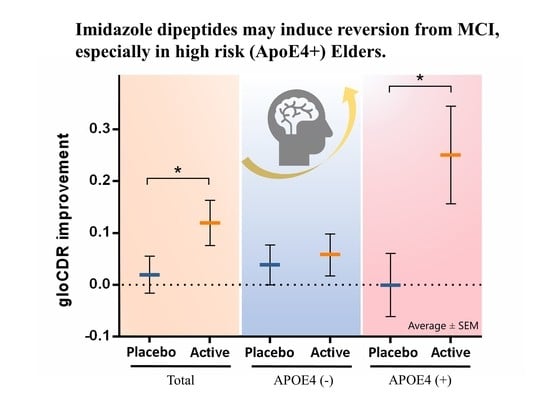
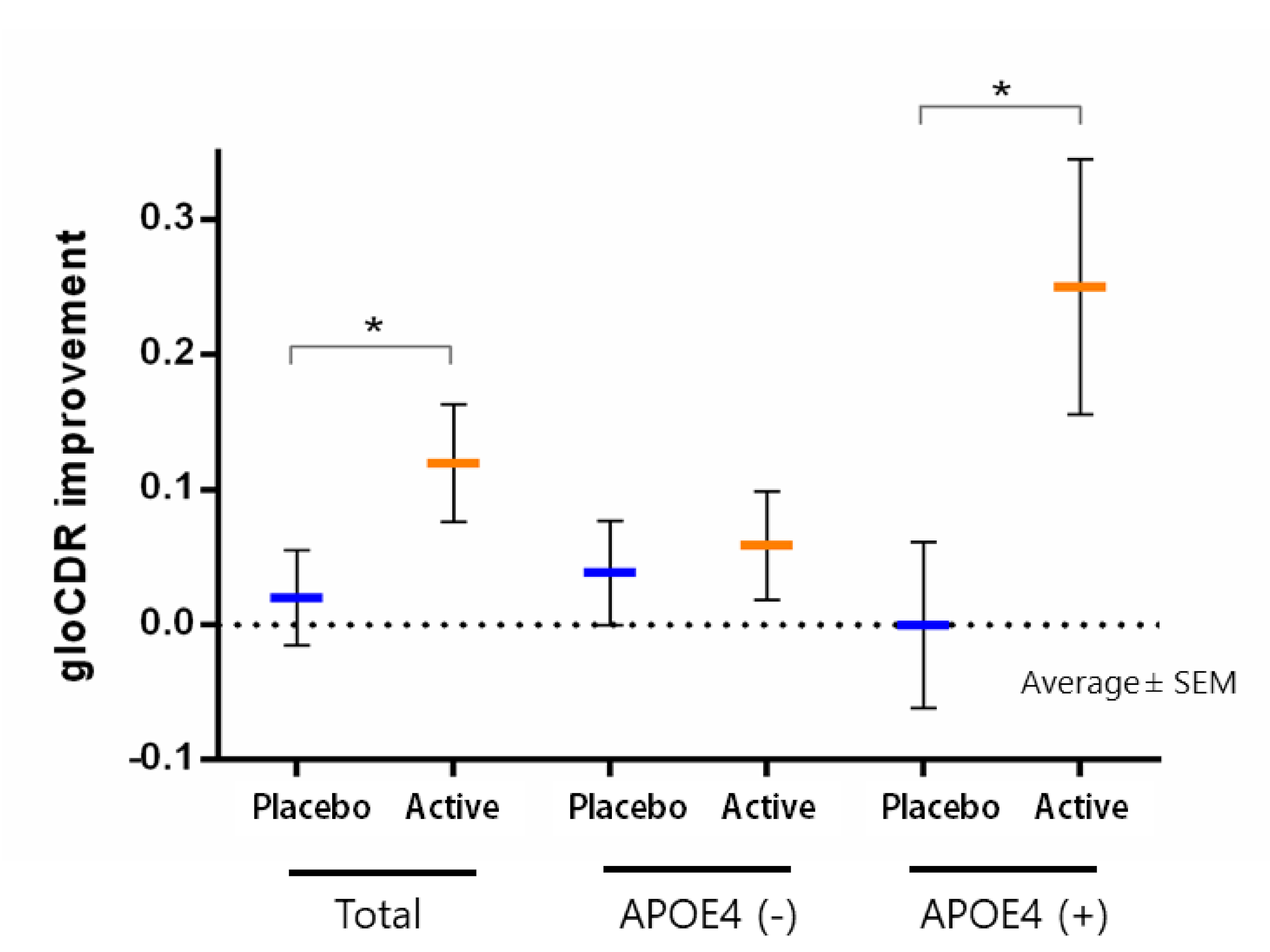
Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review
Abstract
1. Introduction
2. Methodology
3. Biochemical Properties of Imidazole Dipeptides
4. Imidazole Dipeptide on Brain Function
4.1. Imidazole Dipeptide in Animal Models of AD
4.2. Imidazole Dipeptide in Human Interventional Trial Studies
4.3. Suggested Mechanism between Imidazole Dipeptide and Cognitive Reserve
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Eramudugolla, R.; Mortby, M.E.; Sachdev, P.; Meslin, C.; Kumar, R.; Anstey, K.J. Evaluation of a research diagnostic algorithm for DSM-5 neurocognitive disorders in a population-based cohort of older adults. Alzheimers Res. Ther. 2017, 9, 15. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Amos, E.C.; Canick, J.; Ackerley, M.; Raji, C.; Fiala, M.; Ahdidan, J. Reversal of cognitive decline in Alzheimer’s disease. Aging (Albany NY) 2016, 8, 1250–1258. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M. Reversion From Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimers Dis. Assoc. Disord. 2016, 30, 324–330. [Google Scholar] [CrossRef]
- Thomas, K.R.; Edmonds, E.C.; Eppig, J.S.; Wong, C.G.; Weigand, A.J.; Bangen, K.J.; Jak, A.J.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; et al. Alzheimer’s Disease Neuroimaging Initiative. MCI-to-normal reversion using neuropsychological criteria in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2019, 15, 1322–1332. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Lee, S.; Lee, S. Conversion and Reversion Rates in Japanese Older People With Mild Cognitive Impairment. J. Am. Med. Dir. Assoc. 2017, 18, 808.e1–808.e6. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- van Geldorp, B.; Heringa, S.M.; van den Berg, E.; Olde Rikkert, M.G.; Biessels, G.J.; Kessels, R.P. Working memory binding and episodic memory formation in aging, mild cognitive impairment, and Alzheimer’s dementia. J. Clin. Exp. Neuropsychol. 2015, 37, 538–548. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Capurso, C.; Imbimbo, B.P.; Vendemiale, G.; Santamato, A.; D’Onofrio, G.; Seripa, D.; Sancarlo, D.; Pilotto, A.; et al. Metabolic syndrome and cognitive impairment: Current epidemiology and possible underlying mechanisms. J. Alzheimers Dis. 2010, 21, 691–724. [Google Scholar] [CrossRef] [PubMed]
- Osone, A.; Arai, R.; Hakamada, R.; Shimoda, K. Impact of lifestyle-related disease on conversion and reversion in patients with mild cognitive impairment: After 12 months of follow-up. Int. J. Geriatr. Psychiatry 2016, 31, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.S.; Tay, L.; Chan, M.; Lim, W.S.; Ye, R.; Wong, W.C.; Lim, J.P.; Tan, E.K.; Ding, Y.Y. Stage-Specific Relationship between Frailty and Cognitive Impairment in a Specialist Memory Clinic Setting. J. Frailty Aging 2014, 3, 113–119. [Google Scholar] [PubMed]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Di Lena, L.; D’Urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; et al. Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J. Alzheimers Dis. 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Budzen, S.; Kopec, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Canevelli, M.; Lucchini, F.; Quarata, F.; Bruno, G.; Cesari, M. Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients 2016, 8, 144. [Google Scholar] [CrossRef]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018, 15, 32. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Suppresses the Expression of the Inflammatory Chemokine CCL24 in Peripheral Blood Mononuclear Cells from Elderly People. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef]
- Ding, Q.; Tanigawa, K.; Kaneko, J.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation preserves blood flow in the prefrontal brain of elderly people carrying APOE e4. Aging Dis. 2018, 9, 334–345. [Google Scholar] [CrossRef]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef]
- Kaneko, J.; Enya, A.; Enomoto, K.; Ding, Q.; Hisatsune, T. Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged APPswe/PSEN1dE9 Alzheimer’s-model mice. Sci. Rep. 2017, 7, 12571. [Google Scholar] [CrossRef]
- Dobbie, H.; Kermack, W.O. Complex-formation between polypeptides and metals. 2. The reaction between cupric ions and some dipeptides. Biochem. J. 1955, 59, 246–257. [Google Scholar] [CrossRef]
- Nagai, K.; Tanida, M.; Niijima, A.; Tsuruoka, N.; Kiso, Y.; Horii, Y.; Shen, J.; Okumura, N. Role of L-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: Involvement of the circadian clock and histamine. Amino Acids 2012, 43, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tanokura, M.; Tasumi, M.; Miyazawa, T. 1H nuclear magnetic resonance studies of histidine-containing di- and tripeptides. Estimation of the effects of charged groups on the pKa value of the imidazole ring. Biopolymers 1976, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Boldyrev, A.A.; Formazuyk, V.E. Carnosine: Its properties, functions and potential therapeutic applications. Molec. Aspects Med. 1992, 13, 379–444. [Google Scholar] [CrossRef]
- Bush, A.I. Copper, Zinc, and the Metallobiology of Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef]
- Grasso, G.; Pietropaolo, A.; Spoto, G.; Pappalardo, G.; Tundo, G.R.; Ciaccio, C.; Coletta, M.; Rizzarelli, E. Copper(I) and copper(II) inhibit Aβ peptides proteolysis by insulin-degrading enzyme differently: Implications for metallostasis alteration in Alzheimer’s disease. Chemistry 2011, 17, 2752–2762. [Google Scholar] [CrossRef]
- Dobrota, D.; Fedorova, T.; Stvolinsky, S.; Babusikova, E.; Likavcanova, K.; Drgova, A.; Strapkova, A.; Boldyrev, A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: After-stroke-effect. Neurochem. Res. 2005, 30, 1283–1288. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Reduction of Oxidative/Nitrosative Stress in Brain and its Involvement in the Neuroprotective Effect of n-3 PUFA in Alzheimer’s Disease. Curr. Alzheimers Res. 2016, 13, 123–134. [Google Scholar] [CrossRef]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch. Neurosci. 2015, 2, e20078. [Google Scholar]
- Caruso, G.; Fresta, C.G.; Siegel, J.M.; Wijesinghe, M.B.; Lunte, S.M. Microchip electrophoresis with laser-induced fluorescence detection for the determination of the ratio of nitric oxide to superoxide production in macrophages during inflammation. Anal. Bioanal. Chem. 2017, 409, 4529–4538. [Google Scholar] [CrossRef]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Effects of alpha-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr. Res. Pract. 2011, 5, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: Implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 2016, 45, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Worthington, V.C.; Himsworth, D.T.; Herwig, W. Protective effects of carnosine against protein modification mediated by malondialdehyde and hypochlorite. Biochim. Biophys. Acta 1998, 1380, 46–54. [Google Scholar] [CrossRef]
- Karton, A.; O’Reilly, R.J.; Pattison, D.I.; Davies, M.J.; Radom, L. Computational design of effective, bioinspired HOCl antioxidants: The role of intramolecular Cl+ and H+ shifts. J. Am. Chem. Soc. 2012, 134, 19240–19245. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Lei, C.; Li, H.; Inamura, M.; Shiotani, S.; Yanai, N.; Sato, K.; Sakurai, K.; Hisatsune, T. Anserine, HClO-scavenger, protected against cognitive decline in individuals with mild cognitive impairment. Aging (Albany NY) 2021. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Michaelis, J.; Syrris, P.; Kumar, S.; Lam, Y. Carnosine protects proteins against in vitro glycation and cross-linking. Biochem. Soc. Trans. 1994, 22, 399S. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Carnosine and its possible roles in nutrition and health. Adv. Food Nutr. Res. 2009, 57, 87–154. [Google Scholar]
- Aldini, G.; Facino, R.M.; Beretta, G.; Carini, M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: From structural studies to therapeutic perspectives. Biofactors 2005, 24, 77–87. [Google Scholar] [CrossRef]
- Kawahara, M.; Sadakane, Y.; Mizuno, K.; Kato-Negishi, M.; Tanaka, K.I. Carnosine as a Possible Drug for Zinc-Induced Neurotoxicity and Vascular Dementia. Int. J. Mol. Sci. 2020, 21, 2570. [Google Scholar] [CrossRef]
- Chin, Y.; Kishi, M.; Sekino, M.; Nakajo, F.; Abe, Y.; Terazono, Y.; Ohsaki, H.; Kato, F.; Koizumi, S.; Gachet, C.; et al. Involvement of glial P2Y1 receptors in the cognitive deficits after focal cerebral stroke in a rodent model. J. Neuroinflammation 2013, 10, 95. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Breithausen, B.; Keppler, K.; Poll, S.; Schulte, T.; Peter, J.; Plescher, M.; Hansen, J.N.; Blank, N.; et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 2018, 215, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Michaelis, J.; Syrris, P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995, 371, 81–85. [Google Scholar] [CrossRef]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.; Barca, A.; Romano, A.; Guerrieri, S.; Storelli, C.; Rinaldi, R.; Verri, T. Anti-aggregating effect of the naturally occurring dipeptide carnosine on aβ1-42 fibril formation. PLoS ONE 2013, 8, e68159. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A.; Mattson, R.H.; Behar, K.L.; Hyder, F.; Rothman, D.L. Vigabatrin increases human brain homocarnosine and improves seizure control. Ann. Neurol. 1998, 44, 948–952. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, J.J.; Cairns, M.T.; Trainor, M.; Robins, D.J.; Miller, D.J. Analysis of carnosine, homocarnosine, and other histidyl derivatives in rat brain. J. Neurochem. 1990, 55, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Peretto, P.; De Marchis, S.; Fasolo, A. Carnosine-related dipeptides in the mammalian brain. Prog. Neurobiol. 1999, 59, 333–353. [Google Scholar] [CrossRef]
- Stvolinskiĭ, S.L.; Dobrota, D.; Mezeshova, V.; Liptaĭ, T.; Pronaĭova, N.; Zalibera, L.; Boldyrev, A.A. Carnosine and anserine in working muscles--study using proton NMR spectroscopy. Biokhimiia 1992, 57, 1317–1323. [Google Scholar]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Lopachev, A.V.; Lopacheva, O.M.; Abaimov, D.A.; Koroleva, O.V.; Vladychenskaya, E.A.; Erukhimovich, A.A.; Fedorova, T.N. Neuroprotective Effect of Carnosine on Primary Culture of Rat Cerebellar Cells under Oxidative Stress. Biochemistry 2016, 81, 511–520. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, W.-W.; Fan, Y.-Y.; Dai, H.-B.; Fu, Q.-L.; Wei, E.-Q.; Luo, J.-H.; Chen, Z. Carnosine protects against NMDA-induced neurotoxicity in differentiated rat PC12 cells through carnosine-histidine-histamine pathway and H1/H3 receptors. Biochem. Pharmacol. 2007, 73, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, S.; Hayakawa, T.; Yamane, H.; Maemura, H.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Oral administration of chicken breast extract increases brain carnosine and anserine concentrations in rats. Nutr. Neurosci. 2007, 10, 181–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Adornetto, A.; Cavaliere, F.; Varano, G.P.; Rusciano, D.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Mol. Vis. 2015, 21, 718–729. [Google Scholar]
- Ashe, K.H.; Zahs, K.R. Probing the biology of Alzheimer’s disease in mice. Neuron 2010, 66, 631–645. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef]
- Park, J.H.; Widi, G.A.; Gimbel, D.A.; Harel, N.Y.; Lee, D.H.; Strittmatter, S.M. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer’s transgenic mice. J. Neurosci. 2006, 26, 13279–13286. [Google Scholar] [CrossRef]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef]
- Volianskis, A.; Kostner, R.; Molgaard, M.; Hass, S.; Jensen, M.S. Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1deltaE9-deleted transgenic mice model of ss-amyloidosis. Neurobiol Aging 2010, 31, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Hisatsune, T. Cholinergic modification of neurogenesis and gliosis improves the memory of AβPPswe/PSEN1dE9 Alzheimer’s model mice fed a high-fat diet. J. Alzheimers Dis. 2017, 56, 1–23. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Bell, R.D.; Zhao, Z.; Ma, Q.; Winkler, E.A.; Ramanathan, A.; Zlokovic, B.V. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013, 4, 2932. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Gottesman, R.F.; Bernstein, K.E.; Seshadri, S.; McKee, A.; Snyder, H.; Greenberg, S.M.; Yaffe, K.; Schaffer, C.B.; Yuan, C.; et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020, 10, 1002. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Son, D.O.; Satsu, H.; Kiso, Y.; Totsuka, M.; Shimizu, M. Inhibitory effect of carnosine on interleukin-8 production in intestinal epithelial cells through translational regulation. Cytokine 2008, 42, 265–276. [Google Scholar] [CrossRef]
- Cornelli, U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener. Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Small, B.J.; Rawson, K.S.; Martin, C.; Eisel, S.L.; Sanberg, C.D.; McEvoy, C.L.; Sanberg, P.R.; Shytle, R.D.; Tan, J.; Bickford, P.C. Nutraceutical intervention improves older adults’ cognitive functioning. Rejuvenation Res. 2014, 17, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Suttiwan, P.; Yuktanandana, P.; Ngamake, S. Effectiveness of Essence of Chicken on Cognitive Function Improvement: A Randomized Controlled Clinical Trial. Nutrients 2018, 10, 845. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. Embo Molecular Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Everaert, I.; Baron, G.; Barbaresi, S.; Gilardoni, E.; Coppa, C.; Carini, M.; Vistoli, G.; Bex, T.; Stautemas, J.; Blancquaert, W.; et al. Development and validation of a sensitive LC–MS/MS assay for the quantification of anserine in human plasma and urine and its application to pharmacokinetic study. Amino Acids 2019, 51, 103–114. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Hata, J.; Ohara, T.; Katakura, Y.; Shimizu, K.; Yamashita, S.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Shibata, M.; Sakata, S.; et al. Association Between Serum beta-Alanine and Risk of Dementia The Hisayama Study. Am. J. Epidemiol. 2019, 188, 1637–1645. [Google Scholar] [CrossRef]
- Horning, M.S.; Blakemore, L.J.; Trombley, P.Q. Endogenous mechanisms of neuroprotection: Role of zinc, copper, and carnosine. Brain Res. 2000, 852, 56–61. [Google Scholar] [CrossRef]
- Lee, J.M.; Zipfel, G.J.; Park, K.H.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 2002, 115, 871–878. [Google Scholar] [CrossRef]
- Mizuno, D.; Konoha-Mizuno, K.; Mori, M.; Sadakane, Y.; Koyama, H.; Ohkawara, S.; Kawahara, M. Protective activity of carnosine and anserine against zinc-induced neurotoxicity: A possible treatment for vascular dementia. Metallomics 2015, 7, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Wechsler, D. The Wechsler Memory Scale Fourth Edition (WMS-IV); Pearson Assessments: London, UK, 2010. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Sakurai, K.; Li, H.; Inamura, N.; Masuoka, N.; Hisatsune, T. Relationship between elevated impulsivity and cognitive declines in elderly community-dwelling individuals. Sci. Rep. 2020, 10, 21032. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Shen, C.; Ezaki, Y.; Inamura, N.; Fukushima, Y.; Masuoka, N.; Hisatsune, T. Effects of Matcha Green Tea Powder on Cognitive Functions of Community-Dwelling Elderly Individuals. Nutrients 2020, 12, 3639. [Google Scholar] [CrossRef]
- Sakurai, K.; Shen, C.; Shiraishi, I.; Inamura, N.; Hisatsune, T. Consumption of Oleic Acid on the Preservation of Cognitive Functions in Japanese Elderly Individuals. Nutrients 2021, 13, 284. [Google Scholar] [CrossRef]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef]
- Manzano, S.; Agüera, L.; Aguilar, M.; Olazarán, J. A Review on Tramiprosate (Homotaurine) in Alzheimer’s Disease and Other Neurocognitive Disorders. Front. Neurol. 2020, 11, 614. [Google Scholar] [CrossRef]
- Gauthier, S.; Aisen, P.S.; Ferris, S.H.; Saumier, D.; Duong, A.; Haine, D.; Garceau, D.; Suhy, J.; Oh, J.; Lau, W.; et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: Exploratory analyses of the MRI sub-group of the Alphase study. J. Nutr. Health Aging 2009, 13, 550–557. [Google Scholar] [CrossRef]
- Spalletta, G.; Cravello, L.; Gianni, W.; Piras, F.; Iorio, M.; Cacciari, C.; Casini, A.R.; Chiapponi, C.; Sancesario, G.; Fratangeli, C.; et al. Homotaurine effects on hippocampal volume loss and episodic memory in amnestic mild cognitive impairment. J. Alzheimers Dis. 2016, 50, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Abushakra, S.; Porsteinsson, A.; Vellas, B.; Cummings, J.; Gauthier, S.; Hey, J.A.; Power, A.; Hendrix, S.; Wang, P.; Shen, L.; et al. Clinical benefits of tramiprosate in Alzheimer’s disease are associated with higher number of APOE4 alleles: The "APOE4 gene-dose effect". J. Prev. Alzheimers Dis. 2016, 3, 219–228. [Google Scholar] [PubMed]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics:Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2020, 16, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Scipioni, A.; Blasetti Fantauzzi, C.; Giaccari, A.; Salomone, E.; Canevotti, R.; Lapolla, A.; Orioli, M.; et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharmacol. 2012, 166, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Fantauzzi, C.B.; Pugliese, G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: Effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia 2015, 58, 845–853. [Google Scholar] [CrossRef]
| Authors and Year | Type of AD Model Mouse | Start of Treatment, Trial Duration | Sex, Group & Sample Size | Treatment (Doses) | Outcomes of Interest |
|---|---|---|---|---|---|
| Corona et al., 2011 [27] | 3xTg-AD | 1 month, 12 months | MF, control (11), 3× Tg-AD (13), 3× Tg-AD + Carnosine (9) | 10 mg of carnosine per day (400 mg/kg/day) | Morris water maze, Abeta-load, Mitochondria function, Zinc chelating |
| Herculano et al., 2013 [28] | App/psen + High Fat Diet | 4 month, 6 weeks | MF, WT (11); AD (13); AD+HFD (13); AD+HFD + Carnosine (11) | 5 mg of carnosine per day (200 mg/kg/day) | Contextual fear conditioning, Abeta-load, Neuroinflammation |
| Kaneko et al., 2017 [29] | App/psen | 18 month, 8 weeks | MF, WT (10); WT + Ans (11); AD (10); AD + Ans (10) | 10 mg of anserine per day (400 mg/kg/day) | Morris water maze, Abeta-load, Neuroinflammation, Neurovascular damage |
| Authors and Year | Study, Country | Trial Duration, Sample Size (Act: Pla) | Gender, Age (Mean ± SD) Group; | Subjects; Cognitive Function | Imidazole Dipeptide; (Doses) | Outcomes of Interest |
|---|---|---|---|---|---|---|
| Szcześniak et al., 2014 [16] | Double-blind, Placebo-controlled, Randomized controlled trial (DbPcRCT), Poland | 13 weeks 51 (26: 25) | MF, 81.0 ± 7.0 y Active; 80.5 ± 7.5 y Placebo | Residents in a Nursing home; MMSE, >15 | 1 g of anserine/ carnosine (2:1 ratio); once a day | Cognitive function (MMSE, STMS), depression (GDS) |
| Katakura et al., 2017 [24] | DbPcRCT, JAPAN | 12 weeks 60 (30: 30) | MF, 60.4 ± 2.1 y Active; 65.3 ± 1.6 y Placebo | Community-dwelling Healthy volunteers MMSE ≥ 24 | 0.5 g of anserine/ carnosine (3:1 ratio); twice a day | Cognitive function (MMSE), Alzheimer’s disease (ADAS), memory (WMS-LM1, WMS-LM2), depression (BDI) |
| Ding et al., 2018 [25] | DbPcRCT, JAPAN | 12 months (extension of Hisatsune et al., 2016) [23] 68 (31: 37) | MF, 71.3 ± 4.8y Active; 71.8 ± 4.8y Placebo | Community-dwelling Healthy elderly volunteers MMSE > 24 | 0.5 g of anserine/ carnosine (3:1 ratio); twice a day | Memory (WMS-LM1, WMS-LM2), Alzheimer’s disease (ADAS) depressive symptoms (BDI) MRI (ASL, DTI) |
| Hisatsune et al., 2016 [23] | DbPcRCT, JAPAN | (1) 6 months 84 (42: 42) (2) 12 weeks 39 (19: 20) (part of Katakura et al., 2017) [24] | (1) MF, 69.4 ± 5.9 y Active; 70.4 ± 5.7 y Placebo (2) MF, 67.8 ± 5.6 y Intervention, 70.6 ± 5.1 y Control | Community-dwelling Healthy elderly volunteers MMSE ≥ 24 | 0.5 g of anserine/ carnosine (3:1 ratio); twice a day | Memory (WMS-LM1, WMS-LM2), Alzheimer’s disease (ADAS) depressive symptoms (BDI) MRI(ASL) |
| Rokicki et al., 2015 [22] | DbPcRCT, JAPAN | 12 weeks 31 (14: 17) (part of Katakura et al., 2017) [24] | MF, 61.4 y Active; 66.5 y Placebo | Community-dwelling Healthy elderly volunteers MMSE ≥ 24 | 0.5 g of anserine/carnosine (3:1 ratio); twice a day | Memory (WMS-LM1, WMS-LM2), (ADAS) depressive symptoms (BDI) MRI(fMRI) |
| Masuoka et al., 2019 [26] | DbPcRCT, JAPAN | 12 weeks 50 (25: 25) | MF, 72.9 ± 8.8 y Active; 73.6 ± 6.1 y Placebo | Outpatients with MCI, MMSE ≥ 24 | 375 mg anserine and 125 mg carnosine; twice a day | Cognitive function (MMSE), Alzheimer’s disease (ADAS), dementia (CDR), memory (WMS), depressive symptoms (GDS) |
| Cornelli et al., 2010 [81] | DbPcRCT, USA | 6 months 48 (23: 25) | MF, 75.0 ± 4.2 y Active; 74.0 ± 4.9 y Placebo | Patients with diagnosis of probable AD, MMSE score > 21 | Formula F (100 mg carnosine and anti-oxidant*); once per day | Cognitive function (MMSE) |
| Masuoka et al., in press [46] | DbPcRCT, JAPAN | 12 weeks 30 (15; 15) | MF, 74.5 ± 4.6 y Active; 72.0 ± 5.2 y Placebo | Community-dwelling elderly people with MCI, MoCA ≤ 25 | 250 mg anserine; twice a day | Cognitive function (MMSE), Alzheimer’s disease (ADAS); Inflamma-tion (Plasma CRP) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuoka, N.; Lei, C.; Li, H.; Hisatsune, T. Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review. Nutrients 2021, 13, 397. https://doi.org/10.3390/nu13020397
Masuoka N, Lei C, Li H, Hisatsune T. Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review. Nutrients. 2021; 13(2):397. https://doi.org/10.3390/nu13020397
Chicago/Turabian StyleMasuoka, Nobutaka, Chenxu Lei, Haowei Li, and Tatsuhiro Hisatsune. 2021. "Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review" Nutrients 13, no. 2: 397. https://doi.org/10.3390/nu13020397
APA StyleMasuoka, N., Lei, C., Li, H., & Hisatsune, T. (2021). Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review. Nutrients, 13(2), 397. https://doi.org/10.3390/nu13020397