin adducts. Food Chem. Toxicol. 2012, 50, 4163–4168. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Törnqvist, M.; Nishiyama, N.; Kasamatsu, T. Characterization of glycidol-hemoglobin adducts as biomarkers of exposure and in vivo dose. Toxicol. Appl. Pharmacol. 2014, 275, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Dussort, P.; Günther, H.; Hanlon, P.; Honda, H.; Mally, A.; O’Hagan, S.; Scholz, G.; Seidel, A.; Swenberg, J.; et al. Exposure assessment of process-related contaminants in food by biomarker monitoring. Arch. Toxicol. 2018, 92, 15–40. [Google Scholar] [CrossRef] [Green Version]
- Abraham, K.; Hielscher, J.; Kaufholz, T.; Mielke, H.; Lampen, A.; Monien, B. The hemoglobin adduct N-(2, 3-dihydroxypropyl)-valine as biomarker of dietary exposure to glycidyl esters: A controlled exposure study in humans. Arch. Toxicol. 2019, 93, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Aasa, J.; Vryonidis, E.; Abramsson-Zetterberg, L.; Törnqvist, M. Internal doses of glycidol in children and estimation of associated cancer risk. Toxics 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Aasa, J.; Törnqvist, M.; Abramsson-Zetterberg, L. Measurement of micronuclei and internal dose in mice demonstrates that 3-monochloropropane-1, 2-diol (3-MCPD) has no genotoxic potency in vivo. Food Chem. Toxicol. 2017, 109, 414–420. [Google Scholar] [CrossRef]
- Cheng, W.W.; Liu, G.Q.; Wang, L.Q.; Liu, Z.S. Glycidyl fatty acid esters in refined edible oils: A review on formation, occurrence, analysis, and elimination methods. Compr. Rev. Food Sci. Food Saf. 2017, 16, 263–281. [Google Scholar] [CrossRef] [Green Version]
- Landin, H.H.; Tareke, E.; Rydberg, P.; Olsson, U.; Törnqvist, M. Heating of food and haemoglobin adducts from carcinogens: Possible precursor role of glycidol. Food Chem. Toxicol. 2000, 38, 963–969. [Google Scholar] [CrossRef]
- Sillero, M.A.; Sillero, A.; Sols, A. Enzymes involved in fructose metabolism in liver and the glyceraldehyde metabolic crossroads. Eur. J. Biochem. 1959, 10, 345–350. [Google Scholar] [CrossRef]
- Landin, H.H.; Grummt, T.; Laurent, C.; Tates, A. Monitoring of occupational exposure to epichlorohydrin by genetic effects and hemoglobin adducts. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1997, 381, 217–226. [Google Scholar] [CrossRef]
- Ishidao, T.; Kunugita, N.; Fueta, Y.; Arashidani, K.; Hori, H. Effects of inhaled 1-bromopropane vapor on rat metabolism. Toxicol. Lett. 2002, 134, 237–243. [Google Scholar] [CrossRef]
- Landin, H.H.; Osterman-Golkar, S.; Zorcec, V.; Törnqvist, M. Biomonitoring of epichlorohydrin by hemoglobin adducts. Anal. Biochem. 1996, 240, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Schmid, K.; Schaller, B.; Hiddemann-Koca, K.; Drexler, H.; Göen, T. Mercapturic acids as metabolites of alkylating substances in urine samples of German inhabitants. Int. J. Hyg. Environ. Health 2011, 214, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, R.; Cirlini, M.; Mutti, A. Quantification of 3-MCPD and its mercapturic metabolite in human urine: Validation of an LC–MS–MS method and its application in the general population. Anal. Bioanal. Chem. 2015, 407, 4823–4827. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.H.; Kodama, J.K.; Wellington, J.S.; Dunlap, M.K.; Anderson, H.H. The toxicology of glycidoI and some glycidyl ethers. Arch. Indust. Health. 1956, 14, 250–264. [Google Scholar] [PubMed]
- Weil, C.S.; Condra, N.; Haun, C.; Striegel, J.A. Experimental carcinogenicity and acute toxicity of representative epoxides. Am. Ind. Hyg. Assoc. J. 1963, 24, 305–325. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, H.; Zhang, G.; Yin, L. Study on acute toxicity of R, S and (R, S)-3-monchloropropane-1, 2-diol. J. Hyg. Res. 2007, 36, 137–140. [Google Scholar] [PubMed]
- Lawrence, W.H.; Malik, M.; Turner, J.E.; Autian, J. Toxicity profile of epichlorohydrin. J. Pharm. Sci. 1972, 61, 1712–1717. [Google Scholar] [CrossRef]
- World Health Organization. Propylene Oxide-Environmental Health Criteria 56; WHO: Geneva, Switzerland, 1985; pp. 1–53. [Google Scholar]
- American Conference of Governmental Industrial Hygienists (ACGIH). 1-BROMOPROPANE. In TLVs and BELs with 7th Edition Documentation; ACGIH: Washington, DC, USA, 2014. [Google Scholar]
- Dunlap, M.K.; Kodama, J.K.; Wllington, J.S.; Anderson, H.H.; Hine, C.H. The toxicity of allyl alcohol: I. Acute and chronic toxicity. J. Occup. Environ. Med. 1959, 1, 139. [Google Scholar] [PubMed]
- Kirnberger, E.J.; Braun, W.; Stille, G.; Wolf, V. Relation between liver protection and sugar metabolism. Arzneim. Forsch. 1958, 8, 72–76. [Google Scholar] [PubMed]
- Eng, C.P.; Bhatnagar, M.K.; Morgan, J.F. Inhibition of mouse ascites tumors by carbohydrate combined with immunization. Can. J. Physiol. Pharmacol. 1972, 50, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Von Stedingk, H.; Rydberg, P.; Törnqvist, M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC–MS/MS. J. Chromatogr. B 2010, 878, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, P.; von Stedingk, H.; Magnér, J.; Björklund, J. LC/MS/MS analysis of N-terminal protein adducts with improved sensitivity: A comparison of selected Edman isothiocyanate reagents. Int. J. Anal. Chem. 2009, 2009, 153472. [Google Scholar] [CrossRef] [Green Version]
- Landin, H.H.; Segerbäck, D.; Damberg, C.; Osterman-Golkar, S. Adducts with haemoglobin and with DNA in epichlorohydrin-exposed rats. Chem. Biol. Interact. 1999, 117, 49–64. [Google Scholar] [CrossRef]
- Kolman, A.; Chovanec, M.; Osterman-Golkar, S. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: Update review (1990–2001). Mutat. Res. Rev. Mutat. Res. 2002, 512, 173–194. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Ho, C.T. Volatile compounds of deep-oil fried, microwave-heated and oven-baked garlic slices. J. Agric. Food Chem. 1993, 41, 800–805. [Google Scholar] [CrossRef]
- Appel, K.E.; Abraham, K.; Berger-Preiss, E.; Hansen, T.; Apel, E.; Schuchardt, S.; Vogt, C.; Bakhiya, N.; Creutzenberg, O.; Lampen, A. Relative oral bioavailability of glycidol from glycidyl fatty acidesters in rats. Arch. Toxicol. 2013, 87, 1649–1659. [Google Scholar] [CrossRef]
- Mori, N.; Bai, Y.; Ueno, H.; Manning, J.M. Sequence-dependent reactivity of model peptides with glyceraldehyde. Carbohydr. Res. 1989, 189, 49–63. [Google Scholar] [CrossRef]
- Degner, A.; Carlsson, H.; Karlsson, I.; Eriksson, J.; Pujari, S.S.; Tretyakova, N.Y.; Törnqvist, M. Discovery of novel N-(4-Hydroxybenzyl) valine hemoglobin adducts in human blood. Chem. Res. Toxicol. 2018, 31, 1305–1314. [Google Scholar] [CrossRef]


| Administration Chemical Substance | Concentration (mmol/kg bw) | LD50 (mmol/kg bw) | Reference |
|---|---|---|---|
| Glycidol | 0.5, 1.0 | 6.1 | [26] |
| Glycidyl oleate | 0.5, 1.0 | 9.9–10.9 | [27] |
| 3-MCPD | 0.5, 1.0 | 1.7 | [28] |
| Epichlorohydrin | 0.5, 1.0 | 2.6 | [29] |
| Propylene oxide | 5.0 | 10.8 | [30] |
| 1-Bromopropane | 18.0 | 38.2 | [31] |
| Allyl alcohol | 1.0 | 1.5–1.7 | [32] |
| Fructose | 10.0 | 22.2 | [33] |
| Glyceraldehyde | 10.0 | 33.3 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimamura, Y.; Inagaki, R.; Honda, H.; Masuda, S. Does External Exposure of Glycidol-Related Chemicals Influence the Forming of the Hemoglobin Adduct, N-(2,3-dihydroxypropyl)valine, as a Biomarker of Internal Exposure to Glycidol? Toxics 2020, 8, 119. https://doi.org/10.3390/toxics8040119
Shimamura Y, Inagaki R, Honda H, Masuda S. Does External Exposure of Glycidol-Related Chemicals Influence the Forming of the Hemoglobin Adduct, N-(2,3-dihydroxypropyl)valine, as a Biomarker of Internal Exposure to Glycidol? Toxics. 2020; 8(4):119. https://doi.org/10.3390/toxics8040119
Chicago/Turabian StyleShimamura, Yuko, Ryo Inagaki, Hiroshi Honda, and Shuichi Masuda. 2020. "Does External Exposure of Glycidol-Related Chemicals Influence the Forming of the Hemoglobin Adduct, N-(2,3-dihydroxypropyl)valine, as a Biomarker of Internal Exposure to Glycidol?" Toxics 8, no. 4: 119. https://doi.org/10.3390/toxics8040119
APA StyleShimamura, Y., Inagaki, R., Honda, H., & Masuda, S. (2020). Does External Exposure of Glycidol-Related Chemicals Influence the Forming of the Hemoglobin Adduct, N-(2,3-dihydroxypropyl)valine, as a Biomarker of Internal Exposure to Glycidol? Toxics, 8(4), 119. https://doi.org/10.3390/toxics8040119