Reinforcement of Epoxy Resin by Additives of Amine-Functionalized Graphene Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AMG Nanosheets
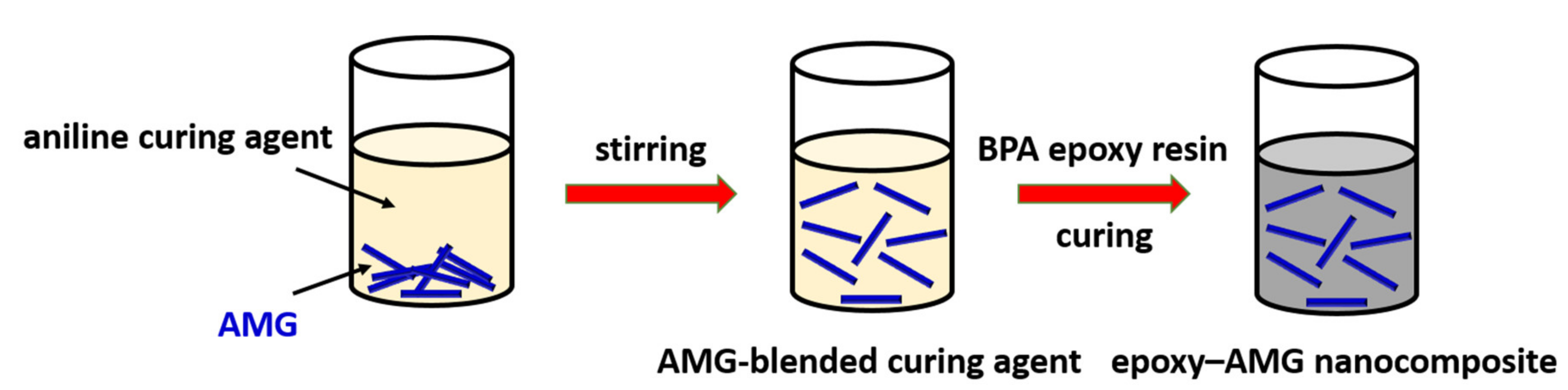
2.3. Preparation of Nanocomposite Cured by Epoxy Resin
2.4. Characterizations
3. Results
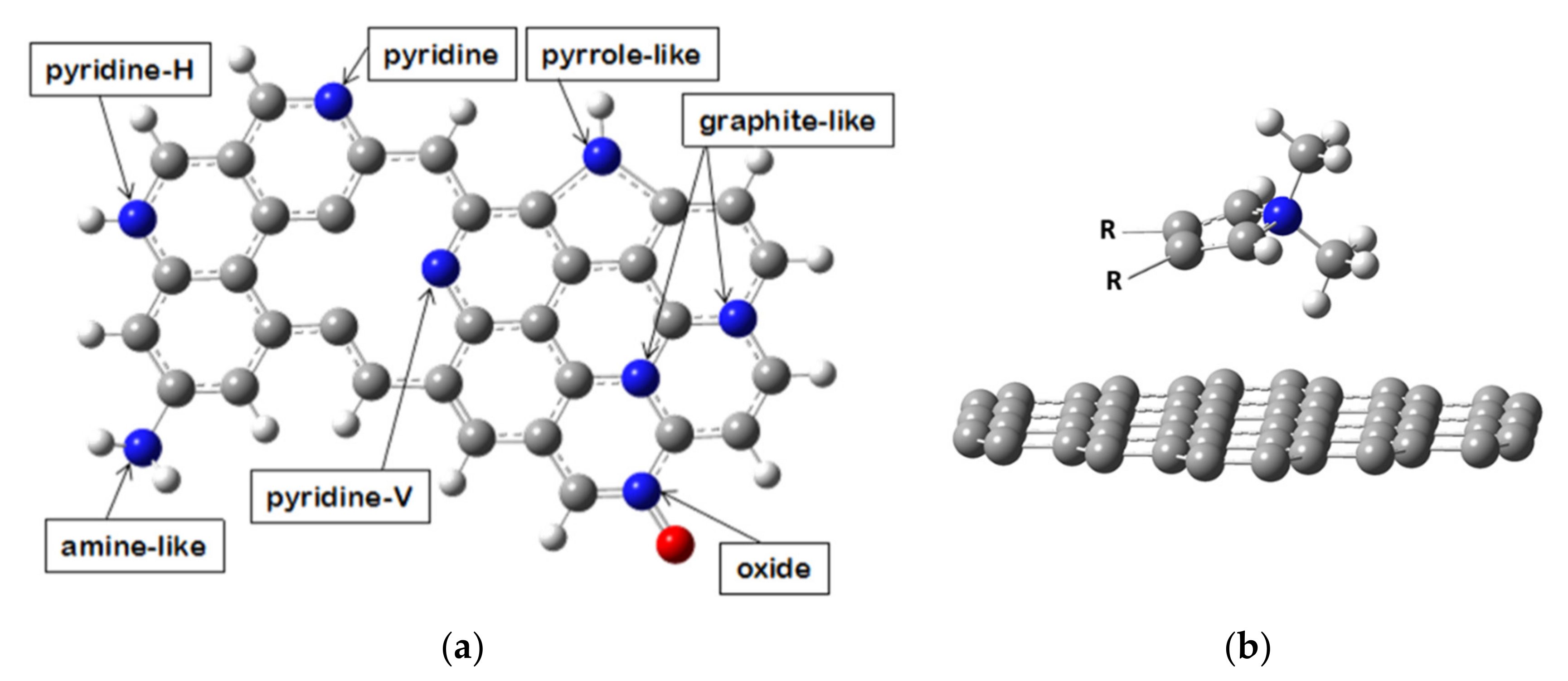
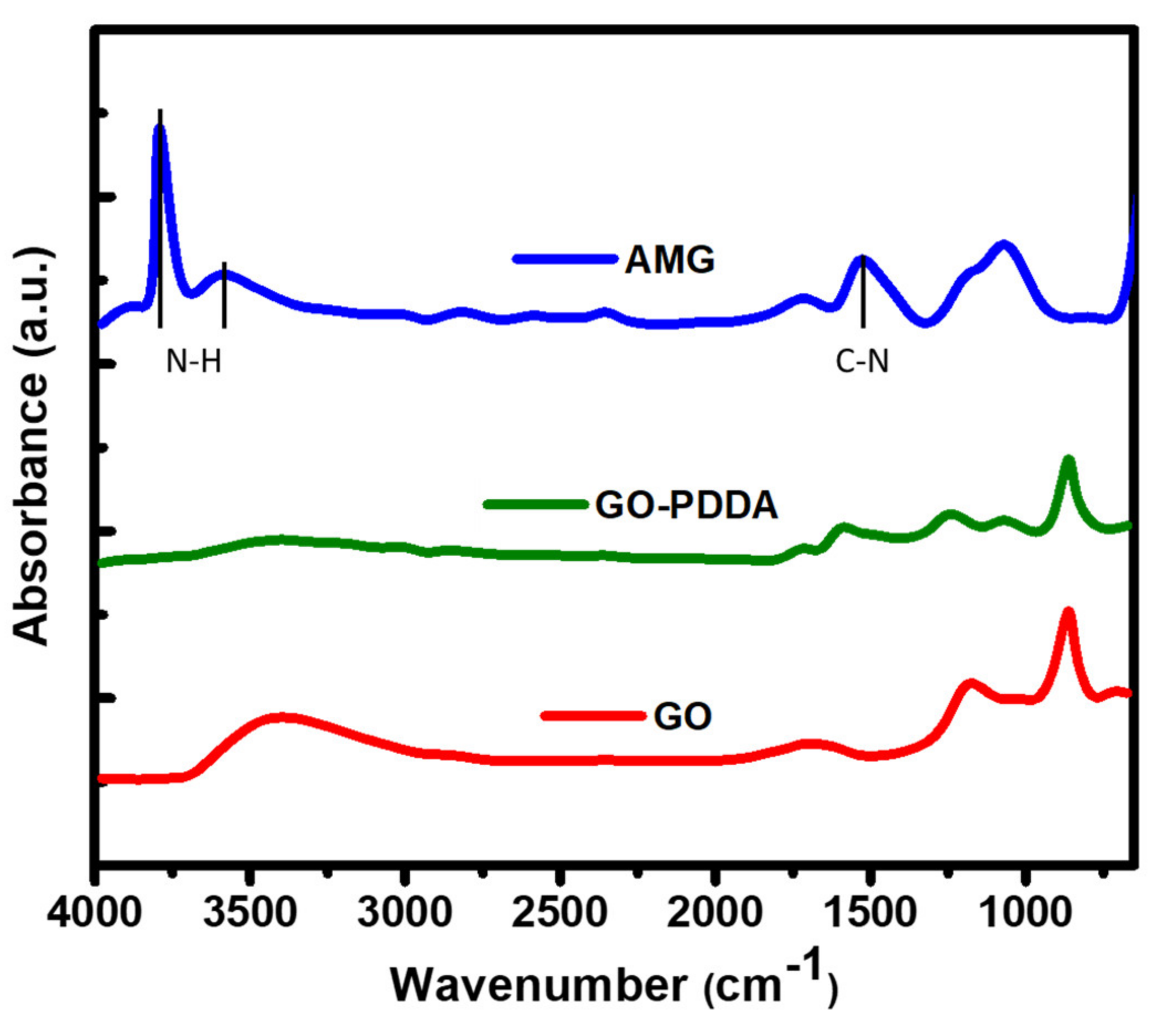
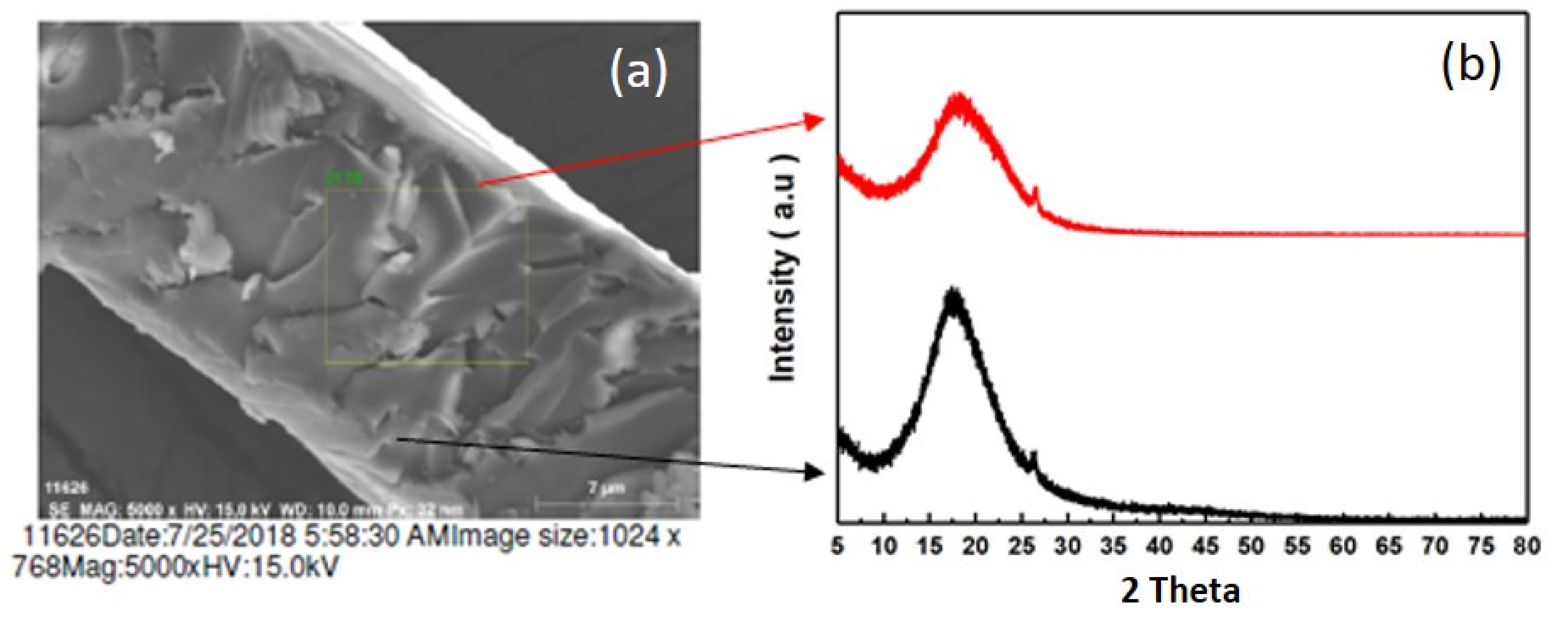
3.1. Structural Categorization
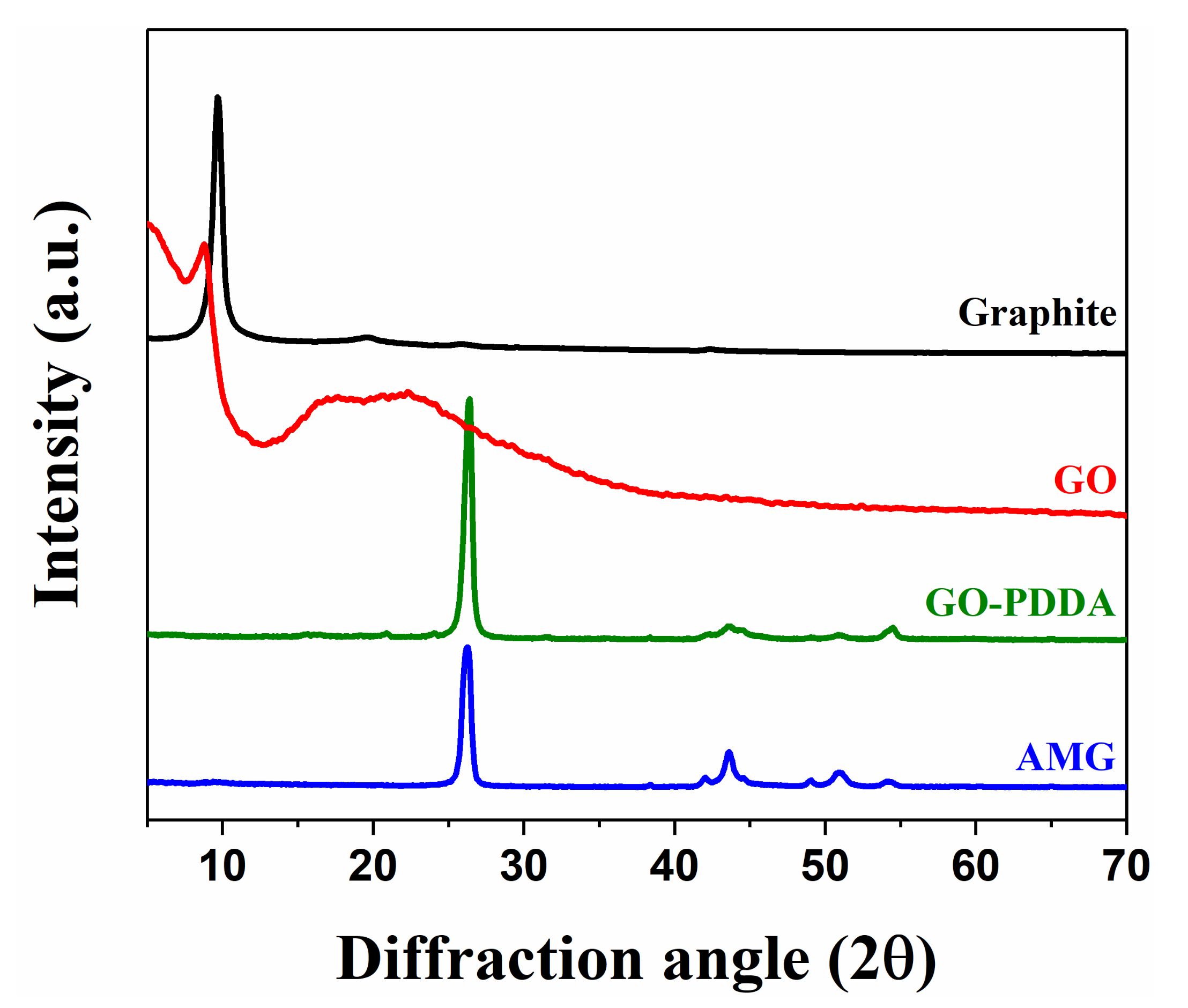
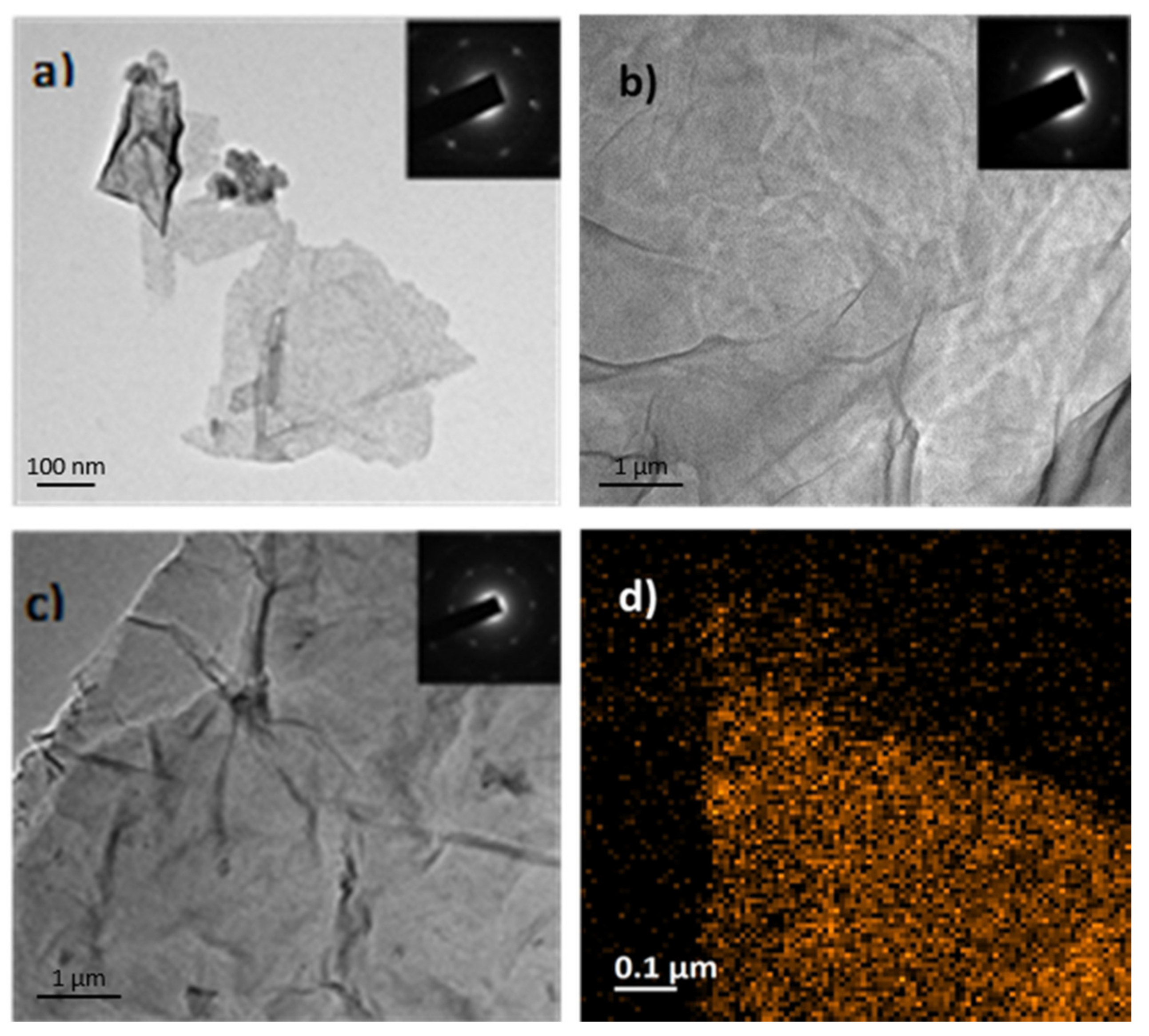
3.2. Characterization of AMG
3.3. Mechanical Tests of the Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daniel, R.D.; Sungjin, P.; Christopher, W.B.; Rodney, S.R. The chemistry of gra-phene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar]
- Byon, H.R.; Gallant, B.M.; Lee, S.W.; Shao-Horn, Y. Role of Oxygen Functional Groups in Carbon Nanotube/Graphene Freestanding Electrodes for High Performance Lithium Batteries. Adv. Funct. Mater. 2013, 23, 1037–1045. [Google Scholar] [CrossRef]
- Deng, W.; Ji, X.; Gomez, M.M.; Lu, F.; Chen, Q.; Banks, C.E. Graphene electrochemical super-capacitors: The influence of oxygen functional groups. Chem. Commun. 2012, 48, 2770–2772. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Luo, Z.; Vora, P.M.; Mele, E.J.; Johnson, A.T.C.; Kikkawa, J.M. Photoluminescence and band gap modulation in graphene oxide. Appl. Phys. Lett. 2009, 94, 111909. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Dilini, G.; Mingchao, W.; Meinan, L.; Nunzio, M.; Eric, W.; Cheng, Y. Recent Advances in Fabrication and Characterization of Graphene-Polymer Nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar]
- Jiacheng, W.; Thuc, V.; Fawad, I. Epoxy/graphene nanocomposites–processing and properties: A review. RSC Adv. 2015, 5, 73510. [Google Scholar]
- Ya, Z.; Le, L.; Yang, C.; Huawei, Z.; Mei, L. Enhanced mechanical properties of epoxy nanocomposites based on graphite oxide with amine-rich surface. RSC Adv. 2015, 5, 98472. [Google Scholar]
- Zhiyi, Z.; Wenhui, Z.; Diansen, L.; Youyi, S.; Zhuo, W.; Chunling, H.; Lu, C.; Yang, C.; Yaqing, L. Mechanical and Anticorrosive Properties of Graphene/Epoxy Resin Composites Coating Prepared by in-Situ Method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar]
- Kavita, S.; Vijayender, B.; Priyanka, S.; Roohi, K.; Shilpa, C.C.; Raman, S. Amine functionalized graphene oxide/CNT nanocomposite for ultrasensitive electrochemical detection of trinitrotoluene. J. Hazard. Mater. 2013, 248, 322–328. [Google Scholar]
- Fengdan, L.; Ling, W.; Yanjie, S.; Wenguang, X.; Kunkun, G. Effect of molecular chain length on the properties of amine-functionalized graphene oxide nanosheets/epoxy resins nanocomposites. RSC Adv. 2015, 5, 45987. [Google Scholar]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-Doping of Graphene Through Electrothermal Reactions with Ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Hulicova, D.; Kodama, M.; Hatori, H. Electrochemical Performance of Nitrogen-Enriched Carbons in Aqueous and Non-Aqueous Supercapacitors. Chem. Mater. 2006, 18, 2318–2326. [Google Scholar] [CrossRef]
- Gunlycke, D.; Li, J.; Mintmire, J.W.; White, C.T. Altering low-bias transport in zigzag-edge graphene nanostrips with edge chemistry. Appl. Phys. Lett. 2007, 91, 112108. [Google Scholar] [CrossRef]
- Pavan, P.; Pravin, B.; Satyendra, M. Influence of amino functionalized graphene oxide on mechanical and thermal properties of epoxy matrix composites. Iran. Polym. J. 2020, 29, 47–55. [Google Scholar]
- Aso, N.; Abdollah, S. Efficient amine functionalization of graphene oxide through the Bucherer reaction: An extraordinary metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 74, 59874–59880. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Wang, S.H.; Liu, C.M.; Chien, M.Y.; Hsu, C.C.; Liu, T.Y. Amino-modified graphene oxide nanoplatelets for photo-thermal and anti-bacterial capability. Surf. Coat. Technol. 2020, 385, 125441. [Google Scholar] [CrossRef]
- Lyaysan, A.; Albina, S.D.B.; Delus, M.; Rustem, A.; Ayrat, M.D. Homogeneous Liquid Phase Transfer of Graphene Oxide into Epoxy Resins. ACS Appl. Mater. Inter.-Faces 2017, 9, 11909–11917. [Google Scholar]
- Jiacheng, W.; Mohd, S.S.; Thuc, V.; Fawad, I. N, N-Dimethylformamide (DMF) Usage in Epoxy/Graphene Nanocomposites: Problems Associated with Reaggregation. Polymers 2017, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Jiacheng, W.; Mohd, S.S.; Thuc, V.; Fawad, I. Dichlorobenzene: An effective solvent for epoxy/graphene nanocomposites preparation. R. Soc. Open Sci. 2017, 4, 170778. [Google Scholar]
- Hontoria, L.C.; López, P.A.J.; López, G.J.D.D.; Rojas, C.M.L.; Martín, A.R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal Suspensions of Highly Reduced Graphene Oxide in a Wide Variety of Organic Solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Yung, T.Y.; Huang, L.Y.; Chan, T.Y.; Wang, K.S.; Liu, T.Y.; Chen, P.T.; Chao, C.Y.; Liu, L.K. Synthesis and characterizations of Ni-NiO nanoparticles on PDDA-modified graphene for oxygen reduction reaction. Nanoscale Res. Lett. 2014, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Seon, G.K.; Ok, K.P.; Joong, H.L.; Bon, C.K. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar]
- Pradeep, K.S.; Kamal, S. Mechanical and Viscoelastic Properties of In-situ Amine Functionalized Multiple Layer Grpahene /epoxy Nanocomposites. Curr. Nanosci. 2018, 14, 252–262. [Google Scholar]
- Prashant, S.K.; Dharmesh, P.H.; Jitendra, B.N.; James, N.; Satyendra, M. Physico-mechanical properties of nano-polystyrene-decorated graphene oxide–epoxy composites. Polym. Intl. 2017, 66, 1402–1409. [Google Scholar]
- Manuel, G.; Akash, M. Investigation of mechanical properties of graphene decorated with graphene quantum dot-reinforced epoxy nanocomposite. J. Appl. Polym. Sci. 2019, 137, 48680. [Google Scholar]


| Mechanical Test, Unit | Epoxy | 1 wt.% AMG | 2 wt.% AMG |
|---|---|---|---|
| Tensile Strength, MPa | 26.32 | 52.15 | 33.57 |
| Tensile Modulus, MPa | 1210 | 3124 | 2206 |
| Elongation, % | 2.34 | 3.51 | 5.72 |
| Bend Strength, MPa | 50.32 | 99.24 | 48.3 |
| Bend Modulus, MPa | 2952 | 3556 | 2252 |
| Compression Strength, MPa | 14.83 | 87.14 | 49.9 |
| Compression Modulus, MPa | 450.4 | 2148 | 1614 |
| AMG Addition | Test 1 | Test 2 | Test 3 | AVG | Improved % |
|---|---|---|---|---|---|
| 0.00 wt.% | 1.167 | 1.167 | 1.275 | 1.203 | 0 |
| 0.50 wt.% | 1.329 | 1.329 | 1.275 | 1.311 | 9.0 |
| 1.00 wt.% | 1.329 | 1.384 | 1.384 | 1.366 | 13.5 |
| 2.00 wt.% | 1.547 | 1.438 | 1.438 | 1.474 | 22.5 |
| 2.67 wt.% | 2.6 | 2.638 | 2.862 | 2.700 | 124.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yung, T.-Y.; Lu, Y.-C.; Chen, J.-S.; Cheng, Y.-W.; Liu, T.-Y.; Chen, P.-T. Reinforcement of Epoxy Resin by Additives of Amine-Functionalized Graphene Nanosheets. Coatings 2021, 11, 35. https://doi.org/10.3390/coatings11010035
Yung T-Y, Lu Y-C, Chen J-S, Cheng Y-W, Liu T-Y, Chen P-T. Reinforcement of Epoxy Resin by Additives of Amine-Functionalized Graphene Nanosheets. Coatings. 2021; 11(1):35. https://doi.org/10.3390/coatings11010035
Chicago/Turabian StyleYung, Tung-Yuan, Yu-Chun Lu, Jeng-Shiung Chen, Yu-Wei Cheng, Ting-Yu Liu, and Po-Tuan Chen. 2021. "Reinforcement of Epoxy Resin by Additives of Amine-Functionalized Graphene Nanosheets" Coatings 11, no. 1: 35. https://doi.org/10.3390/coatings11010035
APA StyleYung, T.-Y., Lu, Y.-C., Chen, J.-S., Cheng, Y.-W., Liu, T.-Y., & Chen, P.-T. (2021). Reinforcement of Epoxy Resin by Additives of Amine-Functionalized Graphene Nanosheets. Coatings, 11(1), 35. https://doi.org/10.3390/coatings11010035







