Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum
Abstract
:1. Introduction
2. Results and Discussion
Bioactivity Analysis
3. Materials and Methods
3.1. Endophytic Fungi Isolation from Piper longum L.
3.2. Molecular Identification of the Endophytic Fungi
3.3. Epigenetic Modifier Experiments
3.4. Bioactivities
3.5. Chemical Analyses
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.T.; Habbu, P.V.; Kulkarni, V.H.; Jagadish, K.S.; Pandey, A.R.; Sutariya, V.N. Endophytic microbes: A novel source for biologically/pharmacologically active secondary metabolites. Asian J. Pharmacol. Toxicol. 2014, 2, 1–6. [Google Scholar]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, D. Volatile Organic Compounds from Endophytic Fungi. In Recent Advancement in White Biotechnology Through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 149–175. [Google Scholar]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Ahmed, F.; Ijaz, B.; Ahmad, Z.; Farooq, N.; Sarwar, M.B.; Husnain, T. Modification of miRNA Expression through plant extracts and compounds against Breast cancer: Mechanism and Translational Significance. Phytomedicine 2020, 68, 153168. [Google Scholar] [CrossRef]
- Zutz, C.; Bandian, D.; Neumayer, B.; Speringer, F.; Gorfer, M.; Wagner, M.; Strauss, J.; Rychli, K. Fungi treated with small chemicals exhibit increased antimicrobial activity against facultative bacterial and yeast pathogens. BioMed. Res. Int. 2014, 2014, 540292. [Google Scholar] [CrossRef] [Green Version]
- Blum, R. Stepping inside the realm of epigenetic modifiers. Biomol. Concepts 2015, 6, 119–136. [Google Scholar] [CrossRef]
- Zutz, C.; Gacek, A.; Sulyok, M.; Wagner, M.; Strauss, J.; Rychli, K. Small chemical chromatin effectors alter secondary metabolite production in Aspergillus clavatus. Toxins 2013, 5, 1723–1741. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-J.; Awakawa, T.; Sun, J.-Y.; Wakimoto, T.; Abe, I. Epigenetic modifier-induced biosynthesis of novel fusaric acid derivatives in endophytic fungi from Datura stramonium L. Natural Products Bioprospecting 2013, 3, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Al Akeel, R. Role of epigenetic reprogramming of host genes in bacterial pathogenesis. Saudi J. Biol. Sci. 2013, 20, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Pfannenstiel, B.T.; Keller, N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Tsai, Y.-J.; Lin, M.-Y.; You, H.-L.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Calebin-A induced death of malignant peripheral nerve sheath tumor cells by activation of histone acetyltransferase. Phytomedicine 2019, 57, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; Van Olphen, A.; Baker, B.J. Epigenetic tailoring for the production of anti-infective cytosporones from the marine fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, W.; Shao, C.-L.; Chi, Z.-M.; Wang, C.-Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. 2016, 18, 409–417. [Google Scholar] [CrossRef]
- Sun, J.; Awakawa, T.; Noguchi, H.; Abe, I. Induced production of mycotoxins in an endophytic fungus from the medicinal plant Datura stramonium L. Bioorganic Med. Chem. Lett. 2012, 22, 6397–6400. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, J.; Singh, D.K.; Mishra, A.; Verma, S.K.; Gond, S.K.; Kumar, A.; Singh, N.; Kharwar, R.N. Induction of Cryptic and Bioactive Metabolites through Natural Dietary Components in an Endophytic Fungus Colletotrichum gloeosporioides (Penz.) Sacc. Front. Microbiol. 2017, 8, 1126. [Google Scholar] [CrossRef]
- Chung, Y.-M.; El-Shazly, M.; Chuang, D.-W.; Hwang, T.-L.; Asai, T.; Oshima, Y.; Ashour, M.L.; Wu, Y.-C.; Chang, F.-R. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, induces the production of anti-inflammatory cyclodepsipeptides from Beauveria felina. J. Natural Products 2013, 76, 1260–1266. [Google Scholar] [CrossRef]
- Chung, Y.-M.; Wei, C.-K.; Chuang, D.-W.; El-Shazly, M.; Hsieh, C.-T.; Asai, T.; Oshima, Y.; Hsieh, T.-J.; Hwang, T.-L.; Wu, Y.-C.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorganic Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2020, 247, 112255. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [Green Version]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price-Whelan, A.; Dietrich, L.E.P.; Newman, D.K. Rethinking’secondary’metabolism: Physiological roles for phenazine antibiotics. Nature Chem. Biol. 2006, 2, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Sarmin, N.M.; Ng, C.L.; Tiong, J.J.L.; Aizat, W.M.; Keong, L.K.; Zin, N.M. Genomic characterization of a new endophytic Streptomyces kebangsaanensis identifies biosynthetic pathway gene clusters for novel phenazine antibiotic production. PeerJ 2017, 5, e3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanabalasingam, D.; Kumar, N.S.; Jayasinghe, L.; Fujimoto, Y. Endophytic Fungus Nigrospora oryzae from a Medicinal plant Coccinia grandis, a High Yielding New Source of Phenazine-l-carboxamide. Natural Product Commun. 2015, 10, 1934578X1501001008. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, K.N.; Jones, J.; Dyer, K.S. Anesthetic-Agent Mass Casualty Incident. In Ciottone’s Disaster Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 692–695. [Google Scholar]
- Othman, A.A.; Kihel, M.; Amara, S. 1, 3, 4-Oxadiazole, 1, 3, 4-thiadiazole and 1, 2, 4-triazole derivatives as potential antibacterial agents. Arabian J. Chem. 2019, 12, 1660–1675. [Google Scholar] [CrossRef] [Green Version]
- Ser, H.-L.; Palanisamy, U.D.; Yin, W.-F.; Abd Malek, S.N.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Presence of antioxidative agent, Pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015, 6, 854. [Google Scholar] [CrossRef] [Green Version]
- George, A.B. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yu, P.H.; Deng, Y.L. Endogenous formaldehyde as a potential factor of vulnerability of atherosclerosis: Involvement of semicarbazide-sensitive amine oxidase-mediated methylamine turnover. Atherosclerosis 1998, 140, 357–363. [Google Scholar] [CrossRef]
- Qureshi, A.; Pradhan, A. Short Review on Thiazole Derivative. J. Drug Deliv. Ther. 2019, 9, 842–847. [Google Scholar]
- Schiestl, F.P.; Roubik, D.W. Odor compound detection in male euglossine bees. J. Chem. Ecol. 2003, 29, 253–257. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Z.; Hou, Y.; Wang, D.; Zhang, H.; Ma, J.; Zhang, L.; Liu, S. Prevalence of Enterobius vermicularis among preschool children in 2003 and 2013 in Xinxiang city, Henan province, Central China. Parasite 2016, 23, 30. [Google Scholar] [CrossRef] [Green Version]
- Machida, S.; Mukai, S.; Kono, R.; Funato, M.; Saito, H.; Uchiyama, T. Synthesis and Comparative Structure--Activity Study of Carbohydrate-Based Phenolic Compounds as $α$-Glucosidase Inhibitors and Antioxidants. Molecules 2019, 24, 4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, A.A.; da Silva, D.B.; Lopes, N.P.; Pupo, M.T. Epigenetic modulation changed the secondary metabolite profile in the endophyte Nigrospora sphaerica SS67. Planta Medica 2012, 78, PL38. [Google Scholar] [CrossRef]
- Magotra, A.; Kumar, M.; Kushwaha, M.; Awasthi, P.; Raina, C.; Gupta, A.P.; Shah, B.A.; Gandhi, S.G.; Chaubey, A. Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): An endophytic fungus from Grewia asiatica L. AMB Express 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Felicio, R.; Pavão, G.B.; de Oliveira, A.L.L.; Erbert, C.; Conti, R.; Pupo, M.T.; Furtado, N.A.J.C.; Ferreira, E.G.; Costa-Lotufo, L.V.; Young, M.C.M.; et al. Antibacterial, antifungal and cytotoxic activities exhibited by endophytic fungi from the Brazilian marine red alga Bostrychia tenella (Ceramiales). Rev. Bras. Farmacogn. 2015, 25, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.C.; Lobkovsky, E.; Gange, A.C.; Singh, S.K.; Prakash, S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J. Antibiot. 2011, 64, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K. Anti-leishmanial and Anti-inflammatory Agents from Endophytes: A Review. Natural Products Bioprospecting 2019, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chithra, S.; Jasim, B.; Sachidanandan, P.; Jyothis, M.; Radhakrishnan, E.K. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine 2014, 21, 534–540. [Google Scholar] [CrossRef]
- Silva, G.H.; Teles, H.L.; Trevisan, H.C.; Bolzani, V. da S.; Young, M.; Pfenning, L.H.; Eberlin, M.N.; Haddad, R.; Costa-Neto, C.M.; Araújo, Â.R. New bioactive metabolites produced by Phomopsis cassiae, an endophytic fungus in Cassia spectabilis. J. Braz. Chem. Soc. 2005, 16, 1463–1466. [Google Scholar] [CrossRef] [Green Version]
- Rajamani, T.; Suryanarayanan, T.S.; Murali, T.S.; Thirunavukkarasu, N. Distribution and diversity of foliar endophytic fungi in the mangroves of Andaman Islands, India. Fungal Ecol. 2018, 36, 109–116. [Google Scholar] [CrossRef]
- Gong, J.L.; Lu, Y.; Wu, W.H.; He, C.P.; Liang, Y.Q.; Huang, X.; Zheng, J.L.; Xi, J.G.; Tang, S.B.; Yi, K.X. First Report of Phomopsis heveicola (Anamorph of Diaporthe tulliensis) Causing Leaf Blight of Coffee (Coffea arabica) in China. Plant Disease 2019, 104, 570. [Google Scholar] [CrossRef]
- Araújo, W.L.; Maccheroni Jr, W.; Aguilar-Vildoso, C.I.; Barroso, P.A.V.; Saridakis, H.O.; Azevedo, J.L. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.J.; De Hoog, G.S.; Starink, M.; Rosete, Y.A.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones & Bartlett Learning: Burlington, MA, USA, 1999. [Google Scholar]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.C.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Strobel, G.A.; Dirkse, E.; Sears, J.; Markworth, C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 2001, 147, 2943–2950. [Google Scholar] [CrossRef] [Green Version]
- Benmerache, A.; Benteldjoune, M.; Alabdul Magid, A.; Abedini, A.; Berrehal, D.; Kabouche, A.; Gangloff, S.C.; Voutquenne-Nazabadioko, L.; Kabouche, Z. Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts. Natural Product Res. 2017, 31, 2828–2835. [Google Scholar] [CrossRef] [PubMed]


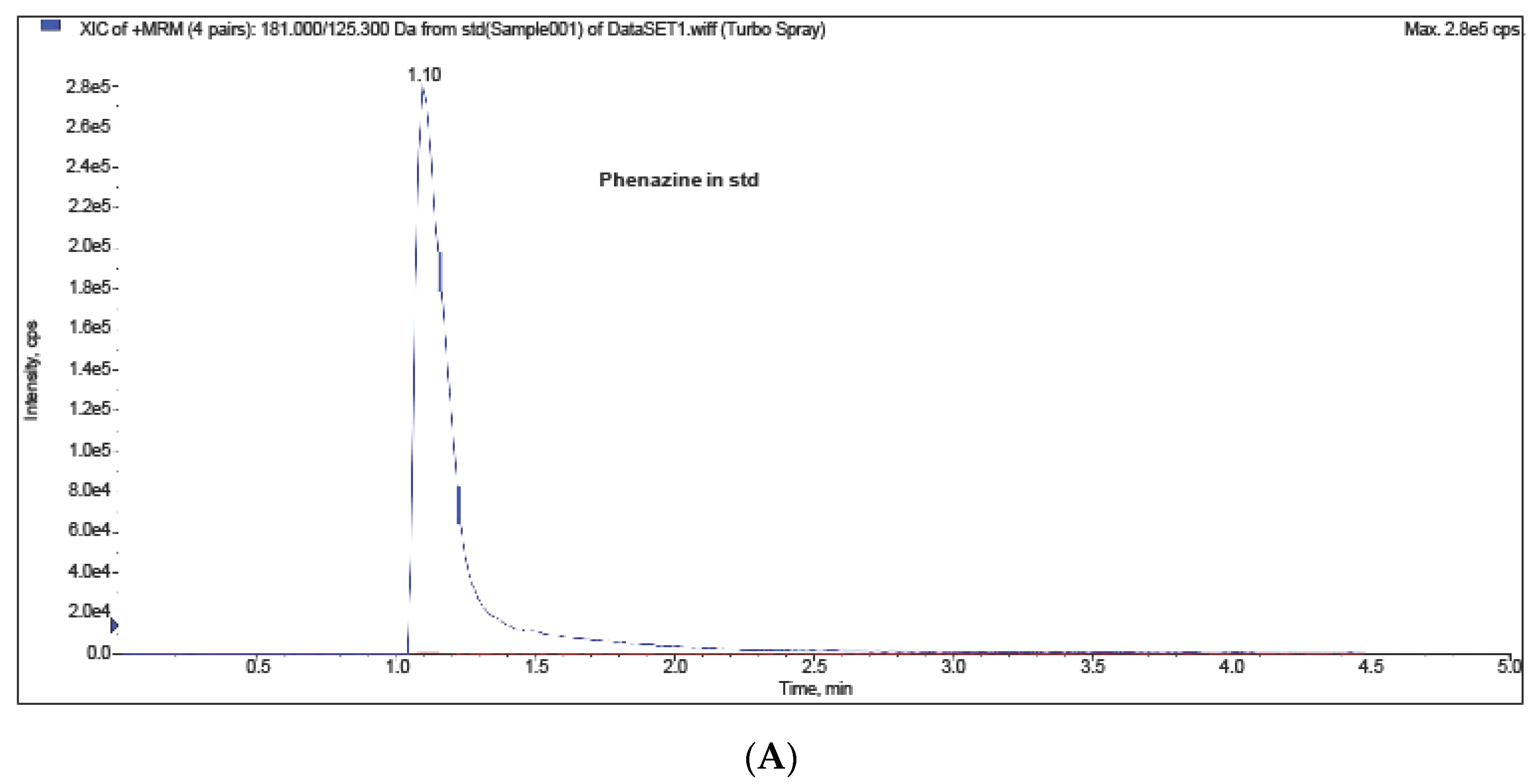
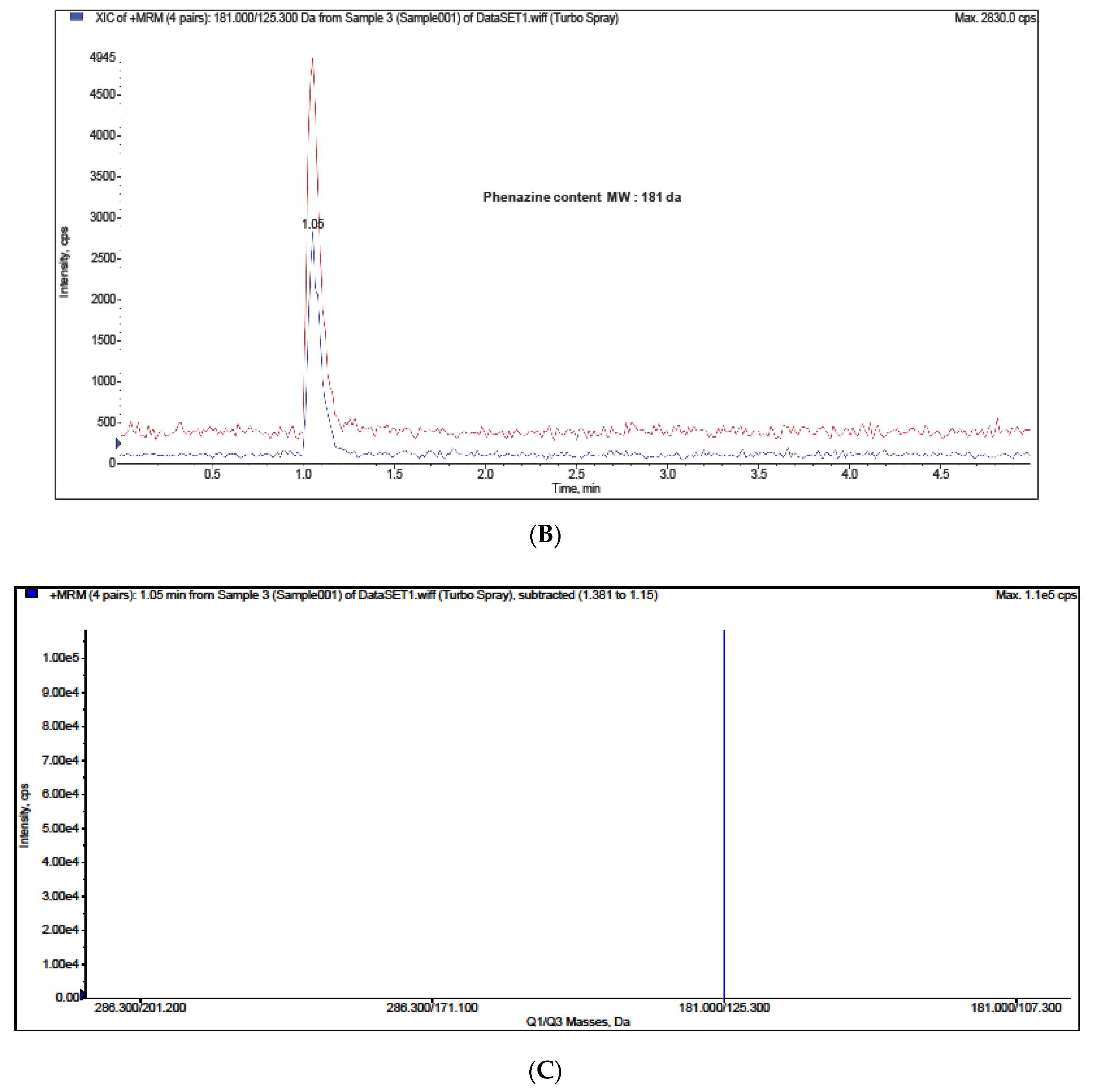
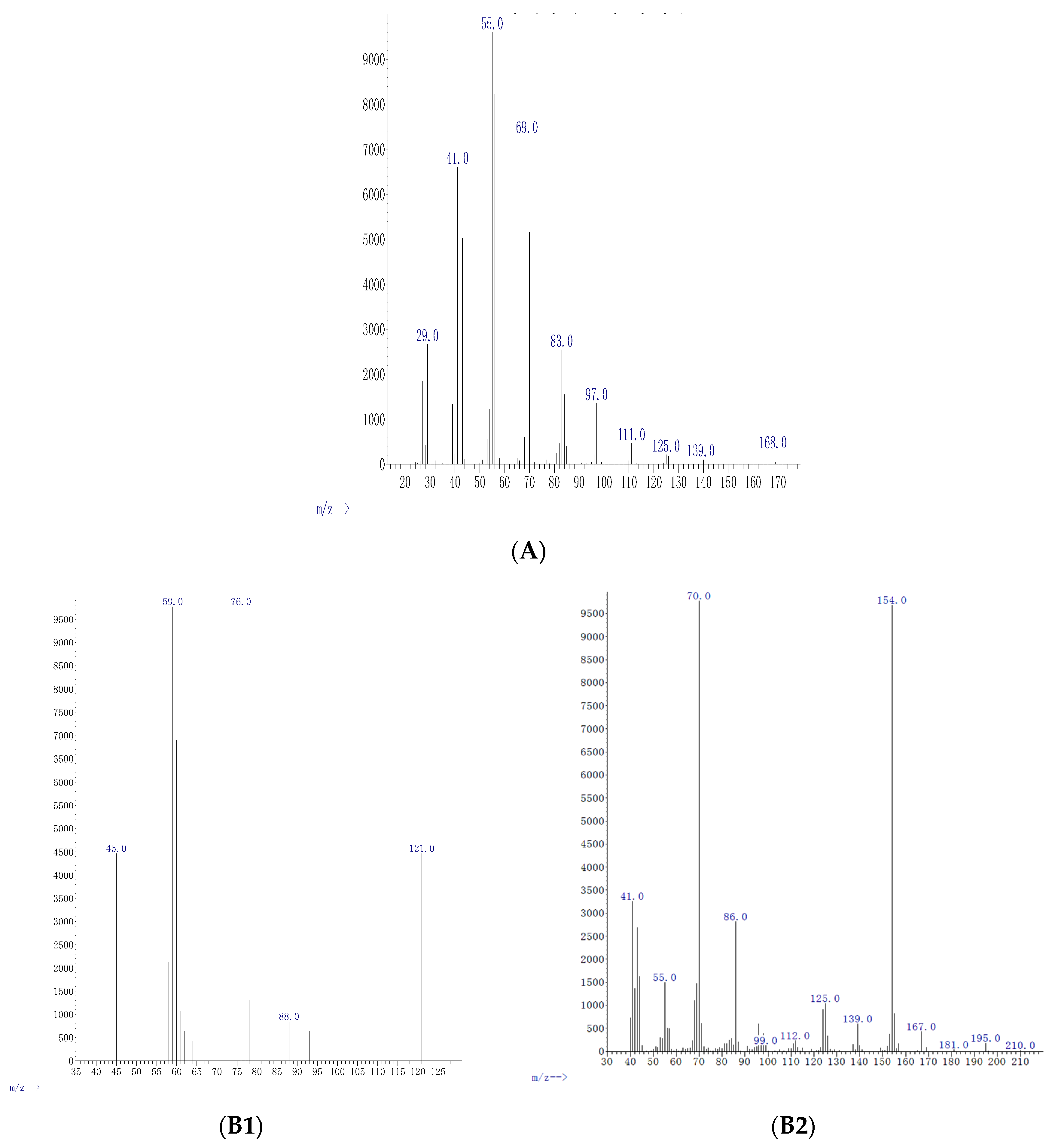

| Epigenetic Modifier | ||||
|---|---|---|---|---|
| Concentration, μg mL−1 | Positive Control | Negative Control | Valproic Acid | Azacytidine |
| 0.5 | 23 ± 1 | 1 ± 2 | 3 ± 2 | 21 ± 5 |
| 5 | 42 ± 1 | 1.4 ± 2 | 28 ± 9 | 51 ± 8 |
| 25 | 73 ± 3 | 1 ± 2 | 68 ± 5 | 58 ± 7 |
| 50 | 83 ± 1 | 1.3 ± 2 | 71 ± 8 | 60 ± 6 |
| Conc μg mL−1 | Compound | Formula | M Da | Fragments | Relative Abundance % | % of Agreement with NIST Database | Functional Uses |
|---|---|---|---|---|---|---|---|
| 0.5 | Cyclo-propane | C3H6 | 42.08 | 29, 41, 42, 55, 69, 83, 97, 111, 125, 139, 168 | 4.9 | 98% | Anesthetic [27] |
| 5 | Oxathiazole 2 thione | C2H3NOS2 | 121.19 | 45, 59, 78, 88, 121 | 37 | 97% | Anti-bacterial agent [28] |
| 5 | Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methyl) | C11H18N2O2 | 210.27 | 41, 55, 70, 86, 99, 112, 125, 139, 154, 167, 181, 196, 210 | 3.7 | 99% | Anti-cancerous/anti-oxidant [22] |
| 25 | Cyclopentadecanolide | C15H28O2 | 240.38 | 40, 55, 83, 98, 124, 152, 180, 197, 222, 240 | 33 | 98% | Flavouring agent [29] |
| 25 | N-allyldecylamine 1-Decanamine - | C13H27N | 197.36 | 28, 41, 55, 70, 84, 97, 126, 140, 154, 197 | 5.0 | 97% | Anti atherosclerosis [30] |
| 25 | Oxazole, 4 ethyl 4,5 di hydro 2, 2hydroxy phenyl | C11H13NO2 | 191.23 | 39, 51, 63, 77, 92, 107, 119, 134, 146, 162, 191 | 85 | 97% | Anti-bacterial [31] |
| 25 | Benzyl ethanoate | C9H10O2 | 150.17 | 15, 27, 39, 51, 77, 91, 105, 119, 135, 150 | 3.8 | 99% | Flavouring agent [32] |
| 50 | Diphenan | C14H13NO2 | 227.26 | 39, 55, 83, 98, 125, 154, 182, 227 | 14 | 98% | Administrated for the treatment of pinworms [33] |
| 50 | Methyl 2,3-anhydro-4,6-O-benzylidenehexopyranoside | C14H16O5 | 264.27 | 29, 55, 83, 111, 137, 165, 193, 222, 264, 284 | 1.3 | 98% | Antioxidant [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, F.; Almansob, A.; Al Tami, M.; Al-Enazi, N.; Al-Sabri, A.; Orfali, R. Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum. Molecules 2021, 26, 29. https://doi.org/10.3390/molecules26010029
Ameen F, Almansob A, Al Tami M, Al-Enazi N, Al-Sabri A, Orfali R. Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum. Molecules. 2021; 26(1):29. https://doi.org/10.3390/molecules26010029
Chicago/Turabian StyleAmeen, Fuad, Abobakr Almansob, Mona Al Tami, Nouf Al-Enazi, Ahmed Al-Sabri, and Raha Orfali. 2021. "Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum" Molecules 26, no. 1: 29. https://doi.org/10.3390/molecules26010029
APA StyleAmeen, F., Almansob, A., Al Tami, M., Al-Enazi, N., Al-Sabri, A., & Orfali, R. (2021). Epigenetic Modifiers Affect the Bioactive Compounds Secreted by an Endophyte of the Tropical Plant Piper longum. Molecules, 26(1), 29. https://doi.org/10.3390/molecules26010029





