Selected Tea and Tea Pomace Extracts Inhibit Intestinal ?-Glucosidase Activity in Vitro and Postprandial Hyperglycemia in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rat α-Glucosidase Inhibitory Activity

| IC50 (mg/mL) | ||||
|---|---|---|---|---|
| Green Tea | Oolong Tea | Black Tea | ||
| Rat intestinal α-glucosidase | TWE | 2.04 ± 0.31 C,cd | 2.33 ± 0.25 B,b | 2.73 ± 0.15 A,a |
| TPE | 1.95 ± 0.37 B,d | 2.22 ± 0.52 A,bc | 2.14 ± 0.21 AB,b−d | |
2.2. Sucrase, Maltase, and Glucoamylase Inhibition Assay
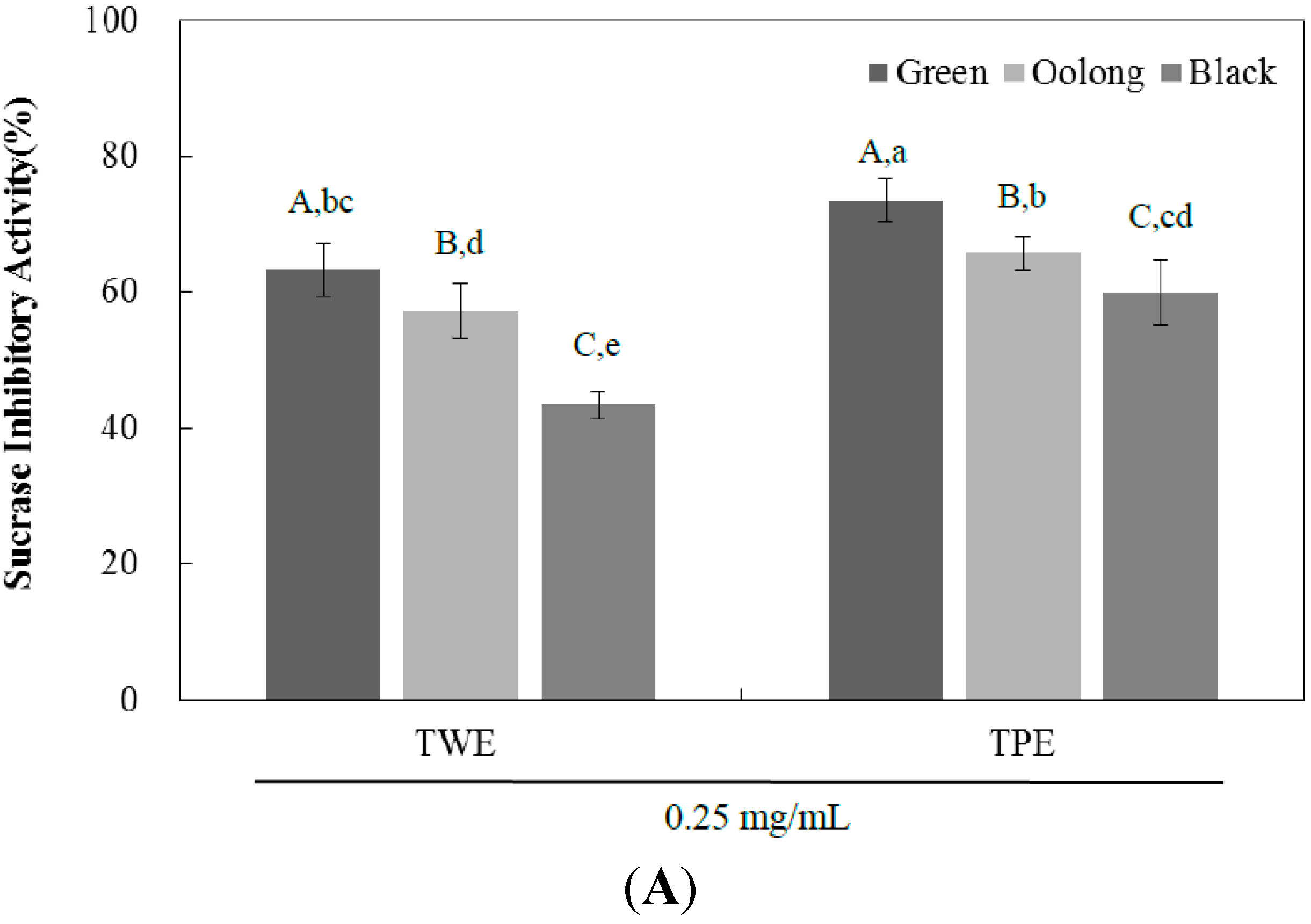

| IC50 (mg/mL) | ||||
|---|---|---|---|---|
| Green Tea | Oolong Tea | Black Tea | ||
| Sucrase | TWE | 0.16 ± 0.01 C,c | 0.19 ± 0.01 B,b | 0.31 ± 0.04 A,a |
| TPE | 0.13 ± 0.01 C,d | 0.16 ± 0.01 B,c | 0.19 ± 0.01 A,b | |
| Maltase | TWE | 0.22 ± 0.01 B,d | 0.22 ± 0.01 B,d | 0.37 ± 0.03 A,a |
| TPE | 0.22 ± 0.01 C,d | 0.26 ± 0.01 B,c | 0.35 ± 0.03 A,b | |
| Glucoamylase | TWE | 0.22 ± 0.01 B,b | 0.23 ± 0.01 B,b | 0.37 ± 0.05 A,a |
| TPE | 0.24 ± 0.02 B,b | 0.25 ± 0.01 B,b | 0.38 ± 0.01 A,a | |
2.3. Total Phenolic Contents of Tea Extracts
| Total Phenolic Contents | ||||
|---|---|---|---|---|
| Green Tea | Oolong Tea | Black Tea | ||
| g/100 g dried wt of extract | TWE | 48.90 ± 1.14 A,d | 48.65 ± 1.24 A,d | 40.14 ± 1.04 B,e |
| (GAE) | TPE | 63.94 ± 1.66 A,a | 61.82 ± 0.38 A,b | 58.24 ± 0.78 B,c |
2.4. Antioxidant Activity by DPPH Radical Scavenging Assay

| IC50 (µg/mL) | ||||
|---|---|---|---|---|
| Green | Oolong | Black | ||
| DPPH | TWE | 15.33 ± 2.61 B,b | 16.14 ± 1.25 B,b | 20.57 ± 0.78 A,a |
| TPE | 9.21 ± 1.86 B,c | 16.06 ± 1.17 B,b | 18.05 ± 0.50 A,a | |

2.5. Sucrose Loading Test


3. Materials and Methods
3.1. Materials
3.2. Preparation of Tea and Tea Pomace Extracts
3.3. Rat α-Glucosidase Inhibition Assay
3.4. Sucrase, Maltase, Glucoamylase Inhibition
3.5. Total Phenolic Contents
3.6. Antioxidant Activity by 2,2-Diphenyl-1-picrylhydrezyl Radical (DPPH) Inhibition Assay
3.7. Sucrose-Loading Test in Rats
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.M.; Jeong, Y.K.; Wang, M.H.; Lee, W.Y.; Rhee, H.I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Yin, P.; Yan, L.; Han, J.; Shi, L.; Zhou, X.; Liu, Y.; Ma, C. α-Glucosidase inhibitory activity of polyphenols from the burs of Castanea mollissima Blume. Molecules 2014, 19, 8373–8386. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rand, J.S.; Coradini, M.; Morton, J.M. Effect of acarbose on postprandial blood glucose concentrations in healthy cats fed low and high carbohydrate diets. J. Feline Med. Surg. 2014. [Google Scholar] [CrossRef]
- Jo, S.H.; Ha, K.S.; Moon, K.S.; Lee, O.H.; Jang, H.D.; Kwon, Y.I. In vitro and in vivo anti-hyperglycemic effects of Omija (Schizandra chinensis) fruit. Int. J. Mol. Sci. 2011, 12, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Shi, J.C.; Mao, X.M. Safety and efficacy of acarbose in the treatment of diabetes in Chinese patients. Ther. Clin. Risk Manag. 2014, 10, 505–511. [Google Scholar] [PubMed]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar] [PubMed]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; van Chuyen, N. α-Glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and alpha-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- De Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(α)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–862. [Google Scholar]
- Hanamura, T.; Hagiwara, T.; Kawagishi, H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC.) fruit. Biosci. Biotechnol. Biochem. 2005, 69, 280–286. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of alpha-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Fukami, H.; Asami, S.; Toyoda, Y.; Nakai, M.; Shibata, H.; Yao, X.S. Effects of oolong tea on plasma antioxidative capacity in mice loaded with restraint stress assessed using the oxygen radical absorbance capacity (ORAC) assay. Biol. Pharm. Bull. 2004, 27, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Wang, M.F.; Liao, M.L.; Chuang, C.K.; Iha, M.; Clevidence, B.; Yamamoto, S. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care 2003, 26, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A.; Grp, J.S. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Juan, C.C.; Hwang, L.S.; Hsu, Y.P.; Ho, P.H.; Ho, L.T. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur. J. Nutr. 2004, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting α-glucosidase. Nutr. Metab. 2010, 7, 71. [Google Scholar] [CrossRef]
- Rhyu, J.; Kim, M.S.; You, M.K.; Bang, M.A.; Kim, H.A. Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Wilkes, S.E.; Rogers, T.J.; Prior, R.L. Effect of dietary blueberry pomace on selected metabolic factors associated with high fructose feeding in growing Sprague-Dawley rats. J. Med. Food 2012, 15, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Striegel, L.; Kang, B.; Pilkenton, S.J.; Rychlik, M.; Apostolidis, E. Effect of black tea and black tea pomace polyphenols on α-glucosidase and α-amylase inhibition, relevant to type 2 diabetes prevention. Front. Nutr. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A. Method for assay of intestinal disaccharidases. Anal. Biochem. 1964, 7, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U.; Bernt, E. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 1205–1212. [Google Scholar]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Jo, S.-H.; Kim, J.S.; Ha, K.-S.; Lee, J.-Y.; Choi, H.-Y.; Yu, S.-Y.; Kwon, Y.-I.; Kim, Y.-C. Selected Tea and Tea Pomace Extracts Inhibit Intestinal ?-Glucosidase Activity in Vitro and Postprandial Hyperglycemia in Vivo. Int. J. Mol. Sci. 2015, 16, 8811-8825. https://doi.org/10.3390/ijms16048811
Oh J, Jo S-H, Kim JS, Ha K-S, Lee J-Y, Choi H-Y, Yu S-Y, Kwon Y-I, Kim Y-C. Selected Tea and Tea Pomace Extracts Inhibit Intestinal ?-Glucosidase Activity in Vitro and Postprandial Hyperglycemia in Vivo. International Journal of Molecular Sciences. 2015; 16(4):8811-8825. https://doi.org/10.3390/ijms16048811
Chicago/Turabian StyleOh, Jungbae, Sung-Hoon Jo, Justin S. Kim, Kyoung-Soo Ha, Jung-Yun Lee, Hwang-Yong Choi, Seok-Yeong Yu, Young-In Kwon, and Young-Cheul Kim. 2015. "Selected Tea and Tea Pomace Extracts Inhibit Intestinal ?-Glucosidase Activity in Vitro and Postprandial Hyperglycemia in Vivo" International Journal of Molecular Sciences 16, no. 4: 8811-8825. https://doi.org/10.3390/ijms16048811
APA StyleOh, J., Jo, S.-H., Kim, J. S., Ha, K.-S., Lee, J.-Y., Choi, H.-Y., Yu, S.-Y., Kwon, Y.-I., & Kim, Y.-C. (2015). Selected Tea and Tea Pomace Extracts Inhibit Intestinal ?-Glucosidase Activity in Vitro and Postprandial Hyperglycemia in Vivo. International Journal of Molecular Sciences, 16(4), 8811-8825. https://doi.org/10.3390/ijms16048811





