Photocatalytic Oxidation of Natural Organic Matter in Water
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Analytical Techniques to Detect and Quantify NOM in Water
3.2. NOM Photocatalytic Treatment
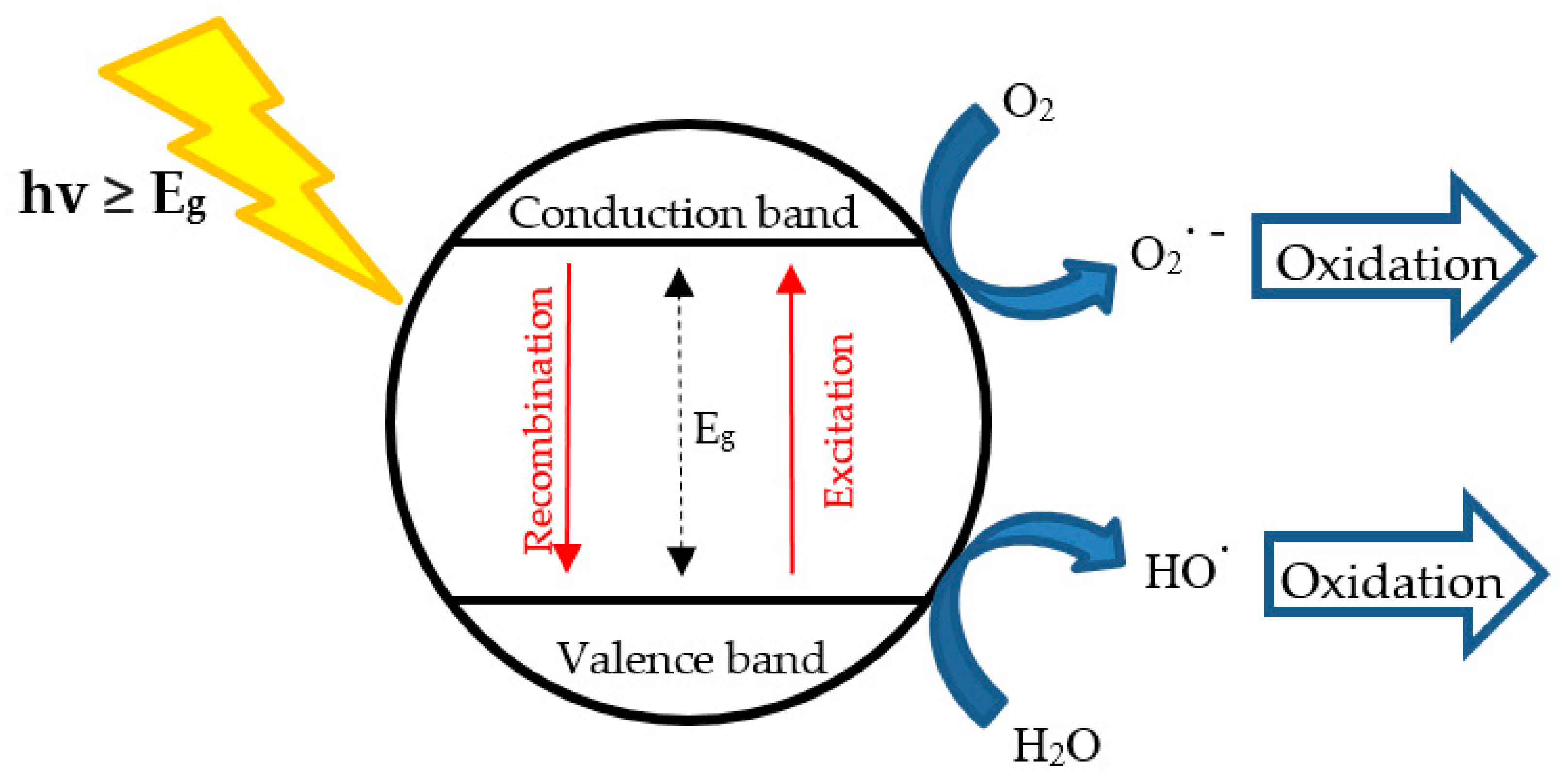
3.2.1. Heterogeneous TiO2 Photocatalysis
Catalyst and NOM Concentration
UV-Light Driven TiO2 Catalysts
Visible Light Driven Modified TiO2 Catalysts
Immobilized Catalysts
Hybrid Processes
3.2.2. Homogeneous Photocatalysis
Photo-Fenton
Hybrid Photolytic Oxidation Processes
Hydrogen Peroxide Based Photocatalysis
Ozone Based Photocatalysis
3.2.3. Energy Efficiency of NOM Treatments
4. Conclusions and Considerations for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Volk, C.; Wood, L.; Johnson, B.; Robinson, J.; Zhu, H.W.; Kaplan, L. Monitoring dissolved organic carbon in surface and drinking waters. J. Environ. Monit. 2002, 4, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Bolto, B.; Dixon, D.; Eldridge, R.; King, S.; Linge, K. Removal of natural organic matter by ion exchange. Water Res. 2002, 36, 5057–5065. [Google Scholar] [CrossRef]
- Reyes, T.G.; Crisosto, J.M. Characterization of Dissolved Organic Matter in River Water by Conventional Methods and Direct Sample Analysis-Time of Flight-Mass Spectrometry. J. Chem. 2016, 2016, 1537370. [Google Scholar] [CrossRef]
- Worrall, F.; Harriman, R.; Evans, C.; Watts, C.; Adamson, J.; Neal, C.; Tipping, E.; Burt, T.; Grieve, I.; Monteith, D.; et al. Trends in Dissolved Organic Carbon in UK Rivers and Lakes. Biogeochemistry 2004, 70, 369–402. [Google Scholar] [CrossRef]
- Zhang, J.; Hudson, J.; Neal, R.; Sereda, J.; Clair, T.; Turner, M.; Jeffries, D.; Dillon, P.; Molot, L.; Somers, K.; et al. Long-term patterns of dissolved organic carbon in lakes across eastern Canada: Evidence of a pronounced climate effect. Limnol. Oceanogr. 2010, 55, 30–42. [Google Scholar] [CrossRef]
- Dong, Q.; Li, P.; Huang, Q.; Abdelhafez, A.A.; Chen, L. Occurrence, polarity and bioavailability of dissolved organic matter in the Huangpu River, China. J. Environ. Sci. 2014, 26, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Eikebrokk, B.; Vogt, R.D.; Liltved, H. NOM increase in Northern European source waters: Discussion of possible causes and impacts on coagulation/contact filtration processes. Water Supply 2004, 4, 47–54. [Google Scholar] [CrossRef]
- Kaal, J.; Plaza, C.; Nierop, K.; Pérez-Rodríguez, M.; Biester, H. Origin of dissolved organic matter in the Harz Mountains (Germany): A thermally assisted hydrolysis and methylation (THM-GC–MS) study. Geoderma 2020, 378, 114635. [Google Scholar] [CrossRef]
- Selle, B.; Knorr, K.-H.; Lischeid, G. Mobilisation and transport of dissolved organic carbon and iron in peat catchments—Insights from the Lehstenbach stream in Germany using Generalised Additive Models. Hydrol. Process. 2019, 33, 3213–3225. [Google Scholar] [CrossRef]
- Korth, A.; Fiebiger, C.; Bornmann, K.; Schmidt, W. NOM increase in drinking water reservoirs—Relevance for drinking water production. Water Sci. Technol. Water Supply 2004, 4, 55–60. [Google Scholar] [CrossRef]
- Grunewald, K.; co-author, as. Increase in Colour and Amount of Organic Matter in Surface Waters, 2003.
- Ritson, J.P.; Graham, N.J.D.; Templeton, M.R.; Clark, J.M.; Gough, R.; Freeman, C. The impact of climate change on the treatability of dissolved organic matter (DOM) in upland water supplies: A UK perspective. Sci. Total Environ. 2014, 473–474, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Leenheer, J.A.; Croué, J.-P. Peer Reviewed: Characterizing Aquatic Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N. Performance and cost estimation of nanofiltration for surface water treatment in drinking water production. Desalination 2006, 196, 55–65. [Google Scholar] [CrossRef]
- Krzeminski, P.; Vogelsang, C.; Meyn, T.; Köhler, S.J.; Poutanen, H.; de Wit, H.A.; Uhl, W. Natural organic matter fractions and their removal in full-scale drinking water treatment under cold climate conditions in Nordic capitals. J. Environ. Manag. 2019, 241, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, R.; Liu, H.; Qu, J. Disinfection by-products formation and precursors transformation during chlorination and chloramination of highly-polluted source water: Significance of ammonia. Water Res. 2013, 47, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, W.; Lee, W. Formation of disinfection byproducts upon chlorine dioxide preoxidation followed by chlorination or chloramination of natural organic matter. Chemosphere 2013, 91, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Li, A.; Xu, B.; Xian, Q.; Shuang, C.; Shi, P.; Zhou, Q. Detection, formation and occurrence of 13 new polar phenolic chlorinated and brominated disinfection byproducts in drinking water. Water Res. 2017, 112, 129–136. [Google Scholar] [CrossRef]
- Costet, N.; Villanueva, C.M.; Jaakkola, J.J.K.; Kogevinas, M.; Cantor, K.P.; King, W.D.; Lynch, C.F.; Nieuwenhuijsen, M.J.; Cordier, S. Water disinfection by-products and bladder cancer: Is there a European specificity? A pooled and meta-analysis of European case–control studies. Occup. Environ. Med. 2011, 68, 379–385. [Google Scholar] [CrossRef]
- Wright, J.M.; Evans, A.; Kaufman, J.A.; Rivera-Núñez, Z.; Narotsky, M.G. Disinfection By-Product Exposures and the Risk of Specific Cardiac Birth Defects. Environ. Health Perspect. 2017, 125, 269–277. [Google Scholar] [CrossRef]
- Grellier, J.; Bennett, J.; Patelarou, E.; Smith, R.B.; Toledano, M.B.; Rushton, L.; Briggs, D.J.; Nieuwenhuijsen, M.J. Exposure to Disinfection By-products, Fetal Growth, and Prematurity: A Systematic Review and Meta-analysis. Epidemiology 2010, 21, 300–313. [Google Scholar] [CrossRef]
- Waller, K.; Swan, S.H.; DeLorenze, G.; Hopkins, B. Trihalomethanes in Drinking Water and Spontaneous Abortion. Epidemiology 1998, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- De Vietro, N.; Tursi, A.; Beneduci, A.; Chidichimo, F.; Milella, A.; Fracassi, F.; Chatzisymeon, E.; Chidichimo, G. Photocatalytic inactivation of Escherichia coli bacteria in water using low pressure plasma deposited TiO2 cellulose fabric. Photochem. Photobiol. Sci. 2019, 18, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Davididou, K.; McRitchie, C.; Antonopoulou, M.; Konstantinou, I.; Chatzisymeon, E. Photocatalytic degradation of saccharin under UV-LED and blacklight irradiation. J. Chem. Technol. Biotechnol. 2018, 93, 269–276. [Google Scholar] [CrossRef]
- Davididou, K.; Nelson, R.; Monteagudo, J.M.; Durán, A.; Expósito, A.J.; Chatzisymeon, E. Photocatalytic degradation of bisphenol-A under UV-LED, blacklight and solar irradiation. J. Clean. Prod. 2018, 203, 13–21. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.; Jiang, Z. Effects of humic acid on enhanced removal of lead ions by polystyrene-supported nano-Fe (0) nanocomposite. Sci. Rep. 2020, 10, 19663. [Google Scholar] [CrossRef]
- Męczykowska, H.; Stepnowski, P.; Caban, M. Impact of humic acids, temperature and stirring on passive extraction of pharmaceuticals from water by trihexyl(tetradecyl)phosphonium dicyanamide. Microchem. J. 2019, 144, 500–505. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Velten, S.; Knappe, D.R.U.; Traber, J.; Kaiser, H.-P.; von Gunten, U.; Boller, M.; Meylan, S. Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res. 2011, 45, 3951–3959. [Google Scholar] [CrossRef]
- Petala, M.D.; Zouboulis, A.I. Vibratory shear enhanced processing membrane filtration applied for the removal of natural organic matter from surface waters. J. Membr. Sci. 2006, 269, 1–14. [Google Scholar] [CrossRef]
- Winter, J.; Barbeau, B.; Bérubé, P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal-Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes (Basel) 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Jun, W.; Katsoyiannis, I.A. Removal of humic acids by flotation. Colloids Surf. A Physicochem. Eng. Asp. 2003, 231, 181–193. [Google Scholar] [CrossRef]
- Boyer, T.H. Removal of Dissolved Organic Matter by Magnetic Ion Exchange Resin. Curr. Pollut. Rep. 2015, 1, 142–154. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Synergistic effects in combined fouling of a loose nanofiltration membrane by colloidal materials and natural organic matter. J. Membr. Sci. 2006, 278, 72–82. [Google Scholar] [CrossRef]
- Alrousan, D.; Afkhami, A.; Bani-Melhem, K.; Dunlop, P. Organic Degradation Potential of Real Greywater Using TiO2-Based Advanced Oxidation Processes. Water 2020, 12, 2811. [Google Scholar] [CrossRef]
- Westerhoff, P.; Aiken, G.; Amy, G.; Debroux, J. Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Res. 1999, 33, 2265–2276. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Li, Y.; Yao, L.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; van Grieken, R.; Marugán, J. Influence of light distribution on the performance of photocatalytic reactors: LED vs. mercury lamps. Appl. Catal. B Environ. 2017, 215, 1–7. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Bowen, C.R.; van Grieken, R.; Marugán, J. Design and validation of a LED-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B Environ. 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Safari, M.; Rezaee, R.; Cheshmeh Soltani, R.D.; Shahmoradi, B.; Zandsalimi, Y. Photocatalytic degradation of humic substances in the presence of ZnO nanoparticles immobilized on glass plates under ultraviolet irradiation. Sep. Sci. Technol. 2016, 51, 2484–2489. [Google Scholar] [CrossRef]
- Maleki, A.; Safari, M.; Shahmoradi, B.; Zandsalimi, Y.; Daraei, H.; Gharibi, F. Photocatalytic degradation of humic substances in aqueous solution using Cu-doped ZnO nanoparticles under natural sunlight irradiation. Environ. Sci. Pollut. Res. 2015, 22, 16875–16880. [Google Scholar] [CrossRef] [PubMed]
- Birben, N.C.; Paganini, M.C.; Calza, P.; Bekbolet, M. Photocatalytic degradation of humic acid using a novel photocatalyst: Ce-doped ZnO. Photochem. Photobiol. Sci. 2017, 16, 24–30. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ioannidi, A.; Petala, A.; Frontistis, Z. Copper phosphide promoted BiVO4 photocatalysts for the degradation of sulfamethoxazole in aqueous media. J. Environ. Chem. Eng. 2020, 8, 104340. [Google Scholar] [CrossRef]
- Tomara, T.; Frontistis, Z.; Petala, A.; Mantzavinos, D. Photocatalytic performance of Ag2O towards sulfamethoxazole degradation in environmental samples. J. Environ. Chem. Eng. 2019, 7, 103177. [Google Scholar] [CrossRef]
- Kumaravel, V.; Rhatigan, S.; Mathew, S.; Bartlett, J.; Nolan, M.; Hinder, S.J.; Sharma, P.K.; Singh, A.; Byrne, J.A.; Harrison, J.; et al. Indium-Doped TiO2 Photocatalysts with High-Temperature Anatase Stability. J. Phys. Chem. C 2019, 123, 21083–21096. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-L. Decolorization of Reactive Red 2 by advanced oxidation processes: Comparative studies of homogeneous and heterogeneous systems. J. Hazard. Mater. 2006, 128, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Peternel, I.T.; Koprivanac, N.; Božić, A.M.L.; Kušić, H.M. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J. Hazard. Mater. 2007, 148, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Santos, E.B.H.; Duarte, A.C. Spectroscopic characteristics of ultrafiltration fractions of fulvic and humic acids isolated from an eucalyptus bleached Kraft pulp mill effluent. Water Res. 2003, 37, 4073–4080. [Google Scholar] [CrossRef]
- Ye, Y.; Bruning, H.; Liu, W.; Rijnaarts, H.; Yntema, D. Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J. Photochem. Photobiol. A Chem. 2019, 371, 216–222. [Google Scholar] [CrossRef]
- Korshin, G.V.; Benjamin, M.M.; Li, C.-W. Use of differential spectroscopy to evaluate the structure and reactivity of humics. Water Sci. Technol. 1999, 40, 9–16. [Google Scholar] [CrossRef]
- Ates, N.; Kitis, M.; Yetis, U. Formation of chlorination by-products in waters with low SUVA—correlations with SUVA and differential UV spectroscopy. Water Res. 2007, 41, 4139–4148. [Google Scholar] [CrossRef]
- Sambo, S.P.; Marais, S.S.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. Quantification of biodegradable natural organic matter (NOM) fractions and its impact on bacterial regrowth in a South African Water Treatment Plant. J. Water Process Eng. 2020, 36, 101332. [Google Scholar] [CrossRef]
- Hua, L.-C.; Chao, S.-J.; Huang, K.; Huang, C. Characteristics of low and high SUVA precursors: Relationships among molecular weight, fluorescence, and chemical composition with DBP formation. Sci. Total Environ. 2020, 727, 138638. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.; Labine, P.; Reidies, A. The chemistry of permanganate in degradative oxidations. In Chemical Oxidation; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1991; pp. 205–219. [Google Scholar]
- Wallace, B.; Purcell, M.; Furlong, J. Total organic carbon analysis as a precursor to disinfection byproducts in potable water: Oxidation technique considerations. J. Environ. Monit. JEM 2002, 4, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kiashemshaki, H.; Mahvi, A.H.; Najafpoor, A.A.; Hosseinzadeh, A. Investigation of the Efficiency of the Conventional Water Treatment Processes Employed to Eliminate TOC in Jalaliyeh Water Treatment Plant, Tehran. Health Scope 2017, 6, e61907. [Google Scholar] [CrossRef]
- Baker, A.; Tipping, E.; Thacker, S.A.; Gondar, D. Relating dissolved organic matter fluorescence and functional properties. Chemosphere 2008, 73, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Bieroza, M.; Baker, A.; Bridgeman, J. Relating freshwater organic matter fluorescence to organic carbon removal efficiency in drinking water treatment. Sci. Total Environ. 2009, 407, 1765–1774. [Google Scholar] [CrossRef]
- Peiris, R.H.; Hallé, C.; Budman, H.; Moresoli, C.; Peldszus, S.; Huck, P.M.; Legge, R.L. Identifying fouling events in a membrane-based drinking water treatment process using principal component analysis of fluorescence excitation-emission matrices. Water Res. 2010, 44, 185–194. [Google Scholar] [CrossRef]
- Khamis, K.; Bradley, C.; Hannah, D.M. High frequency fluorescence monitoring reveals new insights into organic matter dynamics of an urban river, Birmingham, UK. Sci. Total Environ. 2020, 710, 135668. [Google Scholar] [CrossRef]
- Allpike, B.P.; Heitz, A.; Joll, C.A.; Kagi, R.I. A new organic carbon detector for size exclusion chromatography. J. Chromatogr. A 2007, 1157, 472–476. [Google Scholar] [CrossRef]
- Brezinski, K.; Gorczyca, B. An overview of the uses of high performance size exclusion chromatography (HPSEC) in the characterization of natural organic matter (NOM) in potable water, and ion-exchange applications. Chemosphere 2019, 217, 122–139. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Rosario-Ortiz, F.L.; Baik, S.; Vanderford, B.J.; Snyder, S.A. Characterization of fulvic acids by liquid chromatography-quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, J.; Zhang, X.; Shen, J.; Chen, Z.; Chu, W.; Kang, J.; Zhao, S.; Zhou, Y. Application of Fourier transform ion cyclotron resonance mass spectrometry to characterize natural organic matter. Chemosphere 2020, 260, 127458. [Google Scholar] [CrossRef] [PubMed]
- Huck, P.M. Measurement of Biodegradable Organic Matter and Bacterial Growth Potential in Drinking Water. J. Awwa 1990, 82, 78–86. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Bolton, L.; Baker, A. Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations. Water Res. 2007, 41, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Kanokkantapong, V.; Marhaba, T.F.; Panyapinyophol, B.; Pavasant, P. FTIR evaluation of functional groups involved in the formation of haloacetic acids during the chlorination of raw water. J. Hazard. Mater. 2006, 136, 188–196. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Chung, J.; Yoon, J.; Yoon, Y. Characterizing dissolved organic matter and evaluating associated nanofiltration membrane fouling. Chemosphere 2008, 70, 495–502. [Google Scholar] [CrossRef]
- Folhas, D.; Duarte, A.C.; Pilote, M.; Vincent, W.F.; Freitas, P.; Vieira, G.; Silva, A.M.S.; Duarte, R.M.B.O.; Canário, J. Structural Characterization of Dissolved Organic Matter in Permafrost Peatland Lakes. Water 2020, 12, 3059. [Google Scholar] [CrossRef]
- Pelekani, C.; Newcombe, G.; Snoeyink, V.L.; Hepplewhite, C.; Assemi, S.; Beckett, R. Characterization of Natural Organic Matter Using High Performance Size Exclusion Chromatography. Environ. Sci. Technol. 1999, 33, 2807–2813. [Google Scholar] [CrossRef]
- Zhou, Q.; Cabaniss, S.E.; Maurice, P.A. Considerations in the use of high-pressure size exclusion chromatography (HPSEC) for determining molecular weights of aquatic humic substances. Water Res. 2000, 34, 3505–3514. [Google Scholar] [CrossRef]
- Wu, F.C.; Evans, R.D.; Dillon, P.J. Separation and Characterization of NOM by High-Performance Liquid Chromatography and On-Line Three-Dimensional Excitation Emission Matrix Fluorescence Detection. Environ. Sci. Technol. 2003, 37, 3687–3693. [Google Scholar] [CrossRef]
- Bekbölet, M.; Özkösemen, G. A preliminary investigation on the photocatalytic degradation of a model humic acid. Water Sci. Technol. 1996, 33, 189–194. [Google Scholar] [CrossRef]
- Bekbolet, M.; Suphandag, A.S.; Uyguner, C.S. An investigation of the photocatalytic efficiencies of TiO2 powders on the decolourisation of humic acids. J. Photochem. Photobiol. A Chem. 2002, 148, 121–128. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Drikas, M.; Amal, R. Comparison of photocatalytic degradation of natural organic matter in two Australian surface waters using multiple analytical techniques. Org. Geochem. 2010, 41, 124–129. [Google Scholar] [CrossRef]
- Nkambule, T.I.; Kuvarega, A.T.; Krause, R.W.M.; Haarhoff, J.; Mamba, B.B. Synthesis and characterisation of Pd-modified N-doped TiO2 for photocatalytic degradation of natural organic matter (NOM) fractions. Environ. Sci. Pollut. Res. 2012, 19, 4120–4132. [Google Scholar] [CrossRef]
- Gora, S.; Sokolowski, A.; Hatat-Fraile, M.; Liang, R.; Zhou, Y.N.; Andrews, S. Solar photocatalysis with modified TiO2 photocatalysts: Effects on NOM and disinfection byproduct formation potential. Environ. Sci. Water Res. Technol. 2018, 4, 1361–1376. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Hua, D.; Shi, C. Enhanced photocatalytic degradation of humic acids using Al and Fe co-doped TiO2 nanotubes under UV/ozonation for drinking water purification. J. Hazard. Mater. 2013, 262, 527–538. [Google Scholar] [CrossRef]
- Ndlangamandla, N.G.; Kuvarega, A.T.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. A novel photodegradation approach for the efficient removal of natural organic matter (NOM) from water. Phys. Chem. Earth Parts A/B/C 2018, 106, 97–106. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.H.; Fu, W.; Du, A.J.; Sun, D.D. TiO2 nanotube photocatalytic oxidation for water treatment. Water Supply 2009, 9, 45–49. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Shang, Y.; Zhuang, X.; Li, H.; Zhou, Z. Combined process of visible light irradiation photocatalysis-coagulation enhances natural organic matter removal: Optimization of influencing factors and mechanism. Chem. Eng. J. 2019, 374, 748–759. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Rajca, M. The effectiveness of removal of nom from natural water using photocatalytic membrane reactors in PMR-UF and PMR-MF modes. Chem. Eng. J. 2016, 305, 169–175. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; Wang, J.; Zhong, X. The removal of natural organic matter with LiCl–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Huang, X.; Leal, M.; Li, Q. Degradation of natural organic matter by TiO2 photocatalytic oxidation and its effect on fouling of low-pressure membranes. Water Res. 2008, 42, 1142–1150. [Google Scholar] [CrossRef]
- Sun, W.; Chu, H.; Dong, B.; Cao, D.; Zheng, S. The Degradation of Natural Organic Matter in Surface Water by a Nano-TiO2/Diatomite Photocatalytic Reactor. Clean—SoilAirWater 2014, 42, 1190–1198. [Google Scholar] [CrossRef]
- Xue, G.; Liu, H.; Chen, Q.; Hills, C.; Tyrer, M.; Innocent, F. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard. Mater. 2011, 186, 765–772. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Palmer, F.L.; Eggins, B.R.; Coleman, H.M. The effect of operational parameters on the photocatalytic degradation of humic acid. J. Photochem. Photobiol. A Chem. 2002, 148, 137–143. [Google Scholar] [CrossRef]
- Joolaei, H.; Vossoughi, M.; Rashidi Mehr Abadi, A.; Heravi, A. Removal of humic acid from aqueous solution using photocatalytic reaction on perlite granules covered by Nano TiO2 particles. J. Mol. Liq. 2017, 242, 357–363. [Google Scholar] [CrossRef]
- Babel, S.; Sekartaji, P.A.; Sudrajat, H. TiO2 as an effective nanocatalyst for photocatalytic degradation of humic acid in water environment. J. Water Supply Res. Technol.—AQUA 2016, 66, 25–35. [Google Scholar] [CrossRef]
- Song, L.; Zhu, B.; Jegatheesan, V.; Gray, S.R.; Duke, M.C.; Muthukumaran, S. Effect of Hybrid Photocatalysis and Ceramic Membrane Filtration Process for Humic Acid Degradation. In Water Scarcity and Ways to Reduce the Impact: Management Strategies and Technologies for Zero Liquid Discharge and Future Smart Cities; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–113. [Google Scholar]
- Valencia, S.; Marín, J.; Velásquez, J.; Restrepo, G.; Frimmel, F.H. Study of pH effects on the evolution of properties of brown-water natural organic matter as revealed by size-exclusion chromatography during photocatalytic degradation. Water Res. 2012, 46, 1198–1206. [Google Scholar] [CrossRef]
- Tercero Espinoza, L.A.; ter Haseborg, E.; Weber, M.; Karle, E.; Peschke, R.; Frimmel, F.H. Effect of selected metal ions on the photocatalytic degradation of bog lake water natural organic matter. Water Res. 2011, 45, 1039–1048. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Kavurmaci, S.S.; Gürkan, Y.Y.; Turkten, N.; Cinar, Z.; Bekbolet, M. Application of Fe-doped TiO2 specimens for the solar photocatalytic degradation of humic acid. Catal. Today 2017, 281, 78–84. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Han, Y.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2019, 321–322, 9–17. [Google Scholar] [CrossRef]
- Sood, S.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016, 290, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F.; Wang, F.; Luo, S.; Yin, X. Synthesis, characterization, and activities of visible light-driven Bi2O3–TiO2 composite photocatalysts. J. Alloy Compd. 2010, 498, 179–184. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Lin, T.; Ding, M.; Wang, Y.; Tao, H.; Hu, K. Preparation of Fe3O4/TiO2-N-GO sonocatalyst and using for humic acid removal with the assist of ultrasound. Mater. Sci. Semicond. Process. 2019, 102, 104593. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Sakthivel, S.; Janczarek, M.; Kisch, H. Visible Light Activity and Photoelectrochemical Properties of Nitrogen-Doped TiO2. J. Phys. Chem. B 2004, 108, 19384–19387. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.H.; Du, A.J.; Fu, W.; Sun, D.D.; Leckie, J.O. Combination of one-dimensional TiO2 nanowire photocatalytic oxidation with microfiltration for water treatment. Water Res. 2009, 43, 1179–1186. [Google Scholar] [CrossRef]
- Rizzo, L.; Uyguner, C.S.; Selcuk, H.; Bekbolet, M.; Anderson, M. Activation of solgel titanium nanofilm by UV illumination for NOM removal. Water Sci. Technol. 2007, 55, 113–118. [Google Scholar] [CrossRef]
- Lee, S.-A.; Choo, K.-H.; Lee, C.-H.; Lee, H.-I.; Hyeon, T.; Choi, W.; Kwon, H.-H. Use of Ultrafiltration Membranes for the Separation of TiO2 Photocatalysts in Drinking Water Treatment. Ind. Eng. Chem. Res. 2001, 40, 1712–1719. [Google Scholar] [CrossRef]
- Ng, H.K.M.; Sabran, A.H.; Leo, C.P.; Ahmad, A.L.; Abdullah, A.Z. Photocatalysts in polysulfone membrane for the removal of humic acid: The effects of PVP and PVa on membrane morphology, separation performance and catalytic hindrance. J. Membr. Sci. Res. 2016, 2, 95–101. [Google Scholar]
- Rajesh, S.; Senthilkumar, S.; Jayalakshmi, A.; Nirmala, M.T.; Ismail, A.F.; Mohan, D. Preparation and performance evaluation of poly (amide–imide) and TiO2 nanoparticles impregnated polysulfone nanofiltration membranes in the removal of humic substances. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 92–104. [Google Scholar] [CrossRef]
- Ballari, M.d.l.M.; Brandi, R.; Alfano, O.; Cassano, A. Mass transfer limitations in photocatalytic reactors employing titanium dioxide suspensions: II. External and internal particle constrains for the reaction. Chem. Eng. J. 2008, 136, 242–255. [Google Scholar] [CrossRef]
- Yao, P.; Choo, K.-H.; Kim, M.-H. A hybridized photocatalysis–microfiltration system with iron oxide-coated membranes for the removal of natural organic matter in water treatment: Effects of iron oxide layers and colloids. Water Res. 2009, 43, 4238–4248. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Morales-Torres, S.; Likodimos, V.; Romanos, G.E.; Pastrana-Martinez, L.M.; Falaras, P.; Dionysiou, D.D.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Prototype composite membranes of partially reduced graphene oxide/TiO2 for photocatalytic ultrafiltration water treatment under visible light. Appl. Catal. B Environ. 2014, 158–159, 361–372. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kaplan Bekaroglu, S.S.; Yigit, N.O.; Harman, B.I.; Kitis, M. Hybrid Adsorptive and Oxidative Removal of Natural Organic Matter Using Iron Oxide-Coated Pumice Particles. J. Chem. 2016, 2016, 3108034. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Suphandag, S.A.; Kerc, A.; Bekbolet, M. Evaluation of adsorption and coagulation characteristics of humic acids preceded by alternative advanced oxidation techniques. Desalination 2007, 210, 183–193. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Sanabria, J.; Pulgarin, C.; Benítez, N. Simultaneous E. coli inactivation and NOM degradation in river water via photo-Fenton process at natural pH in solar CPC reactor. A new way for enhancing solar disinfection of natural water. Chemosphere 2009, 77, 296–300. [Google Scholar] [CrossRef]
- Gelover, S.; Gómez, L.A.; Reyes, K.; Teresa Leal, M. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef]
- Fernández-Ibáñez, P.; Sichel, C.; Polo-López, M.I.; de Cara-García, M.; Tello, J.C. Photocatalytic disinfection of natural well water contaminated by Fusarium solani using TiO2 slurry in solar CPC photo-reactors. Catal. Today 2009, 144, 62–68. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Murray, C.A.; Parsons, S.A. Advanced oxidation processes: Flowsheet options for bulk natural organic matter removal. Water Supply 2004, 4, 113–119. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Chin, A.; Bérubé, P.R. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res. 2005, 39, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Lamsal, R.; Walsh, M.E.; Gagnon, G.A. Comparison of advanced oxidation processes for the removal of natural organic matter. Water Res. 2011, 45, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Toor, R.; Mohseni, M. UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 2007, 66, 2087–2095. [Google Scholar] [CrossRef]
- Murray, C.A.; Parsons, S.A. Removal of NOM from drinking water: Fenton’s and photo-Fenton’s processes. Chemosphere 2004, 54, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Goslan, E.H.; Gurses, F.; Banks, J.; Parsons, S.A. An investigation into reservoir NOM reduction by UV photolysis and advanced oxidation processes. Chemosphere 2006, 65, 1113–1119. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Pulgarin, C.; Benítez, N. Degradation of DBPs’ precursors in river water before and after slow sand filtration by photo-Fenton process at pH 5 in a solar CPC reactor. Water Res. 2008, 42, 4125–4132. [Google Scholar] [CrossRef]
- Zepp, R.G.; Faust, B.C.; Hoigne, J. Hydroxyl radical formation in aqueous reactions (pH 3-8) of iron(II) with hydrogen peroxide: The photo-Fenton reaction. Environ. Sci. Technol. 1992, 26, 313–319. [Google Scholar] [CrossRef]
- Wardman, P.; Candeias, L.P. Fenton Chemistry: An Introduction. Radiat. Res. 1996, 145, 523–531. [Google Scholar] [CrossRef]
- Park, S.; Yoon, T.-I. The effects of iron species and mineral particles on advanced oxidation processes for the removal of humic acids. Desalination 2007, 208, 181–191. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Fukushima, M.; Tatsumi, K.; Nagao, S. Degradation Characteristics of Humic Acid during Photo-Fenton Processes. Environ. Sci. Technol. 2001, 35, 3683–3690. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-S.; Hsieh, S.-T.; Hong, C.-S. Destruction of humic acid in water by UV light—catalyzed oxidation with hydrogen peroxide. Water Res. 2000, 34, 3882–3887. [Google Scholar] [CrossRef]
- Carr, S.A.; Baird, R.B. Mineralization as a mechanism for TOC removal: Study of ozone/ozone–peroxide oxidation using FT-IR. Water Res. 2000, 34, 4036–4048. [Google Scholar] [CrossRef]
- Otieno, B.; Apollo, S.; Kabuba, J.; Naidoo, B.; Ochieng, A. Ozonolysis Post-Treatment of Anaerobically Digested Distillery Wastewater Effluent. Ozone Sci. Eng. 2019, 41, 551–561. [Google Scholar] [CrossRef]
- Ratpukdi, T.; Siripattanakul, S.; Khan, E. Mineralization and biodegradability enhancement of natural organic matter by ozone–VUV in comparison with ozone, VUV, ozone–UV, and UV: Effects of pH and ozone dose. Water Res. 2010, 44, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Bircher, K.; Tumas, W.; Tolman, C. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Technologies for Both Electric- and Solar-Driven Systems—(IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV–vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Application, 2000; 189.
- Yang, Y.; Pignatello, J.J. Participation of the Halogens in Photochemical Reactions in Natural and Treated Waters. Molecules 2017, 22, 1684. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of Halide Ions and Carbonates on Organic Contaminant Degradation by Hydroxyl Radical-Based Advanced Oxidation Processes in Saline Waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- Treguer, R.; Tatin, R.; Couvert, A.; Wolbert, D.; Tazi-Pain, A. Ozonation effect on natural organic matter adsorption and biodegradation—Application to a membrane bioreactor containing activated carbon for drinking water production. Water Res. 2010, 44, 781–788. [Google Scholar] [CrossRef]
- Yan, W.-Y.; Zhou, Q.; Chen, X.; Yang, Y.; Zhang, Y.; Huang, X.-J.; Wu, Y.-C. Size-Controlled TiO2 nanocrystals with exposed {001} and {101} facets strongly linking to graphene oxide via p-Phenylenediamine for efficient photocatalytic degradation of fulvic acids. J. Hazard. Mater. 2016, 314, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Turkten, N.; Bekbolet, M. Photocatalytic performance of titanium dioxide and zinc oxide binary system on degradation of humic matter. J. Photochem. Photobiol. A Chem. 2020, 401, 112748. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.; Restrepo, G. Photocatalytic Degradation of Humic Acids with Titanium Dioxide Embedded into Polyethylene Pellets to Enhance the Postrecovery of Catalyst. Environ. Eng. Sci. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Asha, R.C.; Vishnuganth, M.A.; Remya, N.; Selvaraju, N.; Kumar, M. Livestock Wastewater Treatment in Batch and Continuous Photocatalytic Systems: Performance and Economic Analyses. Water Air Soil Pollut. 2015, 226, 132. [Google Scholar] [CrossRef]
- Giménez, J.; Bayarri, B.; González, Ó.; Malato, S.; Peral, J.; Esplugas, S. Advanced Oxidation Processes at Laboratory Scale: Environmental and Economic Impacts. ACS Sustain. Chem. Eng. 2015, 3, 3188–3196. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C.H. Application of Photocatalysts and LED Light Sources in Drinking Water Treatment. Catalysts 2013, 3, 726–743. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.; Robertson, P. UV LED Sources for Heterogeneous Photocatalysis. In Environmental Photochemistry Part III; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
| Method | Advantages | Disadvantages | Complexity of Method |
|---|---|---|---|
| Adsorption at 254 nm |
|
| Low High |
| COD |
|
| |
| TOC |
|
| |
| Fluorescence spectroscopy |
|
| |
| FTIR |
|
| |
| HPLC |
|
| |
| GC-MS |
|
|
| Water Matrix | Catalyst Type | Reaction Time | Irradiation Source | Other Operating Parameters | Removal Efficiency | Other Important Findings | Reference |
|---|---|---|---|---|---|---|---|
| Humic acid solution | P25-TiO2 | 120 min | UVA—125 W | Ambient pH [HA] = 50 mg/L [TiO2] = 1 g/L | 88% TOC 99% Vis400 | THMFP * = 14.5 μg/L | Bekbölet et al. (1996) [83] |
| Humic acid solution | P25-TiO2, UV100-TiO2 | 60 min | UVA—125 W λ = 300–420 nm | Ambient pH [HA] = 10 mg/L [TiO2] = 0.25 g/L | P25: 70% TOC UV100: 50% TOC | NOM removal rate constant: P25 = 1.9 × 10−2 min−1 UV100 = 1.2 × 10−2 min−1 | Bekbölet et al. (2002) [84] |
| Reservoir water: M-Myponga site W-Woronora site | P25-TiO2 | 150 min | UVA—20 W λ = 365 nm | pH~7 TOCM = 10.6 mg/L TOCW = 3.5 mg/L [TiO2] = 0.1 g/L | M: 80% TOC 100% UV254 W: 80% TOC 100% UV254 | THMFP: M = < 20 μg/L W = < 20 μg/L | Liu et al. (2010) [85] |
| Sand filtered treatment plant water | N-Pd-TiO2 | 120 min | Solar simulator 500 W | pH~6.73 TOC = 2.38 mg/L [N-Pd-TiO2] = 5 g/L | HPO ** = 71% HPI ** = 35% TPI ** = 15% UV254 | Nkambule et al. (2012) [86] | |
| Reverse osmosis isolate and Alginic acid solution | AgSiO2-TiO2 | 30 min | Solar simulator λ = 400–1100 nm | pH~8.2 TOCI = 3.7 mg/L [TiO2] = 0.1 g/L | 20% TOC 42% UV254 | 219 ± 40 μg THMFP per g TiO2 | Gora et al. (2018) [87] |
| Humic acid solution | Al:Fe-TiO2 (1%) | 15 min | UVC—37 W λ = 254 nm | pH~7 [HA] = 10 mg/L [TiO2] = 0.1 g/L O3 | 63.2% TOC 79.4% UV254 | Increasing HCO3- concentration decrease NOM reduction rate | Yuan et al. (2013) [88] |
| Reservoir water: MV-Midvaal P-Plettenberg bay | MWCNT/N, Pd-TiO2 *** | 120 min | Solar simulator 300 W | [MWCNT/N, Pd-TiO2] = 1 g/L | MV: 69.4% P: 97.7% UV254 | Ndlangamandla et al. (2018) [89] | |
| Humic acid solution | TiO2 nanotubes | 120 min | UVC—11 W λ = 254 nm | [HA] = 50 mg/L [TiO2] = 0.5 g/L | 98.27% DOC 100% UV436 | Humic acid removal rate: 0.0607 molm−3s−1 | Zhang et al. (2009) [90] |
| Landscape surface water | Bi2O3-TiO2 | 10 min | Vis—300 W λ = 400–780 nm | pH~8.13 TOCI = 2.2 mg/L [Bi2O3-TiO2] = 2 g/L | 20.2% TOC 24.4% UV254 | Wang et al. (2019) [91] | |
| Pre-treated (coagulation-flocculation) water | P25-TiO2, TiO2/β-SiC | 220 min | Solar simulator—1500 W | pH~6.7 P25: TOCI = 7.8 mg/L [TiO2] = 0.5 g/L β-SiC: TOCI = 5.5 mg/L [TiO2] = 0.5 g/L | P25: 80% TOC β-SiC: 80% TOC | Ayekoe et al. (2017) [92] | |
| Treatment plant inlet water in immersed ultrafiltration module | P25-TiO2 | 120 min irradiation 43 h total treatment | UVC—15 W λ = 254 nm | pH~7 DOC = 5.48 mg/L [TiO2] = 0.1 g/L | 60% DOC 90% UV254 | THMFP * = 25 μg/L | Rajca et al. (2016) [93] |
| Humic acid solution | LiCl-TiO2 doped PVDF **** membrane | 30 min | UVA—100 W λ = 365 nm | pH~7.5 [HA] = 2 mg/L | 80–84% UV254 | Song et al. (2014) [94] | |
| Extracted river NOM | P25-TiO2 | 120 min | UVC—8 W λ = 254 nm | pH~8.2 TOCI = 10 mg/L [TiO2] = 1 g/L | 80% TOC 100% UV254 | NOM degradation rate constant: 0.0163 min−1 | Huang et al. (2008) [95] |
| River water | Nano-TiO2 on diatomite | 360 min | 3× UVC lamps—16 W λ = 254 nm | pH~8.0–8.5 TOCI = 9.84– 13.18 mg/L [TiO2] = 0.5 g/L | 28.5% TOC 40% UV254 | Sun et al. (2014) [96] | |
| Humic acid solution | TiO2 nanoparticles/granular activated carbon (GAC) | 180 min | UVA—500 W λ = 365 nm | pH~4.2 TOCI = 5.04 mg/L [TiO2/GAC] = 2 g/L | 99.5% UV254 | Significantly lower degradation (70% UV254) at pH = 11 | Xue et al. (2011) [97] |
| Homogeneous Processes | Water Matrix | Catalyst Type | Reaction Time | Irradiation Source | Other Operating Parameters | Removal Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| Hybrid Photolysis | Reservoir water | O3/UV | 60 min | UVA lamp Intensity = 9.7 mW/cm2 | pH~6.6 TOC = 1.8 mg/L O3 dosage = 0.62 g/L | 50% TOC | Chin and Bérubé (2005) [134] |
| River water | H2O2/UV O3/UV | 30 min | UVA lamp—43 W | TOC = 3.1 mg/L [ H2O2] = 23 mg/L O3 dosage = 4 mg/L | H2O2 only: 3–23% DOC 60% UV254 O3 only: 31% TOC 88% UV254 | Lamsal et al. (2011) [135] | |
| Reservoir water | H2O2/UV | - | UVC lamp λ = 254 nm | [H2O2] = 23 mg/L | - | Toor et al. (2005) [136] | |
| Photo-Fenton | Inlet water to water treatment works | FeSO4·7H2O + H2O2 | 20 min | 4× UVA lamps—25 W λ = 365 nm | pH~4 DOC = 9.6 mg/L [Cat] = 5.65 mg/L H2O2:Fe2+ = 5:1 | 90% DOC 95% UV254 | Murray et al. (2002) [132] |
| Water treatment works reservoir water | FeSO4·7H2O + H2O2 | 30 min | 4× UVA lamps—25 W λ = 365 nm | pH~4 DOC = 7.5 mg/L [Fe2+] = 0.1 mM H2O2:Fe2+ = 5:1 | 90% DOC 95% UV254 | Murray et al. (2004) [137] | |
| Reservoir water | FeSO4·7H2O + H2O2 H2O2 only | 1 min | 4× UVC lamp – 12 W λ = 254 nm | pH~4.5 DOC = 17.4 mg/L [H2O2] = 2.0 mM H2O2:Fe2+ = 4:1 | Fe2SO4·7H2O + H2O2: 88% DOC 31% UV254 H2O2: 78% DOC 94% UV254 | Goslan et al. (2006) [138] | |
| River water pre-treated with slow sand filtration | FeCl3·7H2O + H2O2 | After 6.5 KJ/L of solar energy | Solar CPC | pH~5 DOC = 2.7–3.1 mg/L [H2O2] = 20 mg/L [Fe3+] = 1 mg/L | 90% DOC 95% UV254 | Moncayo-Lasso et al. (2008) [139] | |
| River water | FeCl3·7H2O + H2O2 | After 20 KJ/L of solar energy | Solar CPC | pH~6.5 DOC = 5.5 mg/L [H2O2] = 10 mg/L [Fe3+] = 0.6 mg/L | 55% DOC 75% UV254 | Moncayo-Lasso et al. (2009) [128] |
| Process Type | Water Matrix | Catalyst Type | Electrical Power of the Irradiation Source (P)/kW | Reaction Time (t)/min | Volume (V)/L | TOC % | EEO KWh m−3 Order−1 | Reference |
|---|---|---|---|---|---|---|---|---|
| Heterogeneous | Humic acid solution | P25-TiO2 | 0.125 | 120 | 0.05 | 88 | 5430 | Bekbolet et al. (1996) [83] |
| Heterogeneous | Reservoir water | P25-TiO2 | 0.02 | 150 | 0.8 | 100 | 15,625 | Liu et al. (2010) [85] |
| Heterogeneous | Pre-treated (coagulation-flocculation) water | P25-TiO2/βSiC | 1.5 | 220 | 0.1 | 80 | 78,687 | Ayekoe et al. (2017) [92] |
| Homogeneous | River water | H2O2/UV | 0.043 | 30 | 3 | 23 | 63,137 | Lamsal et al. (2010) [135] |
| Homogeneous | River water | O3/UV | 0.043 | 30 | 3 | 31 | 44,472 | Lamsal et al. (2011) [135] |
| Homogeneous | Water treatment works reservoir water | FeSO4.7H2O + H2O2 | 0.1 | 30 | 1 | 90 | 50 | Murray et al. (2004) [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowland, D.C.A.; Robertson, N.; Chatzisymeon, E. Photocatalytic Oxidation of Natural Organic Matter in Water. Water 2021, 13, 288. https://doi.org/10.3390/w13030288
Gowland DCA, Robertson N, Chatzisymeon E. Photocatalytic Oxidation of Natural Organic Matter in Water. Water. 2021; 13(3):288. https://doi.org/10.3390/w13030288
Chicago/Turabian StyleGowland, Dan C. A., Neil Robertson, and Efthalia Chatzisymeon. 2021. "Photocatalytic Oxidation of Natural Organic Matter in Water" Water 13, no. 3: 288. https://doi.org/10.3390/w13030288
APA StyleGowland, D. C. A., Robertson, N., & Chatzisymeon, E. (2021). Photocatalytic Oxidation of Natural Organic Matter in Water. Water, 13(3), 288. https://doi.org/10.3390/w13030288






