Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
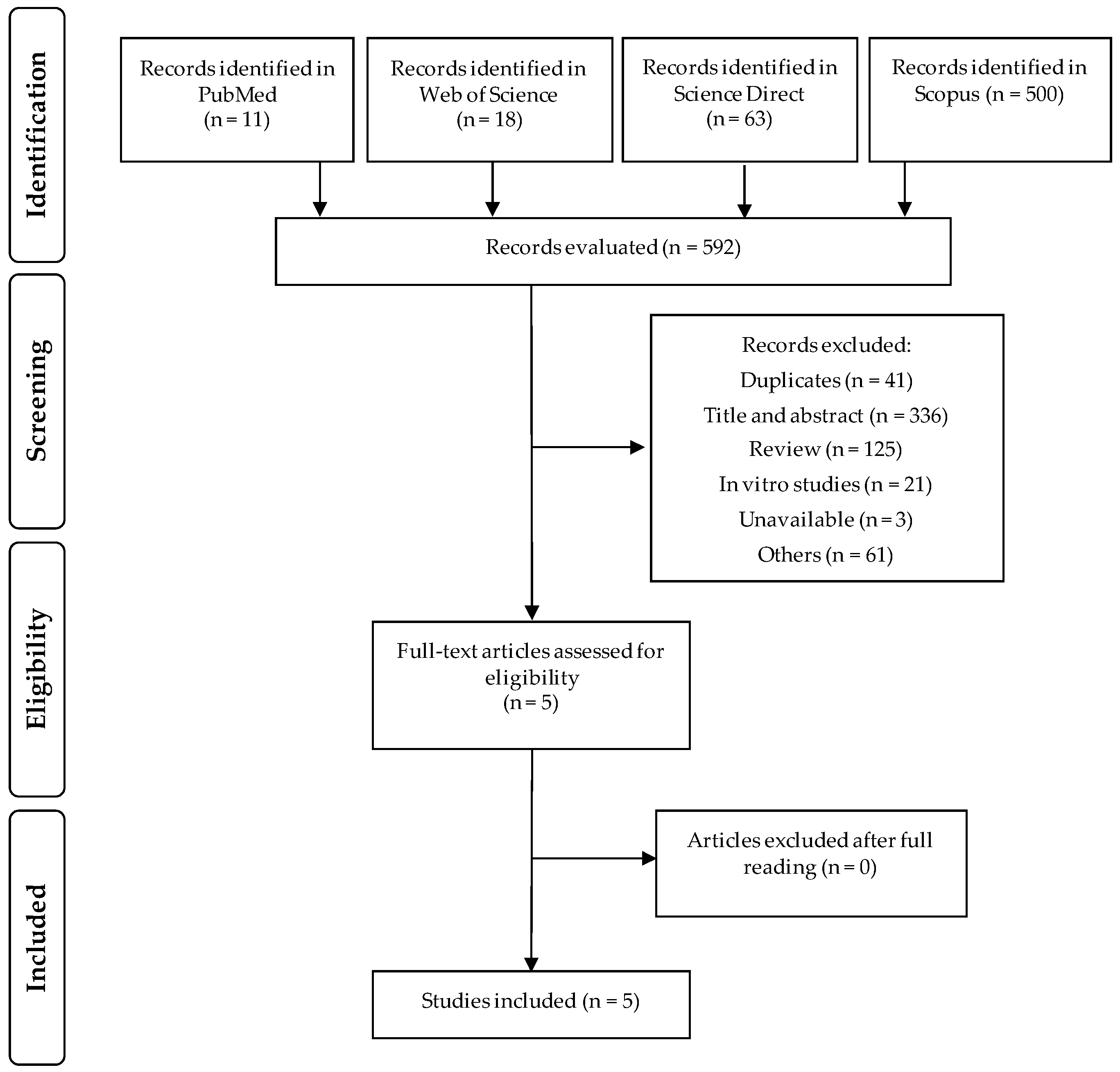
2.3. Screening and Eligibility of Records
2.4. Data Extraction
2.5. Risk-Of-Bias Assessment
3. Results
3.1. Selected Studies
3.2. Characteristics of the Included Studies
3.3. Main Findings
4. Discussion
4.1. Impact of Fe and Zn Biofortification on the Gut Microbiota In Vivo
4.2. Dosages and Reporting Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvestplus. Available online: https://www.harvestplus.org/ (accessed on 25 July 2020).
- Finkelstein, J.L.; Mehta, S.; Udipi, S.A.; Ghugre, P.S.; Luna, S.V.; Wenger, M.J.; Murray-Kolb, L.E.; Przybyszewski, E.M.; Haas, J.D. A Randomized Trial of Iron-Biofortified Pearl Millet in School Children in India. J. Nutr. 2015, 145, 1576–1581. [Google Scholar] [CrossRef] [Green Version]
- Haas, J.D.; Luna, S.V.; Lung’aho, M.G.; Wenger, M.J.; Murray-Kolb, L.E.; Beebe, S.; Gahutu, J.; Egli, I.M. Consuming Iron Biofortified Beans Increases Iron Status in Rwandan Women after 128 Days in a Randomized Controlled Feeding Trial. J. Nutr. 2016, 146, 1586–1592. [Google Scholar] [CrossRef]
- Hotz, C.; Loechl, C.; de Brauw, A.; Eozenou, P.; Gilligan, D.; Moursi, M.; Munhaua, B.; van Jaarsveld, P.; Carriquiry, A.; Meenakshi, J.V. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 2012, 108, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Ndeezi, G.; Nandutu Masawi, A.; Baingana, R.; Carriquiry, A.; de Brauw, A.; Meenakshi, J.V.; et al. Introduction of β-Carotene–Rich Orange Sweet Potato in Rural Uganda Resulted in Increased Vitamin A Intakes among Children and Women and Improved Vitamin A Status among Children. J. Nutr. 2012, 142, 1871–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.J.C.; Lima, S.L.S.; Alves, N.E.G.; Assis, A.; Moreira, M.E.C.; Toledo, R.C.L.; Rosa, C.O.B.; Teixeira, O.R.; Bassinello, P.Z.; De Mejía, E.G.; et al. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, S.L.S.; Gomes, M.J.C.; da Silva, B.P.; Alves, N.E.G.; Toledo, R.C.L.; Theodoro, J.M.V.; de Castro Moreira, M.E.; Bento, J.A.C.; Bassinello, P.Z.; da Matta, S.L.P.; et al. Whole flour and protein hydrolysate from common beans reduce the inflammation in BALB/c mice fed with high fat high cholesterol diet. Food Res. Int. 2019, 122, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.E.G.; Vasconcelos, C.M.; Bassinello, P.Z.; de Mejia, E.G.; Martino, H.S.D. Digested protein isolate from fresh and stored Carioca beans reduced markers of atherosclerosis in oxidized LDL-induced THP-1 macrophages. J. Funct. Foods 2016, 24, 97–111. [Google Scholar] [CrossRef]
- Alves, N.E.G.; de Mejía, E.G.; Vasconcelos, C.M.; Bassinello, P.Z.; Martino, H.S.D. Postharvest storage of Carioca bean (Phaseolus vulgaris L.) did not impair inhibition of inflammation in lipopolysaccharide-induced human THP-1 macrophage-like cells. J. Funct. Foods 2016, 23, 154–166. [Google Scholar] [CrossRef]
- Lindsay, A.; Bruno, B.; Oma, D.; Richard, H. Guidelines on Food Fortification with Micronutrients; World Health Organization, Food and Agricultural Organization of the United Nations: Geneva, Switzerland, 2006; ISBN 92 4 159401 2. [Google Scholar]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [Green Version]
- Abulizi, N.; Quin, C.; Brown, K.; Chan, Y.; Gill, S.; Gibson, D. Gut Mucosal Proteins and Bacteriome Are Shaped by the Saturation Index of Dietary Lipids. Nutrients 2019, 11, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.; Kolba, N.; Binyamin, D.; Ziv, O.; Regini Nutti, M.; Martino, H.; Glahn, R.; Koren, O.; Tako, E. Iron Biofortified Carioca Bean (Phaseolus vulgaris L.)—Based Brazilian Diet Delivers More Absorbable Iron and Affects the Gut Microbiota In Vivo (Gallus gallus). Nutrients 2018, 10, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, B.C.S.; Sarandy, M.M.; Messias, A.C.; Gonçalves, R.V.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Preclinical and clinical relevance of probiotics and synbiotics in colorectal carcinogenesis: A systematic review. Nutr. Rev. 2020, 78, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Jati Kusuma, R.; Ermamilia, A. Fortification of tempeh with encapsulated iron improves iron status and gut microbiota composition in iron deficiency anemia condition. Nutr. Food Sci. 2018, 48, 962–972. [Google Scholar] [CrossRef]
- Leng, B.; Sørensen, M.B.; Kot, W.; Thymann, T.; Krych, L.; Nielsen, D.S. Severe gut microbiota dysbiosis caused by malnourishment can be partly restored during 3 weeks of refeeding with fortified corn-soy-blend in a piglet model of childhood malnutrition. BMC Microbiol. 2019, 19, 277. [Google Scholar] [CrossRef]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Krebs, N.F.; Sherlock, L.G.; Westcott, J.; Culbertson, D.; Hambidge, K.M.; Feazel, L.M.; Robertson, C.E.; Frank, D.N. Effects of Different Complementary Feeding Regimens on Iron Status and Enteric Microbiota in Breastfed Infants. J. Pediatr. 2013, 163, 416–423.e4. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Penders, J.; Shi, Z.; Ren, H.; Cai, K.; Fang, C.; Ding, Q.; Thijs, C.; Blaak, E.E.; Stehouwer, C.D.A.; et al. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Beasley, J.T.; Johnson, A.A.T.; Kolba, N.; Bonneau, J.P.; Glahn, R.P.; Ozeri, L.; Koren, O.; Tako, E. Nicotianamine-chelated iron positively affects iron status, intestinal morphology and microbial populations in vivo (Gallus gallus). Sci. Rep. 2020, 10, 2297. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble extracts from carioca beans (Phaseolus vulgaris L.) affect the gut microbiota and iron related brush border membrane protein expression in vivo (Gallus gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Glahn, R.P.; Koren, O.; Tako, E. Characterizing the gut (Gallus gallus) microbiota following the consumption of an iron biofortified Rwandan cream seeded carioca (Phaseolus vulgaris L.) bean-based diet. PLoS ONE 2017, 12, e0182431. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef]
- Macleod, M.R.; Lawson McLean, A.; Kyriakopoulou, A.; Serghiou, S.; de Wilde, A.; Sherratt, N.; Hirst, T.; Hemblade, R.; Bahor, Z.; Nunes-Fonseca, C.; et al. Risk of Bias in Reports of In Vivo Research: A Focus for Improvement. PLoS Biol. 2015, 13, e1002273. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.; Shlisky, J.; Beaudreault, A.; Bergeron, G.; Blancato, R.; Blumberg, J.; Bourassa, M.; Gomes, F.; et al. Hidden Hunger: Solutions for America’s Aging Populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef] [Green Version]
- Saltzman, A.; Birol, E.; Oparinde, A.; Andersson, M.S.; Asare-Marfo, D.; Diressie, M.T.; Gonzalez, C.; Lividini, K.; Moursi, M.; Zeller, M. Availability, production, and consumption of crops biofortified by plant breeding: Current evidence and future potential. Ann. N. Y. Acad. Sci. 2017, 1390, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.M.; Costa, N.M.B.; Nutti, M.R.; Tako, E.; Martino, H.S.D. Advantages and limitations of in vitro and in vivo methods of iron and zinc bioavailability evaluation in the assessment of biofortification program effectiveness. Crit. Rev. Food Sci. Nutr. 2018, 58, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Tako, E.; Glahn, R.P.; Kolba, N.; de Courcy-Ireland, E.; Stangoulis, J.C.R. Linoleic Acid:Dihomo-γ-Linolenic Acid Ratio Predicts the Efficacy of Zn-Biofortified Wheat in Chicken (Gallus gallus). J. Agric. Food Chem. 2018, 66, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Reed, S.; Anandaraman, A.; Beebe, S.E.; Hart, J.J.; Glahn, R.P. Studies of Cream Seeded Carioca Beans (Phaseolus vulgaris L.) from a Rwandan Efficacy Trial: In Vitro and In Vivo Screening Tools Reflect Human Studies and Predict Beneficial Results from Iron Biofortified Beans. PLoS ONE 2015, 10, e0138479. [Google Scholar] [CrossRef] [Green Version]
- Tako, E.; Bar, H.; Glahn, R. The Combined Application of the Caco-2 Cell Bioassay Coupled with In Vivo (Gallus gallus) Feeding Trial Represents an Effective Approach to Predicting Fe Bioavailability in Humans. Nutrients 2016, 8, 732. [Google Scholar] [CrossRef]
- Hou, T.; Kolba, N.; Glahn, R.; Tako, E. Intra-Amniotic Administration (Gallus gallus) of Cicer arietinum and Lens culinaris Prebiotics Extracts and Duck Egg White Peptides Affects Calcium Status and Intestinal Functionality. Nutrients 2017, 9, 785. [Google Scholar] [CrossRef] [Green Version]
- International Chicken Genome Sequencing Consortium Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004, 432, 695–716. [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef] [PubMed]
- del Campo, R.; Garriga, M.; Pérez-Aragón, A.; Guallarte, P.; Lamas, A.; Máiz, L.; Bayón, C.; Roy, G.; Cantón, R.; Zamora, J.; et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: A double blind prospective study. J. Cyst. Fibros. 2014, 13, 716–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol. Liver Physiol. 2010, 299, G1087–G1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinda, D.; Nakaji, S.; Fukuda, S.; Sakamoto, J.; Shimoyama, T.; Nakamura, T.; Fujisawa, T.; Terada, A.; Sugawara, K. The Fermentation of Different Dietary Fibers Is Associated with Fecal Clostridia Levels in Men. J. Nutr. 2004, 134, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef] [Green Version]
- Lakes, J.E.; Richards, C.I.; Flythe, M.D. Inhibition of Bacteroidetes and Firmicutes by select phytochemicals. Anaerobe 2020, 61, 102145. [Google Scholar] [CrossRef]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef]
- O’Connor, A.; Quizon, P.M.; Albright, J.E.; Lin, F.T.; Bennett, B.J. Responsiveness of cardiometabolic-related microbiota to diet is influenced by host genetics. Mamm. Genome 2014, 25, 583–599. [Google Scholar] [CrossRef] [Green Version]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [Green Version]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enes, B.N.; Moreira, L.P.D.; Silva, B.P.; Grancieri, M.; Lúcio, H.G.; Venâncio, V.P.; Mertens-Talcott, S.U.; Rosa, C.O.B.; Martino, H.S.D. Chia seed (Salvia hispanica L.) effects and their molecular mechanisms on unbalanced diet experimental studies: A systematic review. J. Food Sci. 2020, 85, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Holman, L.; Head, M.L.; Lanfear, R.; Jennions, M.D. Evidence of Experimental Bias in the Life Sciences: Why We Need Blind Data Recording. PLoS Biol. 2015, 13, e1002190. [Google Scholar] [CrossRef]

| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | In vivo animal studies | Human studies; in vitro studies; pregnancy and lactation; pathologies different from obesity and micronutrient deficiency |
| Intervention | Biofortified foods with some micronutrient or their fractions (e.g., flour, soluble extracts) | Do not correlate biofortified foods and gut microbiota; ultra-processed foods; biofortification with compounds different from vitamins and minerals; supplementation |
| Comparison | Standard foods, or their standard fractions; standard diet for rodents, with no biofortified foods | No control group |
| Outcomes | Modulation of the health gut microbiota and decrease of pathogenic bacteria | |
| Study design | Experimental placebo-controlled studies | In vitro studies; reviews; consensus papers; letters to editor; books; book chapters; theses and dissertations; non- animal studies; studies with more than 10 years from publication date |
| Reference | Animal Model | Sex/Age | Number of Animals | Type of Food to Intervention | Method of Administration | Duration of Intervention (wk) |
|---|---|---|---|---|---|---|
| Reed et al., 2018 [31] | Cornish Cross broiler (Gallus gallus) | Male and female/Hatchlings | 30 (n = 15 per group) | Zinc biofortified wheat (Triticum aestivum) | Oral (in diet) | 6 |
| Reed et al., 2017 [30] | Cornish Cross broiler (Gallus gallus) | Male and female/Hatchlings | 28 (n = 14 per group) | Iron biofortified carioca bean (Phaseolus vulgaris L.) | Oral (in diet) | 6 |
| Dias et al., 2018 [15] | Cornish Cross broiler (Gallus gallus) | Male and female/Hatchlings | 28 (n = 14 per group) | Iron biofortified carioca bean (Phaseolus vulgaris L.) | Oral (in diet) | 6 |
| Dias et al., 2019 [29] | Chicken embryos (Gallus gallus) | Male and female/Day 17th of embryonic incubation | 80 eggs (n = 10 per group) | Iron biofortified carioca beans (Phaseolus vulgaris L.) | Intra-amniotic administration (1 mL per egg) | 17th day to 21st day |
| Beasley et al., 2020 [28] | 1st experiment: Chicken embryos (Gallus gallus) 2nd experiment: Cornish Cross broiler (Gallus gallus) | 1st experiment: Male and female/ Day 17th of embryonic incubation 2nd experiment: Male and female/Hatchlings | 1st experiment: 40 eggs (n ≥ 5 per group) 2nd experiment: 30 (n = 15 per group) | Iron biofortified wheat (Triticum aestivum L.) | 1st experiment: Intra-amniotic administration (1 mL per egg) 2nd experiment: Oral (in diet) | 1st experiment: 17th day to 21st day 2nd experiment: 6 |
| Reference | Experimental Groups | Method of Evaluation of the Gut Microbiota | Microbial Activity |
|---|---|---|---|
| Zn-biofortified food | |||
| Reed et al., 2018 [31] | CZn: Standard wheat (75% wheat-based diet; 32.8 ± 0.17 µg Zn/g) BZn: Zn biofortified wheat (75% Zn wheat-based diet; 46.5 ± 0.99 µg Zn/g) | 16S rRNA gene sequencing | Change in β-diversity between the CZn and BZn groups. ↔ no difference in abundance between Firmicutes, Actinobacteria, and Proteobacteria phyla according taxon-based analysis; ↔ no differences between groups at the genus level, according taxon-based analysis. LEfSe method: ↑ Lactobacillus reuteri and members of the Dorea, Clostridiales, Ruminococcus and Lachnospiraceae family in BZn group. |
| Fe-biofortified foods | |||
| Reed et al., 2017 [30] | SFe: Fe standard, 34.6% cream seeded carioca bean based diet (33.7 ± 0.80 μg Fe/g) BFe: Fe biofortified bean, 34.6% cream seeded carioca bean based diet (48.7 ± 1.50 μg Fe/g) | 16S rRNA gene sequencing | No change in β-diversity between the BFe and SFe groups; no difference in α-diversity between groups. ↑ Elusimicrobioa and Euryarchaeota phyla; ↑ Dehalobacteriaceae and Enterococcaceae family; ↑ unclassified Dehalobacteriaceae genus in the BFe group. ↓ Elusimicrobiaceae, Methanobacteriaceae, and Methanomassiliicoccaceae family; ↓ unclassified Elusimicrobiaceae, Methanobrevibacter, vadinCA11, and Enterococcus genus in the BFe group; LEfSe method: ↑ Proteobacteria and Firmicutes; ↓ Elusimicrobiota and Euryarchaeota at phylum level; ↑ Campylobacterales; ↓ Enterobacteriales, Elusimicrobiales, Bacteroidales and E2 at order level; ↑ Helicobacteraceae, Dehalobacteriaceae, and Streptococcaceae; ↓ Enterobacteriaceae, Enterococcaceae, Elusimicrobiaceae, Coriobacteriaceae, Methanomassiliicoccaceae, and Methanobacteriaceae at family level; ↑ Helicobacter, Ruminococcus, Coprococcus, and Streptococcus; ↓ Lachnospira, Enterococcus, vadinCA11, Methanobacterium, and Methanobrevibacter at genus level; ↑ OTUs enriched Faecalibacterium prausnitzii, Barnesiella viscericola, Enterococcus cecorum, and vadinCA11 in the BFe group. |
| Dias et al., 2018 [15] | SC: Fe-standard carioca bean-based diet, 42% BRS Perola bean-based diet (40.47 ± 1.84 μg Fe/g) BC: Fe-biofortified carioca bean-based diet, 42% BRS Cometa bean (47.04 ± 1.52 μg Fe/g) | 16S rRNA gene sequencing | Change in β-diversity between the BFe and SFe groups; no difference in α-diversity between groups; ↔ no significant differences between groups at the genus level; LEfSe method: Predominance of SCFA-producing Firmicutes in BC group; ↑ Eggerthella lenta and Clostridium piliforme; members of the Coriobacteriaceae, Dehalobacteriaceae and Lachnospiraceae in the BC group. |
| Dias et al., 2019 [29] | Non-injected 18 MΩH2O Inulin (40 mg/mL) Perola bean extract (Fe standard carioca bean, 3.2 ± 1.5 μg Fe/g) Cometa bean extract (Fe biofortified carioca bean, 1.8 ± 1.1 μg Fe/g) * Esteio bean extract (Fe standard black bean, 1.1 ± 0.6 μg Fe/g) * SMN 39 bean extract (Fe biofortified black bean, 2.2 ± 0.7 μg Fe/g) * Artico bean extract (Fe standard white bean,) * 6.0 ± 1.1 μg Fe/g | PCR amplification of bacterial 16S rDNA for Lactobacillus, Bifidobacterium, Clostridium and E. coli | ↓ relative abundance of Bifidobacterium in biofortified carioca bean extract compared to standard; ↓ relative abundance of E. coli in biofortified carioca bean extract compared to standard; ↑ relative abundance of Lactobacillus in biofortified black bean extract compared to standard; ↑ relative abundance of Clostridium and E. coli in biofortified black bean extract compared to standard; ↔ relative abundance of Lactobacillus and Clostridium in biofortified carioca bean extract compared to standard; ↔ relative abundance of Bifidobacterium in biofortified black bean extract compared to standard. |
| Beasley et al., 2020 [28] | 1st experiment: NI: non-injected H2O: 18 MΩH2O Fe: Fe solution (1 mg/mL) Fe-EDTA: Fe-EDTA solution (77 μM Fe) Fe-NA: Fe-Nicotinamine solution (1.6 mM) C WF: Control wheat flour extract * (0.91 μg Fe/g of extract) B WF: Fe biofortified wheat flour extract * (0.82 μg Fe/g of extract) * 50 mg/mL 2nd experiment: Control: Fe-standard wheat, 80% wheat based diet (25.9 ± 0.12 μg Fe/g) Biofortified: Fe-biofortified wheat, 80% Fe wheat-based diet (28.9 ± 0.13 μg Fe/g) | 1st experiment: PCR amplification of bacterial 16S rDNA for Lactobacillus, Bifidobacterium, Escherichia and Clostridium 2nd experiment: 16S rRNA gene sequencing | 1st experiment: ↔ relative abundance of Bifidobacterium, Lactobacillus, Escherichia and Clostridium in biofortified wheat flour extract compared to the Control. 2nd experiment: Change in β-diversity and α-diversity between the Control and Biofortified groups; ↑ 1.9-fold the proportion of Actinobacteria; ↓ 1.2- and 2.0-fold, respectively, the proportion of Firmicutes and Proteobacteria in ‘Biofortified’ relative to ‘Control’ group at phyla level; ↑ 1.9- and 1.5-fold, respectively, the proportion of Bifidobacterium and Lactobacillus; ↑ abundance of Enterococcus; ↓ proportion of Streptococcus (1.7-fold), Coprococcus (1.4-fold), Ruminococcus (1.2-fold) Faecalibacterium (2-fold), and Escherichia (2-fold); ↓ Dorea abundance in ‘Biofortified’ relative to ‘Control’ group at genera level; ↓ 1.7-fold the proportion of Lachnospiraceae and ↑ abundance of Enterococcaceae families in ‘Biofortified’ relative to ‘Control’ group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juste Contin Gomes, M.; Stampini Duarte Martino, H.; Tako, E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients 2021, 13, 189. https://doi.org/10.3390/nu13010189
Juste Contin Gomes M, Stampini Duarte Martino H, Tako E. Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients. 2021; 13(1):189. https://doi.org/10.3390/nu13010189
Chicago/Turabian StyleJuste Contin Gomes, Mariana, Hércia Stampini Duarte Martino, and Elad Tako. 2021. "Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review" Nutrients 13, no. 1: 189. https://doi.org/10.3390/nu13010189
APA StyleJuste Contin Gomes, M., Stampini Duarte Martino, H., & Tako, E. (2021). Effects of Iron and Zinc Biofortified Foods on Gut Microbiota In Vivo (Gallus gallus): A Systematic Review. Nutrients, 13(1), 189. https://doi.org/10.3390/nu13010189






