The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature
Abstract
:1. Introduction
2. Nucleic Acid-Based Tests
2.1. Quantitative Reverse Transcription–Polymerase Chain Reaction (RT-qPCR)
2.2. Loop Mediated Isothermal Amplification (LAMP)
2.3. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
2.4. Perspectives and Challenges Associated with Molecular Diagnosis
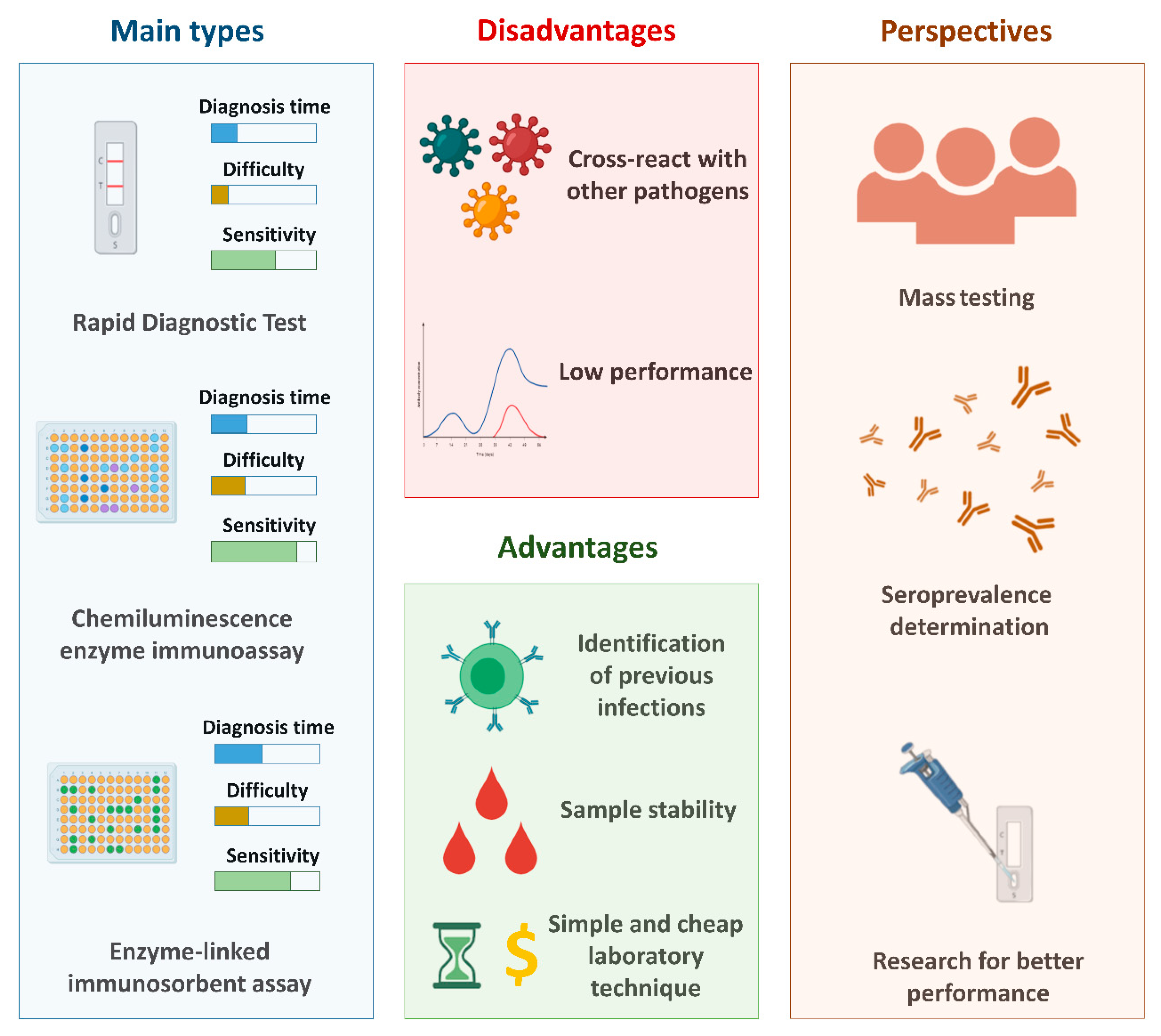
3. Serological Tests
3.1. Enzyme-Linked Immunosorbent Assay (ELISA)
3.2. Chemiluminescence Immunoassay (CLIA)
3.3. Rapid Diagnostic Tests (RDT)
3.4. Antigenic Tests
3.5. Perspectives and Challenges Associated with Serological Diagnosis
4. Nucleic Acid-Based Tests and Serological Tests: Practical Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, M.; Zhou, Y.; Ye, J.; Ahmed, A.; Al-Maskri, A.; Kang, Y.; Zeng, S.; Cai, S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020, 10, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Al-Sadeq, D.W.; AL-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G.K. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Chatziprodromidou, I.P.; Arvanitidou, M.; Guitian, J.; Apostolou, T.; Vantarakis, G.; Vantarakis, A. Global avian influenza outbreaks 2010–2016: A systematic review of their distribution, avian species and virus subtype. Syst. Rev. 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 14 December 2020).
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaushik, S.; Sharma, Y.; Kumar, R.; Yadav, J.P. The Indian perspective of COVID-19 outbreak. Virusdisease 2020, 31, 146–153. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1629–1635. [Google Scholar] [CrossRef]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Wang, M.X.; Ang, I.Y.H.; Tan, S.H.X.; Lewis, R.F.; Chen, J.I.-P.; Gutierrez, R.A.; Gwee, S.X.W.; Chua, P.E.Y.; Yang, Q.; et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J. Clin. Med. 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 2020, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Li, C.; Hu, Y.; Li, K.; Liang, J.; Wang, L.; Du, L.; Jiang, S. Current development of COVID-19 diagnostics, vaccines and therapeutics. Microbes Infect. 2020, 22, 231–235. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, W.; Wang, Y.; Long, C.; Fu, X. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J. Infect. 2020, 81, 147. [Google Scholar] [CrossRef] [PubMed]
- MHRA. COVID-19 Vaccination Programme. Available online: https://www.gov.uk/government/collections/covid-19-vaccination-programme (accessed on 9 December 2020).
- Guglielmi, G. The explosion of new coronavirus tests that could help to end the pandemic. Nature 2020, 583, 506–509. [Google Scholar] [CrossRef]
- Ritchie, H.; Ortiz-Ospina, E.; Beltekian, D.; Mathieu, E.; Hasell, J.; Macdonald, B.; Giattino, C.; Roser, M. Coronavirus (COVID-19) Testing—Statistics and Research. Available online: https://ourworldindata.org/coronavirus-testing#our-checklist-for-covid-19-testing-data (accessed on 29 September 2020).
- Loeffelholz, M.J.; Tang, Y.W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef]
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Hu, B.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; Shi, Z.L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Yurdaisik, I. Effectiveness of Computed Tomography in the Diagnosis of Novel Coronavirus-2019. Cureus 2020, 12, e8134. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Xu, X.; Gan, Q.; Zhang, L.; Luo, L.; Tang, X.; Liu, J. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur. J. Radiol. 2020, 126, 108956. [Google Scholar] [CrossRef] [PubMed]
- Rashid, Z.Z.; Norlia Othman, S.; Samat Abdul, M.N.; Kalsom Ali, U.; Kon Ken, W. Diagnostic performance of COVID-19 serology assays. Malays. J. Pathol. 2020, 42, 13–21. [Google Scholar]
- WHO. Coronavirus Disease (COVID-19) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans; WHO: Washington, DC, USA, 2020. [Google Scholar]
- CDC. Real-Time RT-PCR Diagnostic Panel for Emergency Use Only. Available online: https://www.fda.gov/media/134922/download (accessed on 9 September 2020).
- Wang, P. Combination of serological total antibody and RT-PCR test for detection of SARS-COV-2 infections. J. Virol. Methods 2020, 283, 113919. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [Green Version]
- Lee, V.J.; Chiew, C.J.; Khong, W.X. Interrupting transmission of COVID-19: Lessons from containment efforts in Singapore. J. Travel Med. 2020, 27, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Yun, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Covid-19 in South Korea—Challenges of subclinical manifestations. N. Engl. J. Med. 2020, 382, 1858–1859. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Ghosh, S.; Ali, N. Antibody detection assays for COVID-19 diagnosis: An early overview. Immunol. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Anand, S.; Montez-Rath, M.; Han, J.; Bozeman, J.; Kerschmann, R.; Beyer, P.; Parsonnet, J.; Chertow, G.M. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study. Lancet 2020, 396, 1335–1344. [Google Scholar] [CrossRef]
- Hallal, P.C.; Hartwig, F.P.; Horta, B.L.; Silveira, M.F.; Struchiner, C.J.; Vidaletti, L.P.; Neumann, N.A.; Pellanda, L.C.; Dellagostin, O.A.; Burattini, M.N.; et al. SARS-CoV-2 antibody prevalence in Brazil: Results from two successive nationwide serological household surveys. Lancet Glob. Health 2020, 8, e1390–e1398. [Google Scholar] [CrossRef]
- Murhekar, M.; Bhatnagar, T.; Selvaraju, S.; Rade, K.; Saravanakumar, V.; Vivian Thangaraj, J.; Kumar, M.; Shah, N.; Sabarinathan, R.; Turuk, A.; et al. Prevalence of SARS-CoV-2 infection in India: Findings from the national serosurvey, May-June 2020. Indian J. Med. Res. 2020, 152, 48. [Google Scholar] [CrossRef]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet. Sci. 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F.; et al. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.T.; Tong, Y.X.; Gao, C.; Zhu, L.; Zhang, Y.J.; Zhang, S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: A descriptive study. J. Clin. Virol. 2020, 127, 104346. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020, 26, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Nagura-Ikeda, M.; Imai, K.; Tabata, S.; Miyoshi, K.; Murahara, N.; Mizuno, T.; Horiuchi, M.; Kato, K.; Imoto, Y.; Iwata, M.; et al. Clinical evaluation of self-collected saliva by RT-qPCR, direct RT-qPCR, RT-LAMP, and a rapid antigen test to diagnose COVID-19. J. Clin. Microbiol. 2020, 164, 112316. [Google Scholar] [CrossRef]
- Huang, Z.; Tian, D.; Liu, Y.; Lin, Z.; Lyon, C.J.; Lai, W.; Fusco, D.; Drouin, A.; Yin, X.; Hu, T.; et al. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020, 164, 112316. [Google Scholar] [CrossRef]
- Aguiar, E.R.G.R.; Navas, J.; Pacheco, L.G.C. The COVID-19 Diagnostic Technology Landscape: Efficient Data Sharing Drives Diagnostic Development. Front. Public Health 2020, 8, 309. [Google Scholar] [CrossRef]
- Harahwa, T.A.; Ho, T.; Yau, L.; Lim-Cooke, M.-S.; Al-Haddi, S.; Zeinah, M.; Harky, A. The optimal diagnostic methods for COVID-19. Diagnosis 2020, 7, 349–356. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA J. Am. Med. Assoc. 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Hentzien, M.; Kanagaratnam, L.; Godaert, L. Should RT-PCR be considered a gold standard in the diagnosis of Covid-19? J. Med. Virol. 2020, 92, 2312–2313. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Dong, J.; Wang, N.; Huang, H.; Xu, H.; Xia, C. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: Role of deep-learning-based ct diagnosis and insights from two cases. Korean J. Radiol. 2020, 21, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoska, O.; Stevenson, K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst 2019, 144, 6461–6478. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubina, R.; Dziedzic, A. Molecular and Serological Tests for COVID-19. A Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics. Diagnostics 2020, 10, 434. [Google Scholar] [CrossRef]
- Alizargar, J.; Etemadi Sh, M.; Aghamohammadi, M.; Hatefi, S. Saliva samples as an alternative for novel coronavirus (COVID-19) diagnosis. J. Formos. Med. Assoc. 2020, 119, 1234–1235. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [Green Version]
- James, A.S.; Alwneh, J.I. COVID-19 infection diagnosis: Potential impact of isothermal amplification technology to reduce community transmission of SARS-CoV-2. Diagnostics 2020, 10, 399. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Yip, C.C.Y.; To, K.K.W.; Tang, T.H.C.; Wong, S.C.Y.; Leung, K.H.; Fung, A.Y.F.; Ng, A.C.K.; Zou, Z.; Tsoi, H.W.; et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020, 58, e00310-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A.J.; et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chang, L.; Wang, L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Rev. Med. Virol. 2020, 30, e2106. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Pepper, G.; Naccache, S.N.; Huang, M.L.; Jerome, K.R.; Greninger, A.L. Comparison of Commercially Available and Laboratory Developed Assays for in vitro Detection of SARS-CoV-2 in Clinical Laboratories. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Zhen, W.; Manji, R.; Smith, E.; Berry, G.J. Comparison of Four Molecular In Vitro Diagnostic Assays for the Detection of SARS-CoV-2 in Nasopharyngeal Specimens. J. Clin. Microbiol. 2020, 58, e00743-20. [Google Scholar] [CrossRef]
- Liu, R.; Han, H.; Liu, F.; Lv, Z.; Wu, K.; Liu, Y.; Feng, Y.; Zhu, C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 2020, 505, 172–175. [Google Scholar] [CrossRef]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; de, F. Cobre, A.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control 2020, 49, 21–29. [Google Scholar] [CrossRef]
- Mitchell, S.L.; St George, K.; Rhoads, D.D.; Butler-Wu, S.M.; McNult, P.; Miller, M.B. Understanding, verifying and implementing Emergency Use Authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J. Clin. Microbiol. 2020, 58, e00796-20. [Google Scholar] [CrossRef]
- Chau, C.H.; Strope, J.D.; Figg, W.D. COVID-19 Clinical Diagnostics and Testing Technology. Pharmacotherapy 2020, 40, 857–868. [Google Scholar] [CrossRef]
- Luo, Z.; Jin Yan Ang, M.; Yin Chan, S.; Yi, Z.; Yiing Goh, Y.; Yan, S.; Tao, J.; Liu, K.; Li, X.; Zhang, H.; et al. Combating the Coronavirus Pandemic: Early Detection, Medical Treatment, and a Concerted Effort by the Global Community. Research 2020, 6925296. [Google Scholar] [CrossRef]
- Li, C.; Zhao, C.; Bao, J.; Tang, B.; Wang, Y.; Gu, B. Laboratory Diagnosis of Coronavirus Disease-2019 (COVID-19). Clin. Chim. Acta 2020, 510, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Cepheid Xpert® Xpress SARS-CoV-2. Available online: https://www.cepheid.com/coronavirus (accessed on 5 August 2020).
- Pondaven-Letourmy, S.; Alvin, F.; Boumghit, Y.; Simon, F. How to perform a nasopharyngeal swab in adults and children in the COVID-19 era. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Q.; Hu, J.; Zhou, M.; Yu, M.; Li, K.; Xu, D.; Xiao, Y.; Yang, J.; Lu, Y.; et al. Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load. Front. Med. 2020, 7, 334. [Google Scholar] [CrossRef]
- Lin, C.; Xiang, J.; Yan, M.; Li, H.; Huang, S.; Shen, C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Ko, J.H.; Kim, Y.; Kim, Y.J.; Kim, J.M.; Chung, Y.S.; Kim, H.M.; Han, M.G.; Kim, S.Y.; Chin, B.S. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J. Korean Med. Sci. 2020, 35, e86. [Google Scholar] [CrossRef]
- Pan, Y.; Long, L.; Zhang, D.; Yuan, T.; Cui, S.; Yang, P.; Wang, Q.; Ren, S. Potential False-Negative Nucleic Acid Testing Results for Severe Acute Respiratory Syndrome Coronavirus 2 from Thermal Inactivation of Samples with Low Viral Loads. Clin. Chem. 2020, 66, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Kai-Wang To, K.; Tak-Yin Tsang, O.; Leung, W.-S.; Raymond Tam, A.; Wu, T.-C.; Christopher Lung, D.; Chik-Yan Yip, C.; Cai, J.-P.; Man-Chun Chan, J.; Shiu-Hong Chik, T.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Jiang, L.; Huang, G.; Pu, H.; Gong, B.; Lin, H.; Ma, S.; Chen, X.; Long, B.; Si, G.; et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020, 93, 264–267. [Google Scholar] [CrossRef]
- Ministry of Health. NOTA TÉCNICA No 34/2020-CGLAB/DAEVS/SVS/MS. Available online: http://www.lacen.saude.pr.gov.br/sites/lacen/arquivos_restritos/files/documento/2020-09/nota_tecnica_34_0.pdf (accessed on 2 October 2020).
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA J. Am. Med. Assoc. 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, C.; Han, M.; Ye, J.; Gao, Y.; Liu, Z.; He, T.; Li, T.; Xu, M.; Zhou, L.; et al. Discrimination of False Negative Results in RT-PCR Detection of SARS-CoV-2 RNAs in Clinical Specimens by Using an Internal Reference. Virol. Sin. 2020, 1–10. [Google Scholar] [CrossRef]
- Song, C.-Y.; Yang, D.-G.; Lu, Y.-Q. A COVID-19 patient with seven consecutive false-negative rRT-PCR results from sputum specimens. Intern. Emerg. Med. 2020, 15, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.C.; Risch, L.; Weber, M.; Thiel, S.; Grossmann, K.; Wohlwend, N.; Lung, T.; Hillmann, D.; Ritzler, M.; Bigler, S.; et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: A population-based study. Clin. Chem. Lab. Med. 2020, 58, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [Green Version]
- Visseaux, B.; Le Hingrat, Q.; Collin, G.; Ferré, V.; Storto, A.; Ichou, H.; Bouzid, D.; Poey, N.; de Montmollin, E.; Descamps, D.; et al. Evaluation of the RealStar® SARS-CoV-2 RT-PCR kit RUO performances and limit of detection. J. Clin. Virol. 2020, 129, 104520. [Google Scholar] [CrossRef]
- Yip, C.C.-Y.; Sridhar, S.; Cheng, A.K.-W.; Leung, K.-H.; Choi, G.K.-Y.; Chen, J.H.-K.; Poon, R.W.-S.; Chan, K.-H.; Wu, A.K.-L.; Chan, H.S.-Y.; et al. Evaluation of the commercially available LightMix® Modular E-gene kit using clinical and proficiency testing specimens for SARS-CoV-2 detection. J. Clin. Virol. 2020, 129, 104476. [Google Scholar] [CrossRef]
- Nalla, A.K.; Casto, A.M.; Huang, M.L.W.; Perchetti, G.A.; Sampoleo, R.; Shrestha, L.; Wei, Y.; Zhu, H.; Jerome, K.R.; Greninger, A.L. Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer-Probe Sets and One Assay Kit. J. Clin. Microbiol. 2020, 58, e00557-20. [Google Scholar] [CrossRef] [Green Version]
- Szymczak, W.A.; Goldstein, D.Y.; Orner, E.P.; Fecher, R.A.; Yokoda, R.T.; Skalina, K.A.; Narlieva, M.; Gendlina, I.; Fox, A.S. Utility of Stool PCR for the Diagnosis of COVID-19: Comparison of Two Commercial Platforms. J. Clin. Microbiol. 2020, 58, e01369-20. [Google Scholar] [CrossRef]
- Pujadas, E.; Ibeh, N.; Hernandez, M.M.; Waluszko, A.; Sidorenko, T.; Flores, V.; Shiffrin, B.; Chiu, N.; Young-Francois, A.; Nowak, M.D.; et al. Comparison of SARS-CoV-2 detection from nasopharyngeal swab samples by the Roche cobas 6800 SARS-CoV-2 test and a laboratory-developed real-time RT-PCR test. J. Med. Virol. 2020, 92, 1695–1698. [Google Scholar] [CrossRef]
- Craney, A.R.; Velu, P.; Satlin, M.J.; Fauntleroy, K.A.; Callan, K.; Robertson, A.; Spina, L.; Lei, B.; Chen, A.; Alston, T.; et al. Comparison of Two High-Throughput Reverse Transcription-Polymerase Chain Reaction Systems for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. J. Clin. Microbiol. 2020, 58, e00890-20. [Google Scholar] [CrossRef] [PubMed]
- Perng, C.-L.; Jian, M.-J.; Chang, C.-K.; Lin, J.-C.; Yeh, K.-M.; Chen, C.-W.; Chiu, S.-K.; Chung, H.-Y.; Wang, Y.-H.; Liao, S.-J.; et al. Novel rapid identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by real-time RT-PCR using BD Max Open System in Taiwan. PeerJ 2020, 8, e9318. [Google Scholar] [CrossRef] [PubMed]
- van Kasteren, P.B.; van der Veer, B.; van den Brink, S.; Wijsman, L.; de Jonge, J.; van den Brandt, A.; Molenkamp, R.; Reusken, C.B.E.M.; Meijer, A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 2020, 128, 104412. [Google Scholar] [CrossRef] [PubMed]
- Iglói, Z.; Leven, M.; Abdel-Karem Abou-Nouar, Z.; Weller, B.; Matheeussen, V.; Coppens, J.; Koopmans, M.; Molenkamp, R. Comparison of commercial realtime reverse transcription PCR assays for the detection of SARS-CoV-2. J. Clin. Virol. 2020, 129, 104510. [Google Scholar] [CrossRef]
- Bordi, L.; Piralla, A.; Lalle, E.; Giardina, F.; Colavita, F.; Tallarita, M.; Sberna, G.; Novazzi, F.; Meschi, S.; Castilletti, C.; et al. Rapid and sensitive detection of SARS-CoV-2 RNA using the SimplexaTM COVID-19 direct assay. J. Clin. Virol. 2020, 128, 104416. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Luo, L.; Luo, Z.D.; Lyu, J.X.; Ng, M.Y.; Shen, X.P.; Wen, Z. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir. Med. 2020, 168, 105980. [Google Scholar] [CrossRef]
- Fisher, B.; Seese, L.; Sultan, I.; Kilic, A. The importance of repeat testing in detecting coronavirus disease 2019 (COVID-19) in a coronary artery bypass grafting patient. J. Card. Surg. 2020, 35, 1342–1344. [Google Scholar] [CrossRef]
- Ooi, G.C.; Khong, P.L.; Müller, N.L.; Yiu, W.C.; Zhou, L.J.; Ho, J.C.M.; Lam, B.; Nicolaou, S.; Tsang, K.W.T. Severe acute respiratory syndrome: Temporal lung changes at thin-section CT in 30 patients. Radiology 2004, 230, 836–844. [Google Scholar] [CrossRef]
- Ajlan, A.M.; Ahyad, R.A.; Jamjoom, L.G.; Alharthy, A.; Madani, T.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection: Chest CT Findings. Am. J. Roentgenol. 2014, 203, 782–787. [Google Scholar] [CrossRef]
- Li, Y.; Xia Li, L.Y. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. Am. J. Roentgenol. 2020, 214, 1280–1286. [Google Scholar] [CrossRef]
- Waller, J.V.; Allen, I.E.; Lin, K.K.; Diaz, M.J.; Henry, T.S.; Hope, M.D. The Limited Sensitivity of Chest Computed Tomography Relative to Reverse Transcription Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus-2 Infection A Systematic Review on COVID-19 Diagnostics. Invest. Radiol. 2020, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. J. Infect. Chemother. 2020, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I. Trends and Innovations in Biosensors for COVID-19 Mass Testing. ChemBioChem 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Baek, Y.H.; Lloren, K.K.S.; Choi, W.-S.; Jeong, J.H.; Antigua, K.J.C.; Kwon, H.; Park, S.-J.; Kim, E.-H.; Kim, Y.; et al. Rapid and simple colorimetric detection of multiple influenza viruses infecting humans using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. BMC Infect. Dis. 2019, 19, 676. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.J.R.; Paiva, M.H.S.; Guedes, D.R.D.; Krokovsky, L.; de Melo, F.L.; da Silva, M.A.L.; da Silva, A.; Ayres, C.F.J.; Pena, L.J. Development and Validation of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) for Rapid Detection of ZIKV in Mosquito Samples from Brazil. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Baek, Y.H.; Kim, Y.H.; Choi, Y.K.; Song, M.S.; Ahn, J.Y. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Front. Microbiol. 2017, 7, 2166. [Google Scholar] [CrossRef] [Green Version]
- Poon, L.L.M.; Leung, C.S.W.; Tashiro, M.; Chan, K.H.; Wong, B.W.Y.; Yuen, K.Y.; Guan, Y.; Peiris, J.S.M. Rapid detection of the Severe Acute Respiratory Syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin. Chem. 2004, 50, 1050–1052. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Harrington, A.; Cox, B.; Snowdon, J.; Bakst, J.; Ley, E.; Grajales, P.; Maggiore, J.; Kahn, S. Comparison of abbott id now and abbott m2000 methods for the detection of sars-cov-2 from nasopharyngeal and nasal swabs from symptomatic patients. J. Clin. Microbiol. 2020, 58, 798–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hee-Baek, Y.; Um, J.; Joy Antigua, K.C.; Park, J.-H.; Kim, Y.; Oh, S.; Kim, Y.; Choi, W.-S.; Gyu Kim, S.; Hwan Jeong, J.; et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Wu, X.; Wan, Z.; Li, Y.; Jin, X.; Zhang, C. A novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Pan, W.; Arasthfer, A.; Fang, W.; Ling, L.; Fang, H.; Daneshnia, F.; Yu, J.; Liao, W.; Pei, H.; et al. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Lim, B.; Hsu, C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wu, S.; Hao, X.; Dong, X.; Mao, L.; Vicent, P.; Chen, W.-H.; Yin, X. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin. Chem. 2020, 66, 975–977. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Orihara, Y.; Kawamura, R.; Imai, K.; Sakai, J.; Tarumoto, N.; Matsuoka, M.; Takeuchi, S.; Maesaki, S.; Maeda, T. Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification. J. Clin. Virol. 2020, 129, 104446. [Google Scholar] [CrossRef]
- James, P.; Stoddart, D.; Harrington, E.D.; Beaulaurier, J.; Ly, L.; Reid, S.W.; Turner, D.J.; Juul, S. LamPORE: Rapid, accurate and highly scalable molecular screening for SARS-CoV-2 infection, based on nanopore sequencing. medRxiv 2020. [Google Scholar] [CrossRef]
- Obande, G.A.; Kaur, K.; Singh, B. Current and Future Perspectives on Isothermal Nucleic Acid Amplification Technologies for Diagnosing Infections. Infect. Drug Resist. 2020, 13, 455–483. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Newbigging, A.; Le, C.; Pang, B.; Peng, H.; Cao, Y.; Wu, J.; Abbas, G.; Song, J.; Wang, D.-B.; et al. Molecular diagnosis of COVID-19: Challenges and research needs. Anal. Chem. 2020, 92, 10196–10209. [Google Scholar] [CrossRef]
- McCarthy, M.W. Harnessing the potential of CRISPR-based platforms to advance the field of hospital medicine. Expert Rev. Anti. Infect. Ther. 2020, 18, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Esbin, M.N.; Whitney, O.N.; Chong, S.; Maurer, A.; Darzacq, X.; Tjian, R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 2020, 26, 771–783. [Google Scholar] [CrossRef]
- Nestor, M.W.; Wilson, R.L. Beyond Mendelian Genetics: Anticipatory Biomedical Ethics and Policy Implications for the Use of CRISPR Together with Gene Drive in Humans. J. Bioethical Inq. 2020, 17, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.-G.; Woolley, A.E.; Segel, M.; Barretto, R.P.J.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- FDA. Coronavirus (COVID-19) Update: Daily Roundup May 7, 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-may-7-2020 (accessed on 19 July 2020).
- Sheridan, C. COVID-19 spurs wave of innovative diagnostics. Nat. Biotechnol. 2020, 38, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Abudayyeh, O.O.; Gootenberg, J.S. A Protocol for Detection of Covid-19 Using Crispr Diagnostics. (updated). Available online: https://www.broadinstitute.org/files/publications/special/COVID-19detection.
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgeson, F.P.; Aguinis, H.; Waldman, D.A.; Sengiel, D.S. Recent Biotechnological Tools for Diagnosis of COVID-19 Disease: A review. Biotechnol. Prog. 2020, 9, e3078. [Google Scholar]
- Respiratory Virus Infections Working Group Canadian Public Health Laboratory Network Best Practices for COVID-19. Available online: https://nccid.ca/wp-content/uploads/sites/2/2020/05/COVID-Best-Practices-V1.01-v3.pdf (accessed on 1 October 2020).
- Dias, V.M.; da Cunha, C.A.; de, L. Vidal, C.F.; Ben Corradi, M.F.D.; Michelin, L.; Muglia, V.; Rocha, J.L.L.; Costa, S.F.; de Oliveira, P.R.D.; Carrilho, C.M.; et al. Orientações sobre Diagnóstico, Tratamento e Isolamento de Pacientes com COVID-19. J. Infect. Control 2020, 9, 56–75. [Google Scholar]
- Ferreira, L.; Mota, P.; Ferreira, P.; Freitas, S.; Campainha, S.; Clemente, S.; Areias, V. Recomendações da spp no Diagnóstico e Tratamento de Doenças Difusas do Pulmão Durante a Pandemia por Sars-Cov-2. Available online: https://www.sppneumologia.pt/uploads/subcanais_conteudos_ficheiros/recomendacoes-da-spp-no-diagnostico-e-no-tratamento-de-doencas-difusas-do-pulmao-durante-a-pandemia-por-sars-cov-2.pdf (accessed on 1 October 2020).
- China National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th Edition). Available online: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html (accessed on 1 October 2020).
- Davarpanah, A.H.; Mahdavi, A.; Sabri, A.; Langroudi, F.; Kahkouee, S.; Haseli, S.; Kazemi, M.A.; Mehrian, P.; Mahdavi, A.; Falahati, F.; et al. Novel Screening and Triage Strategy in Iran During Deadly Coronavirus Disease 2019 (COVID-19) Epidemic: Value of Humanitarian Teleconsultation Service. J. Am. Coll. Radiol. 2020, 17, 734–738. [Google Scholar] [CrossRef]
- Abedini, Z.; Sari, A.A.; Foroushani, A.R.; Jaafaripooyan, E. Diffusion of advanced medical imaging technology, CT, and MRI scanners, in Iran: A qualitative study of determinants. Int. J. Health Plann. Manag. 2019, 34, e397–e410. [Google Scholar] [CrossRef] [Green Version]
- Fields, B.K.K.; Demirjian, N.L.; Gholamrezanezhad, A. Coronavirus Disease 2019 (COVID-19) diagnostic technologies: A countrybased retrospective analysis of screening and containment procedures during the first wave of the pandemic. Clin. Imaging 2020, 67, 2019–2225. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, K.; Darzi, A.; Jain, S. ‘Test, re-test, re-test’: Using inaccurate tests to greatly increase the accuracy of COVID-19 testing. Nat. Med. 2020, 26, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Neilan, A.M.; Losina, E.; Bangs, A.C.; Flanagan, C.; Panella, C.; Eskibozkurt, G.E.; Mohareb, A.; Hyle, E.P.; Scott, J.A.; Weinstein, M.C.; et al. Clinical Impact, Costs, and Cost-Effectiveness of Expanded SARS-CoV-2 Testing in Massachusetts. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gopalkrishnan, M.; Krishna, S. Pooling Samples to Increase SARS-CoV-2 Testing. J. Indian Inst. Sci. 2020, 100, 787–792. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Williams, J.; Ju, B.; Shaner, B.; Easton, J.; Wu, G.; Chen, X. A comparison of methods accounting for batch effects in differential expression analysis of UMI count based single cell RNA sequencing. Comput. Struct. Biotechnol. J. 2020, 18, 861–873. [Google Scholar] [CrossRef]
- Lagopati, N.; Tsioli, P.; Mourkioti, I.; Polyzou, A.; Papaspyropoulos, A.; Zafiropoulos, A.; Evangelou, K.; Sourvinos, G.; Gorgoulis, V.G. Sample pooling strategies for SARS-CoV-2 detection. J. Virol. Methods 2020, 289, 114044. [Google Scholar] [CrossRef]
- Mutesa, L.; Ndishimye, P.; Butera, Y.; Souopgui, J.; Uwineza, A.; Rutayisire, R.; Ndoricimpaye, E.L.; Musoni, E.; Rujeni, N.; Nyatanyi, T.; et al. A pooled testing strategy for identifying SARS-CoV-2 at low prevalence. Nature 2020. [Google Scholar] [CrossRef]
- Reta, D.H.; Sisay Tessema, T.; Ashenef, A.S.; Feleke Desta, A.; Labisso, W.L.; Gizaw, S.T.; Abay, S.M.; Melka, D.S.; Reta, F.A. Molecular and Immunological Diagnostic Techniques of Medical Viruses. Int. J. Microbiol. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Winter, A.K.; Hegde, S.T. The important role of serology for COVID-19 control. Lancet Infect. Dis. 2020, 20, 758–759. [Google Scholar] [CrossRef]
- Rogers, R.; O’brien, T.; Aridi, J.; Beckwith, C.G. The COVID-19 Diagnostic Dilemma: A Clinician’s Perspective. J. Clin. Microbiol. 2020, 58, e01287-20. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Veen, W.; Brüggen, M.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, B.; Fang, Z.; Zhao, J.; Liu, X.; Li, Y.; Sun, X.; Liang, H.; Zhong, B.; Huang, Z.; et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001526. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 Serological Tests: How well do they actually perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef]
- La Marca, A.; Capuzzo, M.; Paglia, T.; Roli, L.; Trenti, T.; Nelson, S.M. Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online 2020, 41, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Stratton, C.W.; Tang, Y. An evolving approach to the laboratory assessment of COVID-19. J. Med. Virol. 2020, 92, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yan, M.; Li, H.; Liu, T.; Lin, C.; Huang, S.; Shen, C. Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold-Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461-20. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Chuan, J.; Gong, B.; Shuai, P.; Zhou, Y.; Zhang, Y.; Jiang, Z.; Zhang, D.; Liu, X.; Ma, S.; et al. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci. China Life Sci. 2020, 63, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.F.; Meng, X.J. Antigenic Cross-Reactivity between the Nucleocapsid Protein of Severe Acute Respiratory Syndrome (SARS) Coronavirus and Polyclonal Antisera of Antigenic Group I Animal Coronaviruses: Implication for SARS Diagnosis. J. Clin. Microbiol. 2004, 42, 2351–2352. [Google Scholar] [CrossRef] [Green Version]
- Mazzini, L.; Martinuzzi, D.; Hyseni, I.; Benincasa, L.; Molesti, E.; Casa, E.; Lapini, G.; Piu, P.; Trombetta, C.M.; Marchi, S.; et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunol. Methods 2020, 112937. [Google Scholar] [CrossRef] [PubMed]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2020, 288, 114031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Abduljalil, J.M. Laboratory diagnosis of SARS-CoV-2: Available approaches and limitations. New Microbes New Infect. 2020, 36, 100713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Brochot, E.; Demey, B.; Handala, L.; François, C.; Duverlie, G.; Castelain, S. Comparison of different serological assays for SARS-CoV-2 in real life. J. Clin. Virol. 2020, 130, 104569. [Google Scholar] [CrossRef]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. 2020, 92, 2243–2247. [Google Scholar] [CrossRef]
- Tuaillon, E.; Bolloré, K.; Pisoni, A.; Debiesse, S.; Renault, C.; Marie, S.; Groc, S.; Niels, C.; Pansu, N.; Dupuy, A.M.; et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J. Infect. 2020, 81, e39–e45. [Google Scholar] [CrossRef]
- Lassaunière, R.; Frische, A.; Harboe, Z.B.; Nielsen, A.C.Y.; Fomsgaard, A.; Krogfelt, K.A.; Jørgensen, C.S. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- GeurtsvanKessel, C.H.; Okba, A.N.M.; Igloi, Z.; Bogers, S.; Embregts, E.C.W.; Laksono, B.M.; Leijten, L.; Rokx, C.; Rijnders, B.; Rahamat-Langendoen, J.; et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020, 11, 3436. [Google Scholar] [CrossRef]
- Jääskeläinen, A.J.; Kekäläinen, E.; Kallio-Kokko, H.; Mannonen, L.; Kortela, E.; Vapalahti, O.; Kurkela, S.; Lappalainen, M.; Anne, J.J.; Eliisa, K.; et al. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Eurosurveillance 2020, 25, 2000603. [Google Scholar] [CrossRef] [PubMed]
- Cinquanta, L.; Fontana, D.E.; Bizzaro, N. Chemiluminescent immunoassay technology: What does it change in autoantibody detection? Autoimmun. Highlights 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Karanis, P.; Fallahi, S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection 2018, 46, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Sciacovelli, L.; Faggian, D.; Plebani, M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020, 58, 1081–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Wang, M.; Zuo, Z.; Fan, C.; Ye, F.; Cai, Z.; Wang, Y.; Cui, H.; Pan, K.; Xu, A. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int. J. Infect. Dis. 2020, 94, 49–52. [Google Scholar] [CrossRef]
- Hu, Q.; Cui, X.; Liu, X.; Peng, B.; Jiang, J.; Wang, X.; Li, Y.; Hu, W.; Ao, Z.; Duan, J.; et al. The production of antibodies for SARS-CoV-2 and its clinical implication. medRxiv 2020. [Google Scholar] [CrossRef]
- Cai, X.-F.; Chen, J.; Hu, J.-L.; Long, Q.-X.; Deng, H.-J.; Fan, K.; Liao, P.; Liu, B.-Z.; Wu, G.-C.; Chen, Y.-K.; et al. A Peptide-based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Coronavirus Disease 2019 (COVID-19). J. Infect. Dis. 2020, 222, 189–193. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Yang, Y.; Jiang, D.; Qi, Y.; He, W.; Zhao, C.; Yi, R.; et al. COVID-19 diagnosis and study of serum SARS-CoV-2 specific IgA, IgM and IgG by chemiluminescence immunoanalysis. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Li, Z.; Wang, K.; Li, T.; Liao, P. Performance verification of detecting COVID-19 specific antibody by using four chemiluminescence immunoassay systems. medRxiv 2020. [Google Scholar] [CrossRef]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Liu, R.; Liu, X.; Han, H.; Adnan Shereen, M.; Niu, Z.; Liu, F.; Wu, K.; Luo, Z.; Zhu, C. The comparative superiority of IgM-IgG antibody test to real-time reverse 1 transcriptase PCR detection for SARS-CoV-2 infection diagnosis. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Chung, D.-R.; Kang, M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst 2019, 144, 2460. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.A.; Costa, C.E.; Junior-Delduque, J.; Maia, J.N.B.; Pinto, P.B.O.P. Brazil’s Policy Responses to COVID-19. Available online: https://www.gov.br/economia/pt-br/centrais-de-conteudo/publicacoes/publicacoes-em-outros-idiomas/covid-19/brazil2019s-policy-responses-to-covid-19 (accessed on 1 October 2020).
- Laureano, A.F.S.; Riboldi, M. The different tests for the diagnosis of covid-19-a review in Brazil so far. J. Bras. Reprod. Assist. 2020, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.; Khan, M.; Dombey, D.; Pitel, L. Countries Reject China Pandemic Product Batches. Available online: https://www.ft.com/content/f3435779-a706-45c7-a7e2-43efbdd7777b (accessed on 11 September 2020).
- Baker, S. Coronavirus: Spain Returning 2nd Batch of Faulty Tests to Bioeasy China. Available online: https://www.businessinsider.com/coronavirus-spain-returns-second-batch-faulty-tests-bioeasy-china-2020-4 (accessed on 11 September 2020).
- Chakraborty, B. Netherlands Becomes Latest Country to Reject China-Made Coronavirus Test Kits. Available online: https://www.foxnews.com/world/netherlands-becomes-latest-country-to-reject-china-made-coronavirus-test-kits-gear (accessed on 11 September 2020).
- Adams, E.R.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.I.; Berry, T.; et al. Antibody testing for COVID-19: A report from the National COVID Scientific Advisory Panel. Wellcome Open Res. 2020, 5, 139. [Google Scholar] [CrossRef]
- Döhla, M.; Boesecke, C.; Schulte, B.; Diegmann, C.; Sib, E.; Richter, E.; Eschbach-Bludau, M.; Aldabbagh, S.; Marx, B.; Eis-Hübinger, A.M.; et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health 2020, 182, 170–172. [Google Scholar] [CrossRef]
- Shamsollahi, H.R.; Amini, M.; Alizadeh, S.; Nedjat, S.; Akbari-Sari, A.; Rezaei, M.; Farshad Allameh, S.; Fotouhi, A.; Yunesian, M. Assessment of a serological diagnostic kit of SARS-CoV-2 availble in Iran. medRxiv 2020. [Google Scholar] [CrossRef]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv 2020. [Google Scholar] [CrossRef]
- Imai, K.; Tabata, S.; Ikeda, M.; Noguchi, S.; Kitagawa, Y.; Matuoka, M.; Miyoshi, K.; Tarumoto, N.; Sakai, J.; Ito, T.; et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J. Clin. Virol. 2020, 128, 104393. [Google Scholar] [CrossRef]
- Shen, L.; Wang, C.; Zhao, J.; Tang, X.; Shen, Y.; Lu, M.; Ding, Z.; Huang, C.; Zhang, J.; Li, S.; et al. Delayed specific IgM antibody responses observed among COVID-19 patients with severe progression. Emerg. Microbes Infect. 2020, 9, 1096–1101. [Google Scholar] [CrossRef]
- Andryukov, G.B. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Novazzi, F.; Giardina, F.; Salinaro, F.; Sachs, M.; Perlini, S.; Bruno, R.; Mojoli, F. Performance of VivaDiag COVID—19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID—19 in acute patients referring to emergency room department. J. Med. Virol. 2020, 92, 1724–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Li, X.; Yang, G.; Fan, J.; Tang, Y.; Zhao, J.; Long, X.; Guo, S.; Zhao, Z.; Liu, Y.; et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J. Infect. 2020, 81, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zheng, Y.; Zhang, X.; Zhang, W.; Wang, D.; Jin, J.; Lin, R.; Zhang, Y.; Zhu, G.; Zhu, H.; et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am. J. Transl. Res. 2020, 12, 1348–1354. [Google Scholar] [PubMed]
- Pérez-García, F.; Pérez-Tanoira, R.; Romanyk, J.; Arroyo, T.; Gómez-Herruz, P.; Cuadros-González, J. Rapid diagnosis of SARS-CoV-2 infection by detecting IgG and IgM antibodies 3 with an immunochromatographic device: A prospective single-center study Running title: Rapid serologic test to diagnose SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Spicuzza, L.; Montineri, A.; Manuele, R.; Crimi, C.; Pistorio, M.P.; Campisi, R.; Vancheri, C.; Crimi, N. Reliability and usefulness of a rapid IgM-IgG antibody test for the diagnosis of SARS-CoV-2 infection: A preliminary report. J. Infect. 2020, 81, e53–e54. [Google Scholar] [CrossRef]
- Virgilioparadiso, A.; De Summa, S.; Loconsole, D.; Procacci, V.; Sallustio, A.; Centrone, F.; Silvestris, N.; Cafagna, V.; De Palma, G.; Tufaro, A.; et al. Clinical meanings of rapid serological assay in patients tested for SARS-Co2 RT-PCR. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Demey, B.; Daher, N.; François, C.; Lanoix, J.P.; Duverlie, G.; Castelain, S.; Brochot, E. Dynamic profile for the detection of anti-SARS-CoV-2 antibodies using four immunochromatographic assays. J. Infect. 2020, 81, e6–e10. [Google Scholar] [CrossRef]
- Serrano, M.M.; Rodríguez, D.N.; Palop, N.T.; Arenas, R.O.; Córdoba, M.M.; Mochón, M.D.O.; Cardona, C.G. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J. Clin. Virol. 2020, 129, 104529. [Google Scholar] [CrossRef]
- Mlcochova, P.; Collier, D.; Ritchie, A.; Goodfellow, I.; Gupta, R.K. Combined Point-of-Care Nucleic Acid and Antibody Testing for SARS-CoV-2 following Emergence of D614G Spike Variant. Cell Rep. Med. 2020, 1, 100099. [Google Scholar] [CrossRef]
- Augustine, R.; Das, S.; Hasan, A.; Abhilash, S.; Abdul Salam, S.; Augustine, P.; Dalvi, Y.B.; Varghese, R.; Primavera, R.; Yassine, H.M.; et al. Rapid Antibody-Based COVID-19 Mass Surveillance: Relevance, Challenges, and Prospects in a Pandemic and Post-Pandemic World. J. Clin. Med. 2020, 9, 3372. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Guidance for Antigen Testing for SARS-CoV-2. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 17 December 2020).
- Li, D.; Li, J. Immunologic testing for SARS-CoV-2 infection from the antigen perspective. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X. Chemiluminescent immunometric detection of sars-cov in sera as an early marker for the diagnosis of sars. Biolumin. Chemilumin. 2005, 491–494. [Google Scholar] [CrossRef]
- Che, X.Y.; Qiu, L.W.; Pan, Y.X.; Wen, K.; Hao, W.; Zhang, L.Y.; Di Wang, Y.; Liao, Z.Y.; Hua, X.; Cheng, V.C.C.; et al. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004, 42, 2629–2635. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, C. Coronavirus testing finally gathers speed. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abbott BinaxNOWTM COVID-19 Ag CARD. Available online: www.globalpointofcare.abbott (accessed on 17 December 2020).
- Perchetti, G.; Huang, M.; Mills, M.; Jerome, K.; Greninger, A. Analytical Sensitivity of the Abbott BinaxNOW COVID-19 Ag CARD. J. Clin. Microbiol. 2020. [Google Scholar] [CrossRef]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imöhl, M.; Kleines, M. Comparison of the SARS-CoV-2 Rapid Antigen Test to the Real Star Sars-CoV-2 RT PCR Kit. J. Virol. Methods 2020, 288, 114024. [Google Scholar] [CrossRef]
- Blairon, L.; Wilmet, A.; Beukinga, I.; Tré-Hardy, M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: Experiences of a general hospital. J. Clin. Virol. 2020, 129, 104472. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- CDC Interim Guidelines for COVID-19 Antibody Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 14 September 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA J. Am. Med. Assoc. 2020, 323, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.; Murdoch, D.R. Clinical course and mortality risk of severe COVID-19. Lancet 2020, 395, 1014–1015. [Google Scholar] [CrossRef]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [Green Version]
- Kawase, J.; Asakura, H.; Kurosaki, M.; Oshiro, H.; Etoh, Y.; Ikeda, T.; Watahiki, M.; Kameyama, M.; Hayashi, F.; Kawakami, Y.; et al. Rapid and Accurate Diagnosis Based on Real-Time PCR Cycle Threshold Value for the Identification of Campylobacter jejuni, astA Gene-Positive Escherichia coli, and eae Gene-Positive E. coli. Jpn. J. Infect. Dis. 2018, 71, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Hur, J.; Park, D. Interpreting the COVID-19 Test Results: A Guide for Physiatrists. Am. J. Phys. Med. Rehabil. 2020, 99, 583–585. [Google Scholar] [CrossRef]
- Jang, S.; Rhee, J.-Y.; Wi, Y.M.; Jung, B.K. Viral kinetics of SARS-CoV-2 over the preclinical, clinical, and postclinical period. Int. J. Infect. Dis. 2020, 102, 561–565. [Google Scholar] [CrossRef]
- Louie, J.K.; Stoltey, J.; Scott, H.M.; Trammell, S.; Ememu, E.; Samuel, M.C.; Aragon, T.; Masinde, G. Comparison of Symptomatic and Asymptomatic Infections due to COVID-19 in San Francisco Long Term Care Facilities. Infect. Control Hosp. Epidemiol. 2020, 1–8. [Google Scholar] [CrossRef]
- Singanayagam, A.; Patel, M.; Charlett, A.; Lopez Bernal, J.; Saliba, V.; Ellis, J.; Ladhani, S.; Zambon, M.; Gopal, R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance 2020, 25, 2001483. [Google Scholar] [CrossRef]
- Gorzalski, A.J.; Hartley, P.; Laverdure, C.; Kerwin, H.; Tillett, R.; Verma, S.; Rossetto, C.; Morzunov, S.; Van Hooser, S.; Pandori, M.W. Characteristics of viral specimens collected from asymptomatic and fatal cases of COVID-19. J. Biomed. Res. 2020, 34, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, B.; Li, T.D.; Zheng, S.F.; Su, Y.Y.; Li, Z.Y.; Liu, W.; Yu, F.; Ge, S.X.; Da Zou, Q.; Yuan, Q.; et al. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur. Respir. J. 2020, 56, 2000763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.T.; Gao, C.; Zhang, S. Profile of specific antibodies to SARS-CoV-2: The first report. J. Infect. 2020, 81, 147–178. [Google Scholar] [CrossRef]
- Hamilton, F.; Muir, P.; Attwood, M.; Vipond, A.N.B.; Hopes, R.; Moran, E.; Maskell, N.; Warwick, D.; Albur, M.; Turner, J.; et al. Kinetics and performance of the Abbott architect SARS-CoV-2 IgG antibody assay. J. Infect. 2020, 81, e7–e9. [Google Scholar] [CrossRef]
- Suhandynata, R.T.; Hoffman, M.A.; Kelner, M.J.; McLawhon, R.W.; Reed, S.L.; Fitzgerald, R.L. Longitudinal Monitoring of SARS-CoV-2 IgM and IgG Seropositivity to Detect COVID-19. J. Appl. Lab. Med. 2020, 5, 908–920. [Google Scholar] [CrossRef]
- Wang, H.; Ai, J.; Loeffelholz, M.J.; Tang, Y.-W.; Zhang, W. Meta-analysis of diagnostic performance of serology tests for COVID-19: Impact of assay design and post-symptom-onset intervals. Emerg. Microbes Infect. 2020, 9, 2200–2211. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, L.; Chang, D.; Wang, J.; Hu, Y.; Chen, H.; Guo, L.; Wu, C.; Wang, C.; Wang, Y.; et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun. Biol. 2020, 3, 780–787. [Google Scholar] [CrossRef]
- Li, L.; Liang, Y.; Hu, F.; Yan, H.; Li, Y.; Xie, Z.; Huang, L.; Zhao, J.; Wan, Z.; Wang, H.; et al. Molecular and serological characterization of SARS-CoV-2 infection among COVID-19 patients. Virology 2020, 551, 26–35. [Google Scholar] [CrossRef]
- Brochot, E.; Demey, B.; Touzé, A.; Belouzard, S.; Dubuisson, J.; Schmit, J.-L.; Duverlie, G.; Francois, C.; Castelain, S.; Helle, F. Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Front. Microbiol. 2020, 11, 2468. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Roarty, C.; Tonry, C.; McFetridge, L.; Mitchell, H.; Watson, C.; Waterfield, T.; Waxman, E.; Fairley, D.; Roew-Setz, G.; McKenna, J.; et al. Kinetics and seroprevalence of SARS-CoV-2 antibodies in children. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]



| Country | Institute | Molecular Targets |
|---|---|---|
| China | China Center for Disease Control and Prevention (CDC) | Open Reading Frame (ORF) 1ab and N |
| Germany | Charité | RdRp, E, N |
| Hong Kong | Hong Kong University (HKU) | ORF1b-nsp14, N |
| Japan | National Institute of Infectious Diseases, Department of Virology III | Japan Pancorona and multiple targets, S |
| Thailand | National Institute of Health | N |
| United States | CDC | Two regions in N protein |
| France | Institut Pasteur | Two regions in RdRp |
| Reference | Commercial Kit Name (Manufacturer) | System or Platform | Specimen Type | No. of Patients/Specimens | Gene(s) Target (Reference Method) | Main Findings and/or Conclusions |
|---|---|---|---|---|---|---|
| Visseaux et al. [83] | RealStare Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) RT-PCR kit (Altona Diagnostic) | ABI 7500 real-time PCR system (Applied Biosystems) | Nasopharyngeal swabs | 83 | E and S | The RealStar®® SARS-CoV-2 demonstrated a slightly higher sensitivity than the WHO recommended assay. Sensitivity: 97.8% Specificity: 97.3% No cross reaction with the other human coronaviruses |
| Yip et al. [84] | LightMix®® Modular E-gene kit (Roche) | LightCycler 480 II Real-Time PCR System (Roche) | Nasopharyngeal aspirate | 289 | E | The LightMix®® E-gene kit had similar sensitivity to the in-house assays. Sensitivity: 51.6% No cross reaction with the other human coronaviruses, metapneumovirus, rhinovirus, adenovirus, respiratory syncytial, influenza, and parainfluenza viruses |
| Nalla et al. [85] | BGI RT-PCR detection kit (BGI) | ABI 7500 real-time PCR system (Applied Biosystems) | Nasopharyngeal and oropharyngeal | 375 | ORF1ab | Specificity: 100% Sensitivity: variation according to the serial dilutions of RNA No cross-reactivity with other respiratory viruses |
| Szymczak et al. [86] | Xpert Xpress SARS-CoV-2 (Cepheid) | GeneXpert Infinity (Cepheid) | Stool | 79 | E and N2 | Positive percent agreement between the Cepheid and Hologic assays was 93% (95% CI: 81.1–98.2%) and negative percent agreement was 96% (95% CI: 89–0.99%). No cross-reactivity with bacterial organisms was found in the gastrointestinal tract |
| Hologic Panther Fusion (Hologic) | Panther Fusion System (Hologic) | ORF1a | ||||
| Pujadas et al. [87] | cobas SARS-CoV-2 RT-PCR test (Roche) | Cobas 6800 System (Roche) | Nasopharyngeal swabs | 1006 | ORF1/a and E | A Cohen’s kappa coefficient was calculated between the definitive results from the two platforms and was found to be 0.904 (95% CI, 0.875–0.933), suggesting almost perfect agreement between both platforms |
| Laboratory-developed test (based on a modified CDC protocol) | LightCycler 480 II (Roche) | N1, N2, and N3 | ||||
| Craney et al. [88] | cobas SARS-CoV-2 RT-PCR test (Roche) | Cobas 6800 System (Roche) | Nasopharyngeal swabs | 389 | ORF1/a and E | The overall percent agreement between the platforms was 96.4% (375/389). Cohen’s kappa analysis rated the strength of agreement between the two platforms as “almost perfect” (κ = 0.922; standard error, 0.051). |
| Panther Fusion SARS-CoV-2 RT-PCR test (Hologic) | Panther Fusion System (Hologic) | ORF1ab | ||||
| Xpert Xpress SARS-CoV-2 RT-PCR test (Cepheid) | - | E and N2 | ||||
| Perng et al. [89] | BD MAX (BD) | BD Max Open System (BD) | Throat swab and sputum | 400 | E and RdRp | Concordant results were obtained for both assays in SARS-CoV-2 detection, showing 100% agreement |
| Laboratory-developed test (based on Charité protocol [48]) | Rotor-Gene Q (Qiagen) | ORF1ab and E | ||||
| van Kasteren et al. [90] | RealStar SARS-CoV-2 RT-PCR Kit 1.0 (Altona Diagnostics) | LightCycler 480 II (Roche) | Naso-and/or oropharyngeal swabs | 22 | E and S | PCR efficiency was ≥96% for all assays and the estimated LOD95 varied within a six-fold range None of the assays showed cross-reactivity with other respiratory (corona)viruses, except as expected for the SARS-CoV-1 E-gene |
| Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2 (BGI) | ORF1ab | |||||
| VIASURE SARS-CoV-2 Real Time PCR Detection Kit (CerTest Biotec) | ORF1ab and N | |||||
| RADI COVID-19 Detection kit (KH Medical) | RdRp and S | |||||
| Coronavirus (COVID-19) (PrimerDesign) | RdRp | |||||
| RIDA GENE SARS-CoV-2 RUO (R-Biopharm AG) | E | |||||
| Allplex™ 2019-nCoV Assay (Seegene) | RdRp, N and E | |||||
| Iglói et al. [91] | RealStar SARS-CoV2 1.0 (Altona Diagnostics) | LightCycler 480 (Roche) and Quantstudio5 (Thermo- fisher Scientific) | SARS-CoV-2 cell-cultured virus stock | E, S | All RT-PCR kits included exhibited PCR efficiencies >90%, except for the Sentinel Diagnostics B E-gene assay (80%) Analytical sensitivity varied between 3.3 RNA copies to 330 RNA copies Only one assay (Powercheck 2019-nCoV) cross reacted with another human coronavirus (MERS). | |
| LightMix Sarbeco-E/SARS-CoV-2 RdRp (Tibmolbiol) | E, RdRp | |||||
| Taqman 2019-nCoV Assay kit v1 (ThermoFisher) | ORF1ab, S, N | |||||
| Detection Kit for 2019-nCoV (DAAN Gene) | ORF1ab, N | |||||
| Powercheck 2019-nCoV (Kogene Biotech) | ORF1ab, E | |||||
| 2019-nCoV realtime multiplex RT-PCR (Liferiver) | ORF1ab, N | |||||
| SARS-CoV2 fluorescent PCR (Maccura Biotechnology) | ORF1ab, E, N | |||||
| Ridagene SARS-CoV2 (R-Biopharm) | E | |||||
| 2019-nCoV nucleic acid diagnostic kit (Sansure Biotech) | ORF1ab, N | |||||
| STAT-NAT COVID19 B (Sentinel Diagnostics B) | E, RdRp, N | |||||
| STAT-NAT COVID19 HK (Sentinel Diagnostics HK) | ORF1ab, N | |||||
| SARS-CoV-2 Realtime PCR assay kit (XABT) | ORF1ab, E, N | |||||
| RT PCR kit for detection of 2019-nCoV (Hecin Scientifi) | RdRp, N | |||||
| Bordi et al. [92] | Simplexa™ COVID-19 Direct assay (DiaSorin Molecular) | LIAISON MDX (DiaSorin Molecular) | Nasal and nasopharyngeal swabs | 278 | ORF1ab and S | Cross-reactive analysis performed in 20 nasopharyngeal swabs confirmed 100% of the clinical specificity of the assay Clinical performances of the Simplexa COVID-19 Direct assay were was compared to the Charité protocol [48]. Concordance analysis showed an “almost perfect” agreement in SARS-CoV-2 RNA detection between the two assays, being κ = 0.938; SE = 0.021; 95% CI = 0.896–0.980 |
| Reference | Commercial Kit Name (Manufacturer) | No. of Patients/Samples | Country of the Test Population | Antibodies Detected | Days from Disease Onset | Main Findings and/or Conclusions |
|---|---|---|---|---|---|---|
| Brochot et al. [158] | Euroimmun SARS-CoV-2 IgG (Euroimmun) | 168 | France | IgG (recombinant S protein) | ≥ 9 | Considering hospitalized patients, all these assays showed a sensitivity of 100% from day 9 after the symptoms onset. However, the sensitivity was much lower for patients who did not require hospitalization for COVID-19 confirmed by PCR with 69% for the Euroimmun assay and 91.6% for the Wantai assay |
| Wantai SARS-CoV-2 Ab ELISA (Beijing Wantai) | Total antibodies IgM and IgG (recombinant spike protein receptor binding domain (RBD)) | |||||
| Kohmer et al. [159] | Euroimmun SARS-CoV-2 IgG (Euroimmun) | 33 | Germany | IgG (recombinant S protein) | 5 to 18 | The sensitivity was 58.8% for the Euroimmun assay and 70.6% for the Vircell assay on days 5–9 after confirmation by RT-qPCR. On the other hand, 10–18 days after confirmation, the sensitivity was 93.8% and 100% for the Vircell assay and Euroimmun assay, respectively |
| Vircell COVID-19 ELISA IgG (Vircell) | IgG (recombinant N protein) | |||||
| Tuaillon et al. [160] | ID Screen SARS-CoV-2-N IgG (ID Vet) | 58 | France | IgG (recombinant N protein) | 1 to ≥15 | The commercial ELISAs demonstrated similar sensitivity of 86.7% with 80–100% specificity, depending on the day the samples were collected. However, IgG and IgA assays by the Euroimmun company suffered from a specificity below 90% |
| SARS-CoV-2 IgA and IgG (Euroimmun) | Either IgA or IgG (recombinant subunit protein 1 (S1)) | |||||
| Lassaunière et al. [161] | Wantai SARS-CoV-2 Ab ELISA (Beijing Wantai) | 111 | Denmark | Total antibodies IgM and IgG (recombinant spike protein RBD) | 7 to ≥21 | The diagnostic performance of the commercial assays analyzed may vary The results showed 100% specificity for the Wantai assay, 96% for the Euroimmun IgG assay, and 93% for the Euroimmun IgA assay, with sensitivities of 90%, 65%, and 90%, respectively |
| Anti-SARS-CoV-2 IgG and IgA (Euroimmun) | Either IgA or IgG (recombinant subunit protein 1 (S1)) | |||||
| Geurtsvan-Kessel et al. [162] | Wantai SARS-CoV-2 total Ig and IgM ELISAs (Beijing Wantai) | 147 | Netherlands | Total antibodies IgM and IgG (recombinant spike protein RBD) and IgM and (recombinant spike protein RBD) | >14 | The Wantai assay was able to detect the total immunoglobulins against the receptor binding domain of SARS CoV-2 in different stages and severities of COVID-19 |
| Anti-SARS-CoV-2 IgG and IgA (Euroimmun) | Either IgA or IgG (recombinant subunit protein 1 (S1)) | |||||
| Jääskeläinen et al. [163] | Anti-SARS-CoV-2 IgG and IgA (Euroimmun) | 37 | Finland | Either IgA or IgG (recombinant subunit protein 1 (S1)) | The results showed a higher specificity of SARS-CoV-2 IgG ELISA (91.9%) than SARS-CoV-2 IgA ELISA (73.0%); therefore, it is not suggested to use the IgA assay for initial screening |
| Reference | Commercial Kit Name (Manufacturer) | No. of Patients/Samples | Country of the Test Population | Days from Disease Onset | Antibodies Detected | Main Findings and/or Conclusions |
|---|---|---|---|---|---|---|
| Cassaniti et al. [188] | VivaDiag COVID-19 IgM/IgG Rapid Test (VivaChek) | 50 | Italy | 7 days | IgM and IgG | The majority of patients that diagnosed as positive for COVID-19 by RT-qPCR would have been identified as negative using only the rapid serological assay, leading to a misdiagnosis of COVID-19 disease |
| Pan et al. [189] | Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Lateral Flow) (Zhuhai Livzon Diagnositic) | 105 | China | 1 to >15 | IgM and IgG | The positive rates of IgG and IgM in the early stages are relatively low and gradually increase during disease progression, where the IgM-positive rate increased from 11.1% to 74.2% according to the progression of the disease, as well as the IgG-positive rate, which initially was 3.6% and increased to 96.8% |
| Shen et al. [190] | Test IgM/IgG SARS-CO-2 (Shanghai Outdo Biotech) | 97 | China | 1 to >15 | IgM and IgG | The kit assay for SARS-Cov-2 specific IgM/IgG antibody demonstrated 71.1% and 96.2% for the sensitivity and specificity, respectively, in this survey population, showing the potential for a useful rapid diagnosis test for COVID-19 |
| Pérez-García et al. [191] | AllTest COV-19 IgG/IgM kit | 163 | Spain | 1 to 17 | IgM and IgG | The specificity found was 100% and the sensitivity of the test was 73.9% after 14 days from the onset of symptoms |
| Spicuzza et al. [192] | 2019-nCoV IgG/IgM An- tibody Rapid Test Kit (Beijing Diagreat Biotechnologies) | 37 | Italy | IgM and IgG | The results reported suggest that the rapid IgG/IgM test was reliable in evidencing seroconversion as long as the testing was not performed <6 days before the symptom onset | |
| Virgilio-Paradiso et al. [193] | SARS-CoV-2 rapid IgG-IgM VivaDiag Test (VivaChek) | 191 | Italy | 1 to >15 | IgM and IgG | In general, the performance of the test at the onset of symptoms was low: sensitivity of 30% and a specificity of 89% with respect to the standard assay but these performances improved after 8 days of symptom appearance. After 10 days of symptoms, the predictive value of the rapid serological test was higher than that of the standard assay |
| Demey et al. [194] | Commercial Name Not Informed (Biotime Biotechnology) | 22 | France | 1 to 24 | IgM and IgG | The median antibody detection time was between 8 and 10 days according to the evaluated kit. In general, all the tests showed a sensitivity of 60% to 80% on day 10, with the increase to 100% on day 15. A single cross-reaction was observed with other human coronavirus infections (HCoV-229E) |
| Commercial Name Not Informed (Autobio Diagnostics) | IgM and IgG | |||||
| Commercial Name Not Informed (ISIA BIO-Technology) | IgM and IgG | |||||
| Commercial Name Not Informed (Biolidics) | IgM and IgG | |||||
| Serrano et al. [195] | Commercial Name Not Informed (Hangzhou Alltest Biotech) | 152 | Spain | 1 to 28 | IgM, IgG and IgG/IgM | In general, the test kits showed variable performances, with the specificity ranging from 88.3% to 100%. The overall results were better for the Guangzhou Wondfo Biotech manufacturer. An ELISA assay was also performed, and the values related to its performance included sensitivities for IgG and IgA of 81.5% and 93.1% and specificities of 100% and 80.6%, respectively. The authors suggested that commercial ELISA assays and LFI tests can be used as complementary tools in COVID-19 diagnosis |
| Commercial Name Not Informed (Wuhan UNscience Biotechnology) | IgM, IgG and IgG/IgM | |||||
| Commercial Name Not Informed (Guangzhou Wondfo Biotech) | IgM/IgG | |||||
| Mlcochova et al. [196] | COVIDIX 2019 SARS-CoV-2 IgG/IgM Test (COVIDIX Healthcare) | 128 | United Kingdom | 1 to 28 | IgG and IgM | Antibody detection by LFIA increased according to the progression of the disease, with 100% efficacy beyond the 9th day post-symptoms |
| SureScreen SARS-CoV-2 IgG/IgM Test (SureScreen Diagnostics) | IgG and IgM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, B.A.S.; Hodel, K.V.S.; Barbosa-Júnior, V.G.; Soares, M.B.P.; Badaró, R. The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature. Viruses 2021, 13, 40. https://doi.org/10.3390/v13010040
Machado BAS, Hodel KVS, Barbosa-Júnior VG, Soares MBP, Badaró R. The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature. Viruses. 2021; 13(1):40. https://doi.org/10.3390/v13010040
Chicago/Turabian StyleMachado, Bruna Aparecida Souza, Katharine Valéria Saraiva Hodel, Valdir Gomes Barbosa-Júnior, Milena Botelho Pereira Soares, and Roberto Badaró. 2021. "The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature" Viruses 13, no. 1: 40. https://doi.org/10.3390/v13010040
APA StyleMachado, B. A. S., Hodel, K. V. S., Barbosa-Júnior, V. G., Soares, M. B. P., & Badaró, R. (2021). The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature. Viruses, 13(1), 40. https://doi.org/10.3390/v13010040






