Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study
Abstract
:1. Introduction
2. Study Design
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Experimental Design
2.4. Streptococcus mutans Counts (SM) and Plaque Index Evaluation
2.5. Methods
2.5.1. Microbiological Examination
2.5.2. Plaque Index
3. Statistical Analysis
4. Results
- Placebo Group: 5 males and 5 females.
- Test group: 6 males and 4 females.
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazur, M.; Bietolini, S.; Bellardini, D.; Lussi, A.; Corridore, D.; Maruotti, A.; Ottolenghi, L.; Vozza, I.; Guerra, F. Oral health in a cohort of individuals on a plant-based diet: A pilot study. Clin. Ter. 2020, 171, e142–e148. [Google Scholar] [PubMed]
- Bottalico, L.; Valenzano, A.; Leone, D.; Mangini, F.; Santacroce, L. The incidence of dental caries during childhood. A clinic and epidemiologic study in Matera (Southern Italy). Clin. Ter. 2007, 158, 409–419. [Google Scholar] [PubMed]
- Cirulli, N.; Cantore, S.; Ballini, A.; Perillo, L.; Giannico, O.V.; Tafuri, S.; de Vito, D. Prevalence of caries and dental malocclusions in the apulian paediatric population: An epidemiological study. Eur. J. Paediatr. Dent. 2019, 20, 100–104. [Google Scholar] [PubMed]
- Staszczyk, M.; Jurczak, A.; Magacz, M.; Kościelniak, D.; Gregorczyk-Maga, I.; Jamka-Kasprzyk, M.; Kępisty, M.; Kołodziej, I.; Kukurba-Setkowicz, M.; Krzyściak, W. Effect of polyols and selected dental materials on the ability to create a cariogenic biofilm-on children caries-associated Streptococcus mutans isolates. Int. J. Environ. Res. Public Health 2020, 17, 3720. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Argimón, S.; Schön, C.N.; Saraithong, P.; Caufield, P.W. Characterizing diversity of lactobacilli associated with severe early childhood caries: A study protocol. Adv. Microbiol. 2015, 5, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Ballini, A.; Cantore, S.; Saini, R.; Pettini, F.; Fotopoulou, E.A.; Saini, S.R.; Georgakopoulos, I.P.; Dipalma, G.; Isacco, C.G.; Inchingolo, F. Effect of activated charcoal probiotic toothpaste containing Lactobacillus paracasei and xylitol on dental caries: A randomized and controlled clinical trial. J. Biol. Regul. Homeost. Agents 2019, 33, 977–981. [Google Scholar]
- Ballini, A.; Gnoni, A.; de Vito, D.; Dipalma, G.; Cantore, S.; Isacco, C.G.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar]
- Lippolis, R.; Siciliano, R.A.; Mazzeo, M.F.; Abbrescia, A.; Gnoni, A.; Sardanelli, A.M.; Papa, S. Comparative secretome analysis of four isogenic Bacillus clausii probiotic strains. Proteome Sci. 2013, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Lippolis, R.; Gnoni, A.; Abbrescia, A.; Panelli, D.; Maiorano, S.; Paternoster, M.S.; Sardanelli, A.M.; Papa, S.; Gaballo, A. Comparative proteomic analysis of four Bacillus clausii strains: Proteomic expression signature distinguishes protein profile of the strains. J. Proteomics 2011, 74, 2846–2855. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Marrelli, M.; Mirgaldi, R.; de Vito, D.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar] [PubMed]
- Mangini, F.; Santacroce, L.; Bottalico, L. Periodontitis and systemic diseases. Clin. Ter. 2006, 157, 541–548. [Google Scholar] [PubMed]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral microbiota and immune system crosstalk: A translational research. Biology 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; de Vito, D. Cytokine gene polymorphisms associate with microbiogical agents in periodontal disease: Our experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Ballini, A.; Scattarella, A.; Crincoli, V.; Carlaio, R.G.; Papa, F.; Perillo, L.; Romanazzo, T.; Bux, M.V.; Nardi, G.M.; Dituri, A.; et al. Surgical treatment of gingival overgrowth with 10 years of follow-up. Head Face Med. 2010, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Scattarella, A.; Ballini, A.; Grassi, F.R.; Carbonara, A.; Ciccolella, F.; Dituri, A.; Nardi, G.M.; Cantore, S.; Pettini, F. Treatment of oroantral fistula with autologous bone graft and application of a non-reabsorbable membrane. Int. J. Med. Sci. 2010, 7, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Codispoti, B.; Makeeva, I.; Benincasa, C.; Spagnuolo, G. From Mouth to Brain: Neuroendocrine Markers Play as a Crosstalk among Oral and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 378. [Google Scholar] [CrossRef]
- Santacroce, L.; Cagiano, R.; Carlaio, R.G.; del Prete, R.; Bottalico, L. Dentistry oral hygiene and endocarditis. Pathophysiology andprophilactic therapy [Rischio di endocarditi infettive correlate a trattamenti odontoiatrici e di igiene orale]. Recenti Progress. Med. 2008, 99, 516–521. [Google Scholar]
- Inchingolo, F.; Martelli, F.S.; Isacco, C.G.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; de Vito, D.; Aityan, S.K.; et al. Chronic periodontitis and immunity, towards the implementation of a personalized medicine: A translational research on gene Single Nucleotide Polymorphisms (SNPs) linked to chronic oral dysbiosis in 96 caucasian patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and Porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Pawlaczyk-Kamieńska, T.; Torlińska-Walkowiak, N.; Borysewicz-Lewicka, M. The relationship between oral hygiene level and gingivitis in children. Adv. Clin. Exp. Med. 2018, 27, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Kamieńska, T.; Borysewicz-Lewicka, M.; Batura-Gabryel, H. Salivary biomarkers and oral microbial load in relation to the dental status of adults with cystic fibrosis. Microorganisms 2019, 7, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantore, S.; Ballini, A.; Saini, R.; de Vito, D.; Altini, V.; Saini, S.R.; Pustina-Krasniqi, T.; Xhajanka, E.; Isacco, C.G.; Dipalma, G.; et al. Efficacy of a combined sea salt-based oral rinse with xylitol against dental plaque, gingivitis, and salivary Streptococcus mutans load. J. Biol. Regul. Homeost. Agents 2018, 32, 1593–1597. [Google Scholar] [PubMed]
- Laganà, G.; Abazi, Y.; Nastasi, E.B.; Vinjolli, F.; Fabi, F.; Divizia, M.; Cozza, P. Oral health conditions in an Albanian adolescent population: An epidemiological study. BMC Oral Health 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, M.; Westland, S.; Guerra, F.; Corridore, D.; Vichi, M.; Maruotti, A.; Nardi, G.M.; Ottolenghi, L. Objective and subjective aesthetic performance of icon® treatment for enamel hypomineralization lesions in young adolescents: A retrospective single center study. J Dent. 2018, 68, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantore, S.; Ballini, A.; Saini, R.; Altini, V.; de Vito, D.; Pettini, F.; Dipalma, V.; Inchingolo, F. Effects of sea salt rinses on subjects undergone to oral surgery: A single blinded randomized controlled trial. Clin. Ter. 2020, 171, 46–52. [Google Scholar]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Honkala, E.; Honkala, S.; Shyama, M.; Al-Mutawa, S.A. Field trial on caries prevention with xylitol candies among disabled school children. Caries Res. 2006, 40, 508–513. [Google Scholar] [CrossRef]
- Milgrom, P.; Ly, K.A.; Roberts, M.C.; Rothen, M.; Mueller, G.; Yamaguchi, D.K. Mutans streptococci dose response to xylitol chewing gum. J. Dent. Res. 2006, 8, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Tapiainen, T.; Sormunen, R.; Kaijalainen, T.; Kontiokari, T.; Ikäheimo, I.; Uhari, M. Ultrastructure of Streptococcus pneumoniae after exposure to xylitol. J. Antimicrob. Chemother. 2004, 54, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Hujoel, P.P.; Mäkinen, K.K.; Bennett, C.A.; Isotupa, K.P.; Isokangas, P.J.; Allen, P.; Mäkinen, P.L. The optimum time to initiate habitual xylitol gum-chewing for obtaining long-term caries prevention. J. Dent. Res. 1999, 78, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.M.; Maier, G.; Axmann, D.; Brecx, M.; von Ohle, C. Effect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int. 2008, 39, 17–22. [Google Scholar] [PubMed]
- Michel, J.F.; Michel, M.G.; Nadan, J.; Nowzari, H. The street children of Manila are affected by early-in-life periodontal infection: Description of a treatment modality: Sea salt. Refu at Ha Peh Veha Shinayim 1993, 201, 6–13. [Google Scholar]
- Mani, A.; Mani, S.; Anarthe, R. A clinical pilot study to evaluate the efficacy of sea salt based oral rinse in gingivitis patients. Int. J. Exp. Den. Sci. 2015, 4, 116–118. [Google Scholar]
- Hoover, J.; Tovar, E.; Zlatnik, T.; Karunanayake, C. Efficacy of a rinse containing sea salt and lysozyme on biofilm and gingival health in a group of young adults: A pilot study. Int. J. Dent. Med. 2017, 4056708. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Score | CFU/ML | Caries Susceptibility |
|---|---|---|
| Class O | <103 | Negligible |
| Class 1 | 103–104 | Low |
| Class 2 | 104–105 | Moderate |
| Class 3 | >105 | High |
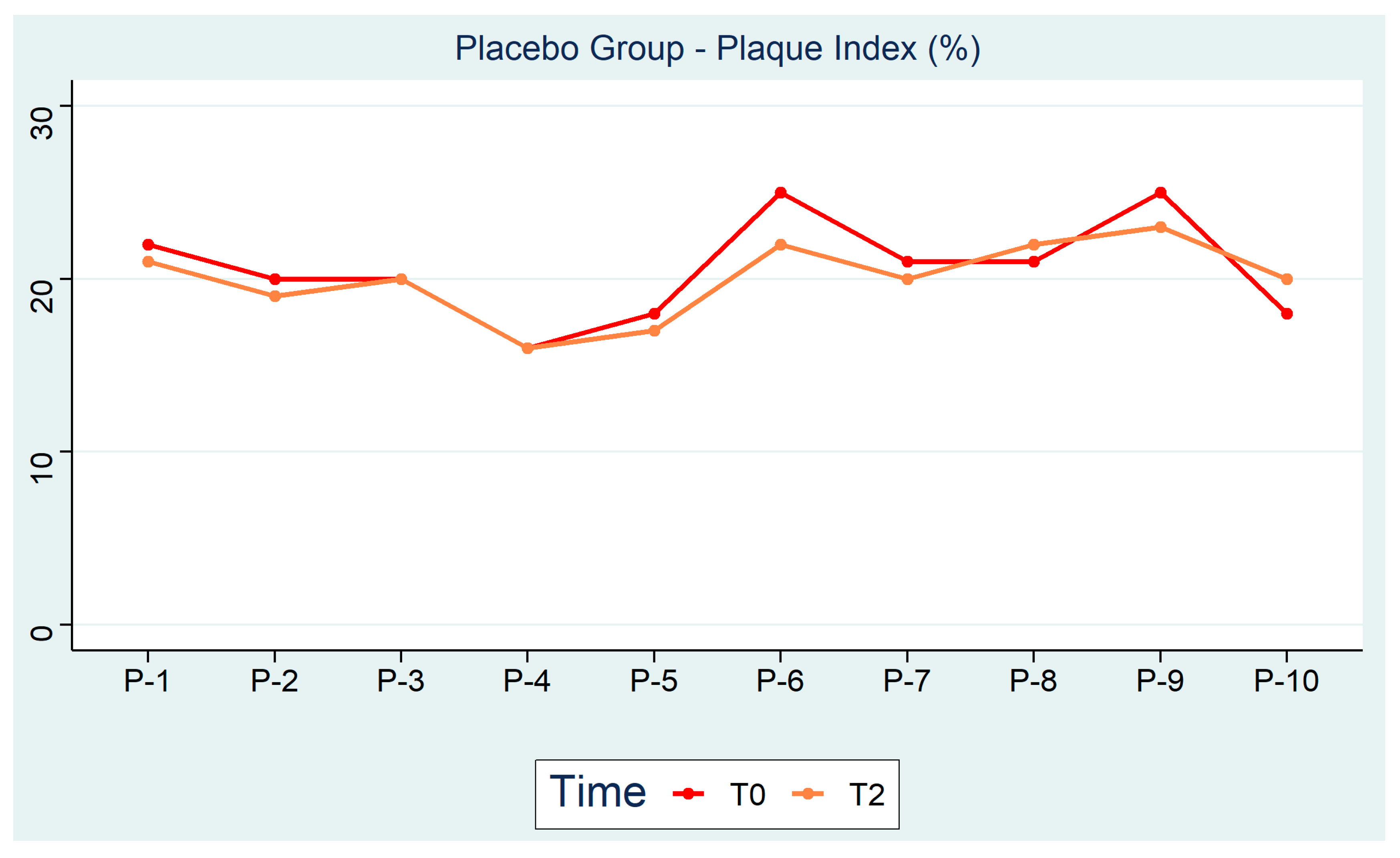
| Test Group Plaque Index (%) | Placebo Group Plaque Index (%) | ||||||
|---|---|---|---|---|---|---|---|
| ID | T0 Baseline | T1 1 Month after the First Rinse | T2 3 Months after the First Rinse | ID | T0 Baseline | T1 1 Month after the First Rinse | T2 3 Months after the First Rinse |
| T-1 | 22 | 20 | 17 | P-1 | 22 | 22 | 21 |
| T-2 | 13 | 10 | 10 | P-2 | 20 | 19 | 19 |
| T-3 | 21 | 17 | 15 | P-3 | 20 | 20 | 20 |
| T-4 | 25 | 22 | 20 | P-4 | 16 | 17 | 16 |
| T-5 | 21 | 20 | 19 | P-5 | 18 | 17 | 17 |
| T-6 | 21 | 18 | 18 | P-6 | 25 | 24 | 22 |
| T-7 | 20 | 17 | 16 | P-7 | 21 | 21 | 20 |
| T-8 | 19 | 18 | 15 | P-8 | 21 | 22 | 22 |
| T-9 | 15 | 11 | 11 | P-9 | 25 | 24 | 23 |
| T-10 | 19 | 18 | 14 | P-10 | 18 | 20 | 20 |
| Test Group S. mutans (Score) | Placebo Group S. mutans (Score) | ||||||
|---|---|---|---|---|---|---|---|
| ID | T0 Baseline | T1 1 Month after the First Rinse | T2 3 Months after the First Rinse | ID | T0 Baseline | T1 1 Month after the First Rinse | T2 3 Months after the First Rinse |
| T-1 | 2 | 2 | 0 | P-1 | 3 | 3 | 2 |
| T-2 | 3 | 2 | 1 | P-2 | 3 | 3 | 2 |
| T-3 | 2 | 1 | 0 | P-3 | 2 | 2 | 2 |
| T-4 | 2 | 1 | 0 | P-4 | 2 | 2 | 2 |
| T-5 | 3 | 1 | 0 | P-5 | 3 | 3 | 2 |
| T-6 | 3 | 2 | 1 | P-6 | 3 | 3 | 3 |
| T-7 | 3 | 2 | 0 | P-7 | 2 | 2 | 2 |
| T-8 | 2 | 2 | 1 | P-8 | 3 | 3 | 3 |
| T-9 | 3 | 2 | 1 | P-9 | 2 | 2 | 2 |
| T-10 | 2 | 2 | 0 | P-10 | 3 | 3 | 2 |
| Test Group Plaque Index (%) | Placebo Group Plaque Index (%) | Test Group S. mutans (Score) | Placebo Group S. mutans (Score) |
|---|---|---|---|
| p < 0.001 | p = 0.25 | p < 0.01 | p = 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballini, A.; Cantore, S.; Signorini, L.; Saini, R.; Scacco, S.; Gnoni, A.; Inchingolo, A.D.; De Vito, D.; Santacroce, L.; Inchingolo, F.; et al. Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 44. https://doi.org/10.3390/ijerph18010044
Ballini A, Cantore S, Signorini L, Saini R, Scacco S, Gnoni A, Inchingolo AD, De Vito D, Santacroce L, Inchingolo F, et al. Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(1):44. https://doi.org/10.3390/ijerph18010044
Chicago/Turabian StyleBallini, Andrea, Stefania Cantore, Luca Signorini, Rajiv Saini, Salvatore Scacco, Antonio Gnoni, Alessio Danilo Inchingolo, Danila De Vito, Luigi Santacroce, Francesco Inchingolo, and et al. 2021. "Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 1: 44. https://doi.org/10.3390/ijerph18010044










