Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents
Abstract
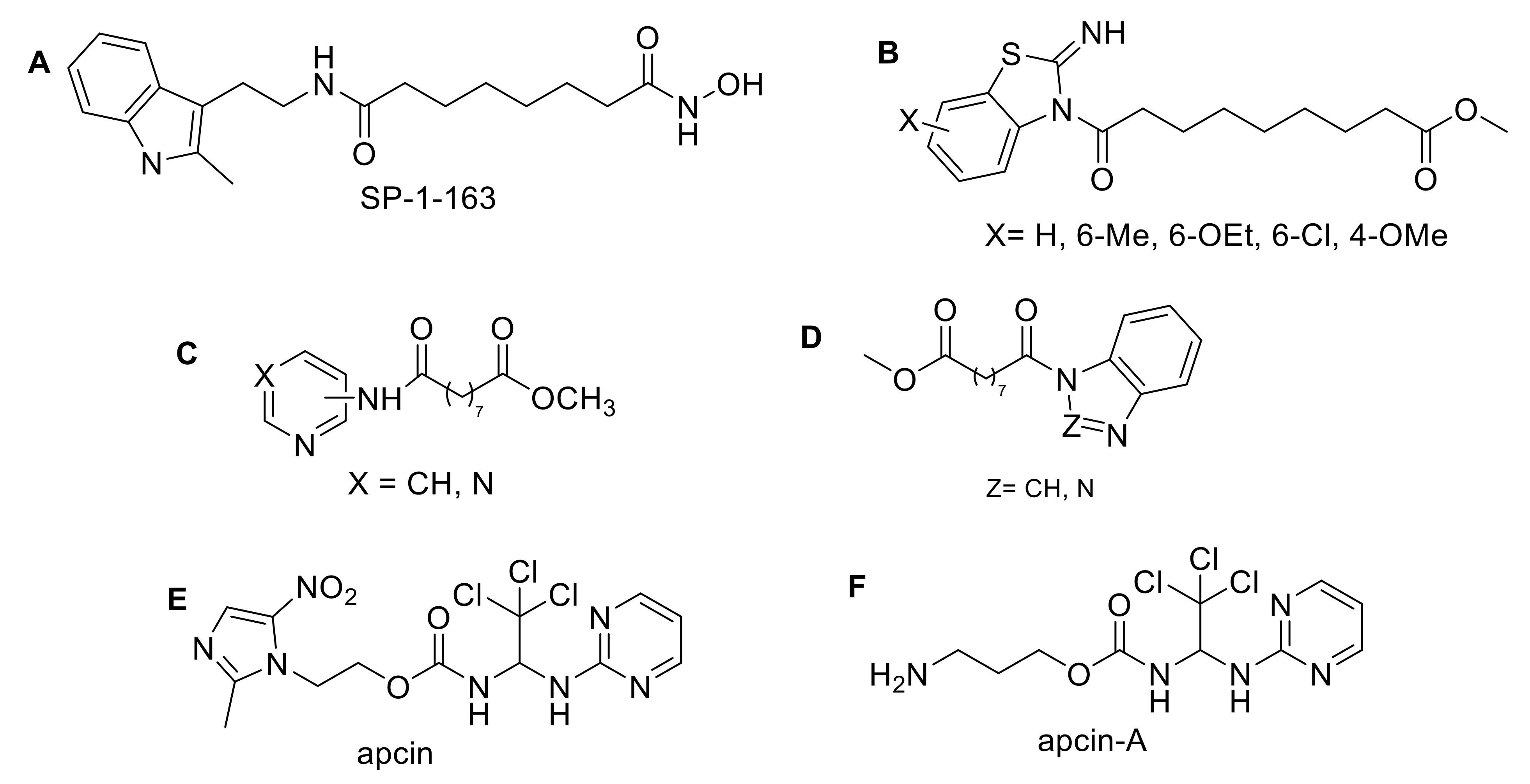
:1. Introduction
2. Results and Discussion
2.1. Chemistry
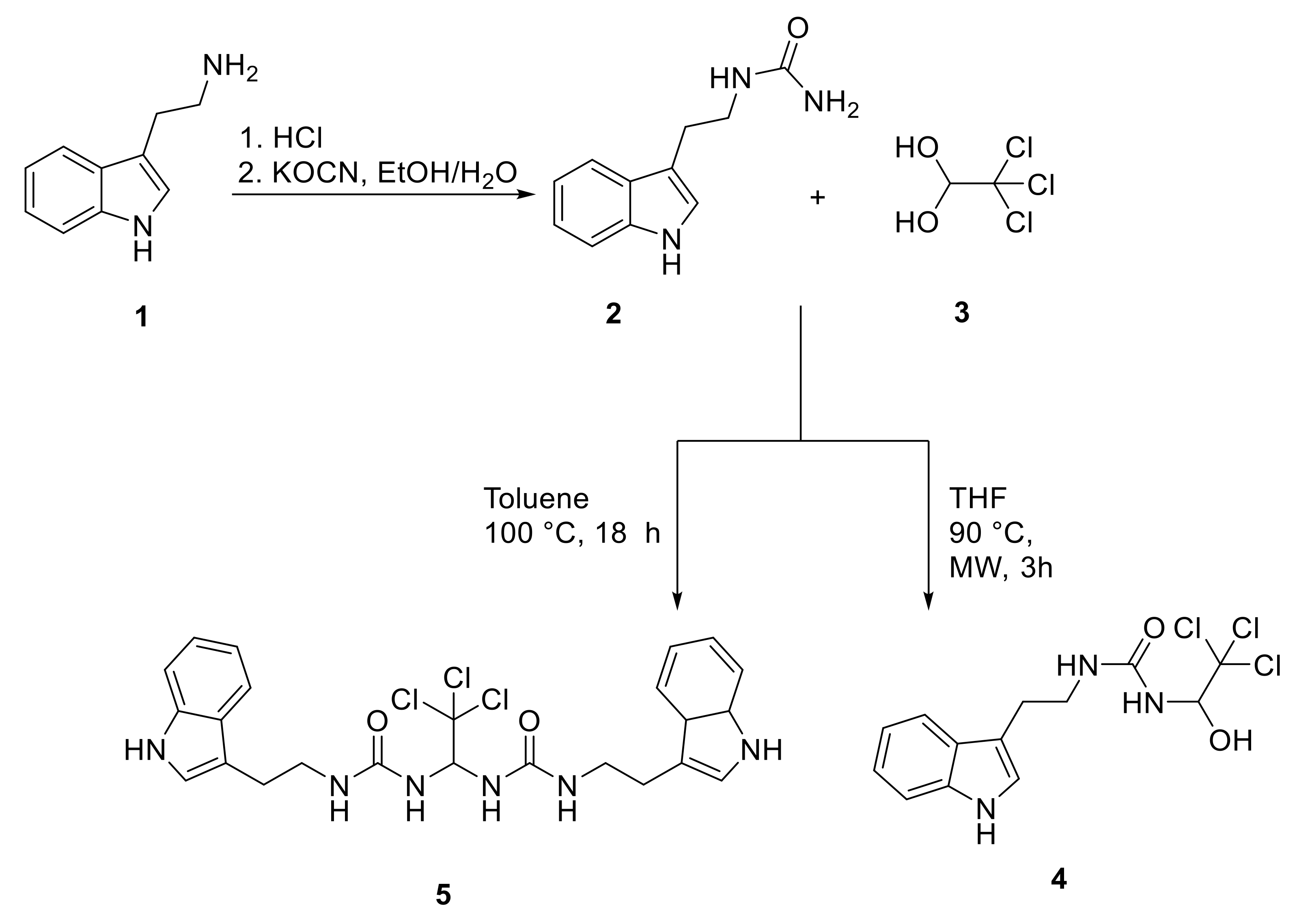
2.1.1. Synthesis of Ureido Derivatives 4 and 5, Bearing Tryptamine and Trichloromethyl Moieties
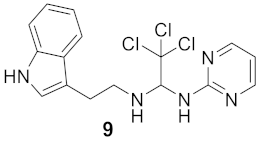
2.1.2. Synthesis of N-(2-(1H-indol-3-yl)ethyl)-2,2,2-trichloro-N′-(pyrimidin-2yl)etan-1,1-diamine (9)
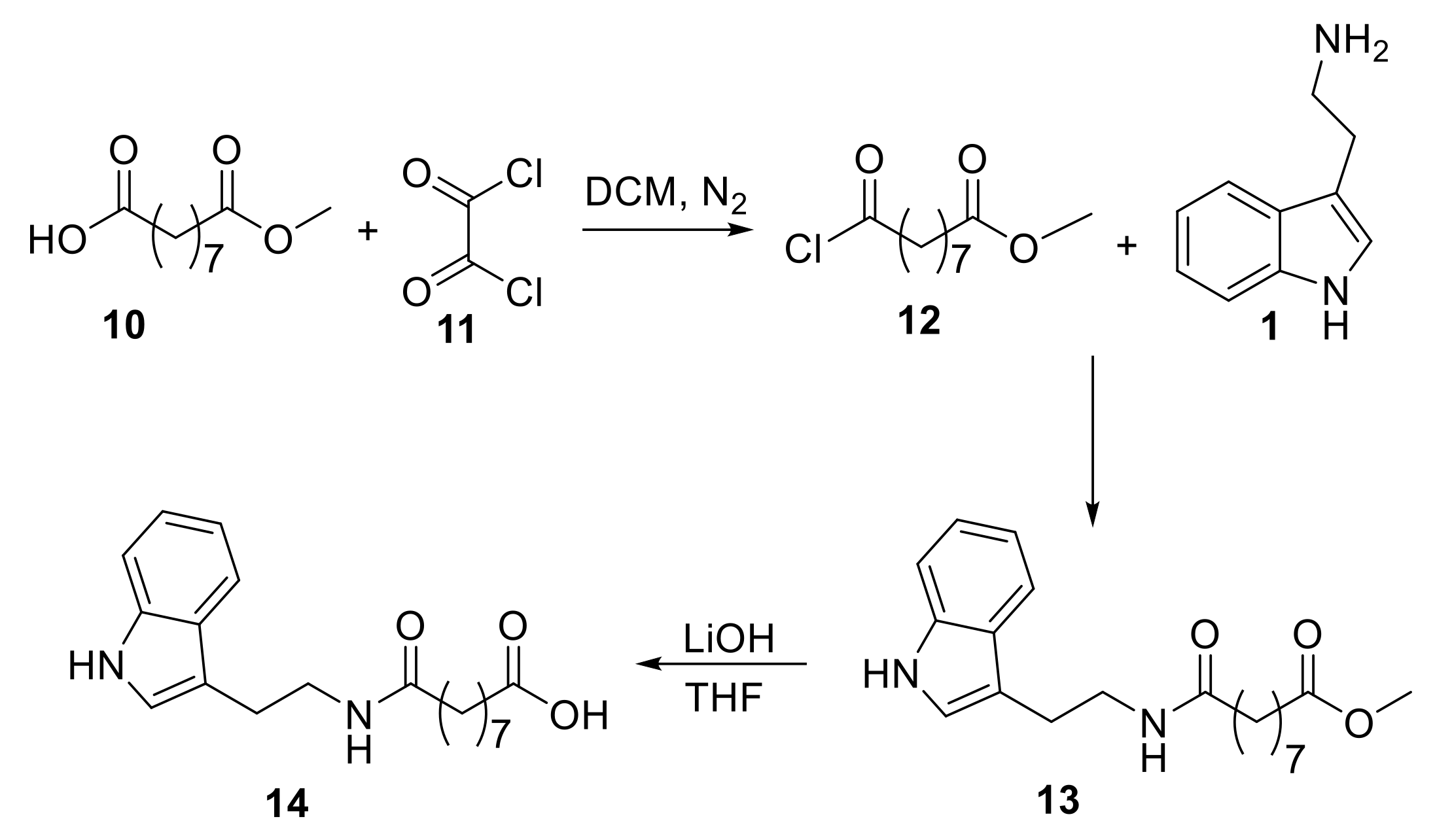
2.1.3. Synthesis of Hybrids Containing Tryptamine and Azelaoyl Moieties
2.2. Biological Activity
Effect of Compounds on Cells Viability
3. Materials and Methods
3.1. Chemical Syntheses
3.1.1. 1-(2-(1H-Indol-3-yl)ethyl)urea (Compound 2)
3.1.2. 1-(2-(Indolin-3-yl)ethyl)-3-(2,2,2-trichloro-1-hydroxyethyl)urea (Compound 4)
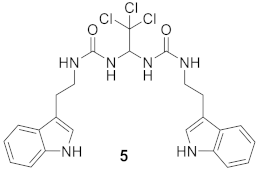
3.1.3. 1-(2-(1H-Indol-3-yl)ethyl)-3-(2,2,2-trichloro-1-(3-(2-(3a,7a-dihydro-1H-indol-3-yl)ethyl)ureido)ethyl)urea (Compound 5).
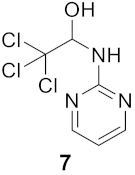
3.1.4. 2,2,2-Trichloro-1-(pyrimidin-2-ylamino)ethan-1-ol (Compound 7)
3.1.5. N-(1,2,2,2-tetrachloroethyl)pyrimidin-2-amine (Compound 8)
3.1.6. 2,2,2-Trichloro-N-(2-(3a,7a-dihydro-1H-indol-3-yl)ethyl)-N′-(pyrimidin-2-yl)ethane-1,1-diamine (Compound 9)
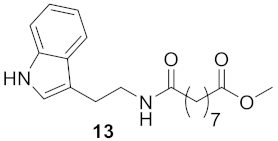
3.1.7. Methyl 9-((2-(1H-indol-3-yl)ethyl)amino)-9-oxononanoate (Compound 13)
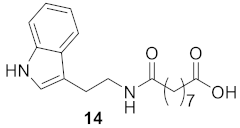
3.1.8. 9-((2-(1H-Indol-3-yl)ethyl)amino)-9-oxononanoic Acid (Compound 14)
3.2. Cell Culture and Treatments
3.2.1. Cell Culture
3.2.2. Cell Viability Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gribble, G.W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles in Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 26. [Google Scholar]
- O’Connor, S.E. Comprehensive Natural Products II; Mander, L., Liu,, H.-W., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, p. 977. [Google Scholar]
- Aniszewski, T. Alkaloids Chemistry, Biology, Ecology and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Han, S.; Movassaghi, M.J. Concise total synthesis and stereochemical revision of all (−)-Trigonoliimines. Am. Chem. Soc. 2011, 133, 10768–10771. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.B.; Simmons, B.; Mastracchio, A.; MacMillan, D.W.C. Collective synthesis of natural products by means of organocascade catalysis. Nature 2011, 475, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Eleutherakis-Papaiakovou, E.; Kanellias, N.; Kastritis, E.; Gavriatopoulou, M.; Terpos, E.; Dimopoulos, M.A. Efficacy of Panobinostat for the Treatment of Multiple Myeloma. J. Oncol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Grindrod, S.; Jung, M.; Brown, M.; Dritschilo, A. Dual Function Molecules for hIstone Deacetylase Inhibition and Ataxia Telangiectasia Mutated Activation and Methods of Use Thereof; US 2016/0257649 Al; Shuttle Pharmaceuticals, LLC.: Rockville, MD, USA, 2016. [Google Scholar]
- Bertucci, C.; Hudaib, M.; Boga, C.; Calonghi, N.; Cappadone, C.; Masotti, L. Gas chromatography/mass apectrometry assay of endogenous cellular lipid peroxidation products: Quantitative analysis of 9- and 10-hydroxystearic acids. Rapid Commun. Mass Spectrom. 2002, 16, 859–864. [Google Scholar] [CrossRef]
- Calonghi, N.; Cappadone, C.; Pagnotta, E.; Farruggia, G.; Buontempo, F.; Boga, C.; Brusa, G.; Santucci, M.; Masotti, L. 9-Hydroxystearic acid upregulates p21WAF1 in HT29 cancer cells. Biochem. Biophys. Res. Commun. 2004, 314, 138–142. [Google Scholar] [CrossRef]
- Calonghi, N.; Cappadone, C.; Pagnotta, E.; Boga, C.; Bertucci, C.; Fiori, J.; Tasco, G.; Casadio, R.; Masotti, L. Histone deacetylase 1: a target of 9-hydroxystearic acid in the inhibition of cell growth in human colon cancer. J. Lipid Res. 2005, 46, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Calonghi, N.; Pagnotta, E.; Parolin, C.; Tognoli, C.; Boga, C.; Masotti, L. 9-Hydroxystearic acid interferes with EGF signalling in a human colon adenocarcinoma. Biochem. Biophys. Res. Commun. 2006, 342, 585–588. [Google Scholar] [CrossRef]
- Calonghi, N.; Pagnotta, E.; Parolin, C.; Molinari, C.; Boga, C.; Piaz, F.D.; Brusa, G.; Santucci, M.; Masotti, L. Modulation of apoptotic signalling by 9-hydroxystearic acid in osteosarcoma cells. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2007, 1771, 139–146. [Google Scholar] [CrossRef]
- Parolin, C.; Calonghi, N.; Presta, E.; Boga, C.; Caruana, P.; Naldi, M.; Andrisano, V.; Masotti, L.; Sartor, G. Mechanism and stereoselectivity of HDAC I inhibition by (R)-9-hydroxystearic acid in colon cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 1334–1340. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Boga, C.; Micheletti, G.; Cassani, M.C.; Fini, M.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Hydroxyapatite as Anti-Proliferative and Cytotoxic Agent towards Osteosarcoma Cells. Langmuir 2016, 32, 188–194. [Google Scholar] [CrossRef]
- Busi, A.; Aluigi, A.; Guerrini, A.; Boga, C.; Sartor, G.; Calonghi, N.; Sotgiu, G.; Posati, T.; Corticelli, F.; Fiori, J.; et al. Unprecedented Behavior of (9R)-9-Hydroxystearic Acid-Loaded Keratin Nanoparticles on Cancer Cell Cycle. Mol. Pharm. 2019, 16, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Calonghi, N.; Boga, C.; Telese, D.; Bordoni, S.; Sartor, G.; Torsello, C.; Micheletti, G. Synthesis of 9-Hydroxystearic Acid Derivatives and Their Antiproliferative Activity on HT 29 Cancer Cells. Molecules 2019, 24, 3714. [Google Scholar] [CrossRef] [Green Version]
- Boga, C.; Micheletti, G.; Orlando, I.; Strocchi, E.; Vitali, B.; Verardi, L.; Sartor, G.; Calonghi, N. New Hybrids with 2-aminobenzothiazole and Azelayl Scaffolds: Synthesis, Molecular Docking and Biological Evaluation. Curr. Org. Chem. 2018, 22, 1649–1660. [Google Scholar] [CrossRef]
- Micheletti, G.; Calonghi, N.; Farruggia, G.; Strocchi, E.; Palmacci, V.; Telese, D.; Bordoni, S.; Frisco, G.; Boga, C. Synthesis of Novel Structural Hybrids between Aza-Heterocycles and Azelaic Acid Moiety with a Specific Activity on Osteosarcoma Cells. Molecules 2020, 25, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sackton, K.L.; Dimova, N.; Zeng, X.; Tian, W.; Zhang, M.; Sackton, T.B.; Meaders, J.L.; Pfaff, K.L.; Sigoillot, F.D.; Yu, H.; et al. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. Nat. Cell Biol. 2014, 514, 646–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Zhou, Z.; Chi, J.; Schiltz, G. Bifunctional Compounds Comprising Apcin-a and Their Use in the Treatment of Cancer; WO 2020/214555 A1; Northwestern University: Evanston, IL, USA, 2020. [Google Scholar]
- Simonetti, G.; Bruno, S.; Padella, A.; Tenti, E.; Martinelli, G. Aneuploidy: Cancer strength or vulnerability? Int. J. Cancer 2019, 144, 8–25. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, G.; Padella, A.; Valle, I.F.D.; Fontana, M.C.; Fonzi, E.; Bruno, S.; Baldazzi, C.; Guadagnuolo, V.; Manfrini, M.; Ferrari, A.; et al. Aneuploid acute myeloid leukemia exhibits a signature of genomic alterations in the cell cycle and protein degradation machinery. Cancer 2018, 125, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-J.; Hu, K.-S.; Wang, D.-S.; Zeng, Z.-L.; Zhang, D.; Chen, D.-L.; Bai, L.; Xu, R. CDC20 overexpression predicts a poor prognosis for patients with colorectal cancer. J. Transl. Med. 2013, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Moura, I.M.B.; Delgado, M.L.; Silva, P.M.A.; Lopes, C.A.; Amaral, J.B.D.; Monteiro, L.S.; Bousbaa, H. High CDC20 expression is associated with poor prognosis in oral squamous cell carcinoma. J. Oral Pathol. Med. 2013, 43, 225–231. [Google Scholar] [CrossRef]
- Ouellet, V.; Guyot, M.-C.; Le Page, C.; Filali-Mouhim, A.; Lussier, C.; Tonin, P.N.; Provencher, D.; Mes-Masson, A.-M. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. Int. J. Cancer 2006, 119, 599–607. [Google Scholar] [CrossRef]
- Lub, S.; Maes, A.; Maes, K.; De Veirman, K.; De Bruyne, E.; Menu, E.; Fostier, K.; Kassambara, A.; Moreaux, J.; Hose, D.; et al. Inhibiting the anaphase promoting complex/cyclosome induces a metaphase arrest and cell death in multiple myeloma cells. Oncotarget 2015, 7, 4062–4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Dai, X.; Gan, W.; Wan, L.; Li, M.; Mitsiades, N.; Wei, W.; Ding, Q.; Zhang, J. Prostate cancer-associated mutation in SPOP impairs its ability to target Cdc20 for poly-ubiquitination and degradation. Cancer Lett. 2017, 385, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, K.; Grubb, T.M.; Zalenski, A.A.; Pfaff, K.E.; Pal, D.; Majumder, S.; Summers, M.K.; Venere, M. Hyperphosphorylation of CDH1 in Glioblastoma Cancer Stem Cells Attenuates APC/CCDH1 Activity and Pharmacologic Inhibition of APC/CCDH1/CDC20 Compromises Viability. Mol. Cancer Res. 2019, 17, 1519–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhang, B.; Wang, Y.; Shang, G. Cdc20 inhibitor apcin inhibits the growth and invasion of osteosarcoma cells. Oncol. Rep. 2018, 40, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Le, X.; Huang, F.; Yang, J.; Yang, H.; Ma, J.; Hu, G.; Li, Q.; Chen, Z. Discovery of a Dual Tubulin Polymerization and Cell Division Cycle 20 Homologue Inhibitor via Structural Modification on Apcin. J. Med. Chem. 2020, 63, 4685–4700. [Google Scholar] [CrossRef]
- Di Rorà, A.G.L.; Martinelli, G.; Simonetti, G. The balance between mitotic death and mitotic slippage in acute leukemia: a new therapeutic window? J. Hematol. Oncol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, T.-J.; Kim, S.H.; Choi, Y.H.; Lee, S.-H.; Lee, J.M.; Kim, Y.-H.; Park, J.-W.; Kwon, T.K. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008, 13, 1494–1504. [Google Scholar] [CrossRef]
- Okamoto, S.; Tsujioka, T.; Suemori, S.; Kida, J.; Kondo, T.; Tohyama, Y.; Tohyama, K. Withaferin A suppresses the growth of myelodysplasia and leukemia cell lines by inhibiting cell cycle progression. Cancer Sci. 2016, 107, 1302–1314. [Google Scholar] [CrossRef]
- Sanchez-Martin, M.; Ambesi-Impiombato, A.; Qin, Y.; Herranz, D.; Bansal, M.; Girardi, T.; Paietta, E.; Tallman, M.S.; Rowe, J.M.; De Keersmaecker, K.; et al. Synergistic antileukemic therapies inNOTCH1-induced T-ALL. Proc. Natl. Acad. Sci. USA 2017, 114, 2006–2011. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Manero, G.; Yang, H.; Bueso-Ramos, C.; Ferrajoli, A.; Cortes, J.; Wierda, W.G.; Faderl, S.; Koller, C.; Morris, G.; Rosner, G.; et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008, 111, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Abaza, Y.; Kadia, T.; Jabbour, E.J.; Konopleva, M.Y.; Borthakur, G.; Ferrajoli, A.; Estrov, Z.; Wierda, W.G.; Alfonso, A.; Chong, T.H.; et al. Phase 1 dose escalation multicenter trial of pracinostat alone and in combination with azacitidine in patients with advanced hematologic malignancies. Cancer 2017, 123, 4851–4859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aillaud, I.; Barber, D.M.; Thompson, A.L.; Dixon, D.J. Enantioselective Michael Addition/Iminium Ion Cyclization Cascades of Tryptamine-Derived Ureas. Org. Lett. 2013, 15, 2946–2949. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Edwards, L.D.; Christian, E.J.; Jenkins, G.L. The Study, Testing and Synthesis of Certain Organic Compounds for Rodenticidal Activity. J. Am. Pharm. Assoc. 1947, 36, 349–352. [Google Scholar] [CrossRef] [PubMed]


 |  |  |  |  |  | |
|---|---|---|---|---|---|---|
| A431 | n.a | n.a | n.a | n.a | >250 | 0.0072 |
| HT29 | 0.0115 | 212 | n.a | n.a | 0.006 | 0.096 |
| IGROV1 | >250 | n.a | n.a | n.a | >250 | 0.0015 |
| U2OS | 22.54 | n.a | 0.383 | 235 | 222 | 0.469 |
| KG-1 | n.a | n.a | n.a | n.a | 32.44 | n.a |
| MV-4-11 | n.a | n.a | n.a | 28.95 | 102.50 | n.a |
| REH | n.a | n.a | n.a | 65.32 | 29.32 | n.a |
| Jurkat 6 | n.a | n.a | 61.12 | 0.570 | n.a | n.a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, G.; Boga, C.; Durante, J.; Micheletti, G.; Telese, D.; Caruana, P.; Ghelli Luserna di Rorà, A.; Mantellini, F.; Bruno, S.; Martinelli, G.; et al. Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents. Molecules 2021, 26, 683. https://doi.org/10.3390/molecules26030683
Simonetti G, Boga C, Durante J, Micheletti G, Telese D, Caruana P, Ghelli Luserna di Rorà A, Mantellini F, Bruno S, Martinelli G, et al. Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents. Molecules. 2021; 26(3):683. https://doi.org/10.3390/molecules26030683
Chicago/Turabian StyleSimonetti, Giorgia, Carla Boga, Joseph Durante, Gabriele Micheletti, Dario Telese, Paolo Caruana, Andrea Ghelli Luserna di Rorà, Fabio Mantellini, Samantha Bruno, Giovanni Martinelli, and et al. 2021. "Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents" Molecules 26, no. 3: 683. https://doi.org/10.3390/molecules26030683
APA StyleSimonetti, G., Boga, C., Durante, J., Micheletti, G., Telese, D., Caruana, P., Ghelli Luserna di Rorà, A., Mantellini, F., Bruno, S., Martinelli, G., & Calonghi, N. (2021). Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents. Molecules, 26(3), 683. https://doi.org/10.3390/molecules26030683










