Vagally Mediated Gut-Brain Relationships in Appetite Control-Insights from Porcine Studies
Abstract
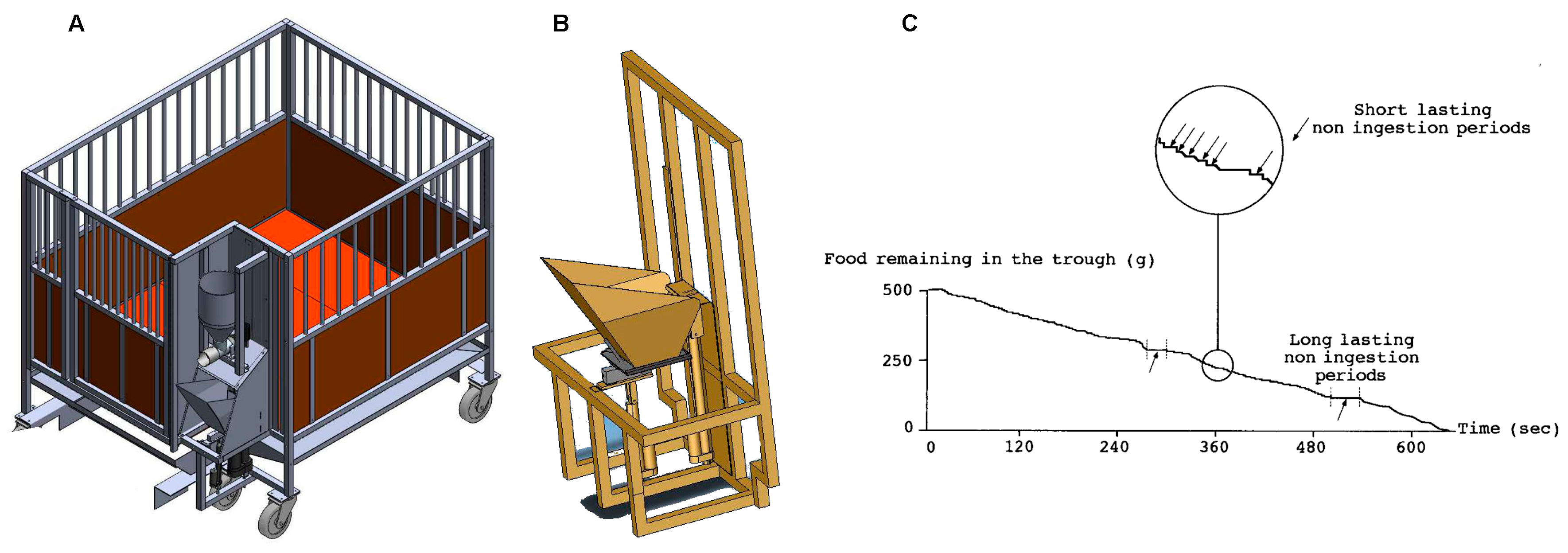
:1. Introduction
2. Appetite, Satiety, and Their Measurements
3. Gastric Emptying and Meal Distribution
4. Gastric signals
4.1. Acute Gastric Distension
- a.
- Vagal afferents during gastric distention
- b.
- Central processing
4.2. Chronic Gastric Distension
5. Intestinal Signals
5.1. Transpyloric Flow
5.2. Jejunal vs. Portal Signals
6. Mimicking Abdominal Afferent Vagal Signaling
6.1. Importance of Vagal Afferents for Appetite
6.2. Vagal Afferents Plasticity
6.3. Early Outcomes of Abdominal Vagal Stimulation
6.4. Targeting the Appropriate Neuronal Type
6.5. Central Effects of VNS
6.6. VNS Improves Insulin Sensitivity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOLD | Blood-oxygen-level dependent |
| DAT | Dopamine active transporter |
| DVC | Dorsal vagal complex |
| fMRI | functional magnetic resonance imaging |
| GLP-1r | Glucagon like peptide-1 receptor |
| HMPAO | hexa-methyl-propyl-amineoxime |
| PET | Positron emission tomography |
| SPECT | Single photon emission computed tomography |
| SERT | sodium-dependent serotonin transporter |
| VNS | vagal nerve stimulation |
| VOI | volume of interest |
References
- Duclaux, R.; Mei, N.; Ranieri, F. Conduction velocity along the afferent vagal dendrites: A new type of fibre. J. Physiol. 1976, 260, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iggo, A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q. J. Exp. Physiol. Cogn. Med. Sci. 1957, 42, 398–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottrell, D.F.; Iggo, A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J. Physiol. 1984, 354, 497–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Mesgarzadeh, S.; Ramesh, K.S.; Huey, E.L.; Liu, Y.; Gray, L.A.; Aitken, T.J.; Chen, Y.; Beutler, L.R.; Ahn, J.S.; et al. Genetic identification of vagal sensory neurons that control feeding. Cell 2019, 179, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Zagorodnyuk, V.P.; Chen, B.N.; Costa, M.; Brookes, S.J. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J. Physiol. 2003, 553, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Sclocco, R.; Beissner, F.; Desbordes, G.; Polimeni, J.R.; Wald, L.L.; Kettner, N.W.; Kim, J.; Garcia, R.G.; Renvall, V.; Bianchi, A.M.; et al. Neuroimaging brainstem circuitry supporting cardiovagal response to pain: A combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150189. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.Y.; Bodhit, A.; Derequito, R.; Ansari, S.; Abukhalil, F.; Thenkabail, S.; Ganji, S.; Saravanapavan, P.; Shekar, C.C.; Bidari, S.; et al. Vagus nerve stimulation in ischemic stroke: Old wine in a new bottle. Front. Neurol. 2014, 5, 107. [Google Scholar] [CrossRef] [Green Version]
- Saikali, S.; Meurice, P.; Sauleau, P.; Eliat, P.-A.; Bellaud, P.; Randuineau, G.; Verin, M.; Malbert, C.-H. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J. Neurosci. Methods 2010, 192, 102–109. [Google Scholar] [CrossRef]
- Sauleau, P.; Lapouble, E.; Val-Laillet, D.; Malbert, C.-H. The pig model in brain imaging and neurosurgery. Animal 2009, 3, 1138–1151. [Google Scholar] [CrossRef] [Green Version]
- Malbert, C.H. AniMate. An open source software for absolute PET quantification. In Proceedings of the Annual Congress of the European Association of Nuclear Medicine 43, Barcelona, Spain, 15–19 October 2016. [Google Scholar]
- Gibbons, C.; Hopkins, M.; Beaulieu, K.; Oustric, P.; Blundell, J.E. Issues in measuring and interpreting human appetite (sa-tiety/satiation) and its contribution to obesity. Curr. Obes. Rep. 2019, 8, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Koopmans, S.J.; Schuurman, T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur. J. Pharmacol. 2015, 759, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Doulah, A.; Farooq, M.; Yang, X.; Parton, J.; McCrory, M.A.; Higgins, J.A.; Sazonov, E. Meal microstructure characterization from sensor-based food intake detection. Front. Nutr. 2017, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biraben, A.; Guerin, S.; Bobillier, E.; Malbert, C.H. Central activation after chronic vagus nerve stimulation in pigs: Contribution of functional imaging. Bull. Acad. Vet. Fr. 2008, 161, 441–448. [Google Scholar]
- Lepionka, L.; Malbert, C.-H.; LaPlace, J.P. Proximal gastric distension modifies ingestion rate in pigs. Reprod. Nutr. Dev. 1997, 37, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malbert, C.-H.; Bobillier, E.; Picq, C.; Divoux, J.-L.; Guiraud, D.; Henry, C. Effects of chronic abdominal vagal stimulation of small-diameter neurons on brain metabolism and food intake. Brain Stimul. 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Malbert, C.-H.; Genissel, M.; Divoux, J.-L.; Henry, C. Chronic abdominal vagus stimulation increased brain metabolic connectivity, reduced striatal dopamine transporter and increased mid-brain serotonin transporter in obese miniature pigs. J. Transl. Med. 2019, 17, 78. [Google Scholar] [CrossRef]
- Malbert, C.-H. Brain Imaging during Feeding Behaviour; Wiley Online Library: Angers, France, 2013. [Google Scholar]
- De Graaf, C.; Blom, W.A.M.; Smeets, P.A.M.; Stafleu, A.; Hendriks, H.F.J. Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [CrossRef] [Green Version]
- Warwick, J.M. Imaging of brain function using SPECT. Metab. Brain Dis. 2004, 19, 113–123. [Google Scholar] [CrossRef]
- Murase, K.; Tanada, S.; Fujita, H.; Sakaki, S.; Hamamoto, K. Kinetic behavior of technetium-99m-HMPAO in the human brain and quantification of cerebral blood flow using dynamic SPECT. J. Nucl. Med. 1992, 33, 135–143. [Google Scholar]
- Lapouble, E.; Chauvin, A.; Guerin, S.; Malbert, C.-H. Regional Brain Activation during Proximal Gastric Distension in Pigs. In Joint International Society Meeting in Neurogastroenterology and GI Motility; Wiley-Blackwell: Boston, MA, USA, 2006. [Google Scholar]
- Malbert, C.-H. Porc miniature modèle pour l’innovation thérapeutique—Stimulation vagale et syndrome métabolique. Bull. Académie Vétérinaire Fr. 2018. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, M.; Malbert, C.-H.; Meurice, P.; Val-Laillet, D. Effects of chronic consumption of sugar-enriched diets on brain metabolism and insulin sensitivity in adult Yucatan minipigs. PLoS ONE 2016, 11, e0161228. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Alloosh, M.; Saxena, R.; Van Alstine, W.; Watkins, B.A.; Klaunig, J.E.; Sturek, M.; Chalasani, N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 2009, 50, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahri, S.; Horowitz, M.; Malbert, C.-H. Inward glucose transfer accounts for insulin-dependent increase in brain glucose metabolism associated with diet-induced obesity. Obesity 2018, 26, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, S.J.; Mroz, Z.; Dekker, R.; Corbijn, H.; Ackermans, M.; Sauerwein, H. Association of insulin resistance with hyperglycemia in streptozotocin-diabetic pigs. Metabolism 2006, 55, 960–971. [Google Scholar] [CrossRef]
- Horowitz, M.; Jones, K.; Edelbroek, M.A.L.; Smout, A.J.P.M.; Read, N.W. The effect of posture on gastric emptying and intra-gastric distribution of oil and aqueous meal components and appetite. Gastroenterology 1993, 105, 382–390. [Google Scholar] [CrossRef]
- Janssen, P.; Berghe, P.V.; Verschueren, S.; Lehmann, A.; Depoortere, I.; Tack, J. Review article: The role of gastric motility in the control of food intake. Aliment. Pharmacol. Ther. 2011, 33, 880–894. [Google Scholar] [CrossRef] [Green Version]
- Abell, T.L.; Camilleri, M.; Donohoe, K.; Hasler, W.L.; Lin, H.C.; Maurer, A.H.; McCallum, R.W.; Nowak, T.; Nusynowitz, M.L.; Parkman, H.P.; et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am. J. Gastroenterol. 2008, 103, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.L.; Bartholomeusz, F.D.; Kirkwood, I.D.E.; Chatterton, B.; Summersides, G.; Penglis, S.; Kuchel, T.; Sansom, L. Liquid gastric emptying in the pig: Effect of concentration of inhaled isoflurane. J. Nucl. Med. 2002, 43, 968–971. [Google Scholar]
- Malbert, C.-H.; Mathis, C.; Bobillier, E.; LaPlace, J.P.; Horowitz, M. Measurement of gastric emptying by intragastric gamma scintigraphy. Neurogastroenterol. Motil. 1997, 9, 157–165. [Google Scholar] [CrossRef]
- Blat, S.; Guerin, S.; Chauvin, A.; Bobillier, E.; Le Cloirec, J.; Bourguet, P.; Malbert, C.-H. Role of vagal innervation on intragastric distribution and emptying of liquid and semisolid meals in conscious pigs. Neurogastroenterol. Motil. 2001, 13, 73–80. [Google Scholar] [CrossRef]
- Blat, S.; Guerin, S.; Chauvin, A.; Bobillier-Chaumont, E.; Malbert, C.-H. Dorsal vagal trunk has a preponderant role to control gastric emptying in pigs. Neurogastroenterol. Motil. 1998, 10, 467. [Google Scholar]
- Malbert, C.-H.; Biraben, A.; Guerin, S.; Chauvin, A. Gastric Emptying is Not Altered by Chronic Vagal Stimulation. In Joint International Society Meeting in Neurogastroenterology and GI Motility; Wiley-Blackwell: Boston, MA, USA, 2006. [Google Scholar]
- Ménard, O.; Famelart, M.-H.; Deglaire, A.; Le Gouar, Y.; Guérin, S.; Malbert, C.-H.; Dupont, D. Gastric emptying and dynamic in vitro digestion of drinkable yogurts: Effect of viscosity and composition. Nutrients 2018, 10, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, R.J.; Powley, T. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996, 271, R766–R769. [Google Scholar] [CrossRef] [PubMed]
- Read, N.; French, S.; Cunningham, K. The role of the gut in regulating food intake in man. Nutr. Rev. 2009, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Geliebter, A.; Melton, P.M.; Gage, D.; McCray, R.S.; Hashim, S.A. Gastric balloon to treat obesity: A double-blind study in nondieting subjects. Am. J. Clin. Nutr. 1990, 51, 584–588. [Google Scholar] [CrossRef]
- Oesch, S.; Rüegg, C.; Fischer, B.; Degen, L.; Beglinger, C. Effect of gastric distension prior to eating on food intake and feelings of satiety in humans. Physiol. Behav. 2006, 87, 903–910. [Google Scholar] [CrossRef]
- Sturm, K.; Parker, B.; Wishart, J.; Feinle-Bisset, C.; Jones, K.L.; Chapman, I.; Horowitz, M. Energy intake and appetite are related to antral area in healthy young and older subjects. Am. J. Clin. Nutr. 2004, 80, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Distrutti, E.; Azpiroz, F.; Soldevilla, A.; Malagelada, J. Gastric wall tension determines perception of gastric distention. Gastroenterology 1999, 116, 1035–1042. [Google Scholar] [CrossRef]
- Settell, M.L.; Pelot, N.A.; Knudsen, B.E.; Dingle, A.M.; McConico, A.L.; Nicolai, E.N.; Trevathan, J.K.; Ezzell, J.A.; Ross, E.K.; Gustafson, K.J.; et al. Functional vagotopy in the cervical vagus nerve of the domestic pig: Implications for the study of vagus nerve stimulation. J. Neural Eng. 2020, 17, 026022. [Google Scholar] [CrossRef]
- Malbert, C.H.; Horowitz, M. The pig as a model for human digestive motor activity. In Digestive Physiology in Pigs; Laplace, J.P., Fevrier, C., Barbeau, A., Eds.; EAAP Publication: Paris, France, 1997; pp. 3–13. [Google Scholar]
- Lepionka, L.; Malbert, C. Are fundic receptors sensitive to circumferential wall tension in vivo? Gastroenterology 1998, 114, A787. [Google Scholar] [CrossRef]
- Phillips, R.J.; Powley, T.L. Tension and stretch receptors in gastrointestinal smooth muscle: Re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Rev. 2000, 34, 1–26. [Google Scholar] [CrossRef]
- Wang, Y.B.; De Lartigue, G.; Page, A.J. Dissecting the role of subtypes of gastrointestinal vagal afferents. Front. Physiol. 2020, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Verheijden, S.; Boeckxstaens, G.E. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 2017, 152, 730–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladabaum, U.; Minoshima, S.; Hasler, W.L.; Cross, D.; Chey, W.D.; Owyang, C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 2001, 120, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Roberts, T.P.L.; Mcgonigle, D.J. Gastric fundic distension activates fronto-limbic structures but not primary somatosensory cortex: A functional magnetic resonance imaging study. NeuroImage 2007, 34, 724–732. [Google Scholar] [CrossRef]
- Wang, G.-J.; Tomasi, D.; Backus, W.; Wang, R.; Telang, F.; Geliebter, A.; Korner, J.; Bauman, A.; Fowler, J.S.; Thanos, P.K.; et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage 2008, 39, 1824–1831. [Google Scholar] [CrossRef]
- Alger, S.E.; Payne, J.D. The differential effects of emotional salience on direct associative and relational memory during a nap. Cogn. Affect. Behav. Neurosci. 2016, 16, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- McClure, S.M.; York, M.K.; Montague, P.R. The neural substrates of reward processing in humans: The modern role of fMRI. Neuroscience 2004, 10, 260–268. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Lenard, N.R.; Shin, A.C. Food reward, hyperphagia, and obesity. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1266–R1277. [Google Scholar] [CrossRef] [Green Version]
- Geliebter, A. Neuroimaging of gastric distension and gastric bypass surgery. Appetite 2013, 71, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Lapouble, E.; Guérin, S.; Malbert, C.H. Vagal versus non vagal gastric afferent signals processing in the brain. Gastroenterology 2007, 51, 61–62. [Google Scholar]
- Kumbhari, V.; Oberbach, A.; Nimgaonkar, A. Primary endoscopic therapies for obesity and metabolic diseases. Curr. Opin. Gastroenterol. 2015, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.M.; Geliebter, A. Intragastric balloon treatment for obesity: Review of recent studies. Adv. Ther. 2017, 34, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Layec, S.; Val-Laillet, D.; Heresbach, D.; Malbert, C.-H. Gastric tone, volume and emptying after implantation of an intragastric balloon for weight control. Neurogastroenterol. Motil. 2010, 22, 1016.e266. [Google Scholar] [CrossRef] [PubMed]
- Layec, S.; Lapouble, E.; Val-Laillet, D.; Guérin, S.; Chauvin, A.; Heresbach, D.; Malbert, C.-H. T1805 Chronic but not accute gastric distension activates brain reward circuit. Gastroenterology 2009, 136, A583. [Google Scholar] [CrossRef]
- Sabatinelli, D.; Bradley, M.M.; Lang, P.J.; Costa, V.D.; Versace, F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J. Neurophysiol. 2007, 98, 1374–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roura, E.; Fu, M. Taste, nutrient sensing and feed intake in pigs (130 years of research: Then, now and future). Anim. Feed Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Maltecca, C.; Bergamaschi, M.; Tiezzi, F. The interaction between microbiome and pig efficiency: A review. J. Anim. Breed. Genet. 2020, 137, 4–13. [Google Scholar] [CrossRef]
- Malbert, C.-H.; Horowitz, M.; Young, R.L. Low-calorie sweeteners augment tissue-specific insulin sensitivity in a large animal model of obesity. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2380–2391. [Google Scholar] [CrossRef]
- Malbert, C.H.; Mathis, C. Antro-Pyloric modulation of the transpyloric flow of liquids in pigs. Gastroenterology 1994, 107, 37–46. [Google Scholar] [CrossRef]
- Jones, K.L.; O’Donovan, D.; Horowitz, M.; Russo, A.; Lei, Y.; Hausken, T. Effects of posture on gastric emptying, transpyloric flow, and hunger after a glucose drink in healthy humans. Dig. Dis. Sci. 2006, 51, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Malbert, C.-H.; Leitner, L.-M. Mechanoreceptors sensitive to flow at the gastroduodenal junction of the cat. Am. J. Physiol. Gastrointest. Liver Physiol. 1993, 265, G310–G313. [Google Scholar] [CrossRef] [PubMed]
- Vozzo, R.; Su, Y.-C.; Fraser, R.J.; Wittert, G.A.; Horowitz, M.; Malbert, C.-H.; Shulkes, A.; Volombello, T.; Chapman, I.M. Antropyloroduodenal, cholecystokinin and feeding responses to pulsatile and non-pulsatile intraduodenal lipid infusion. Neurogastroenterol. Motil. 2002, 14, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Peters, T.; Beglinger, C.; Peterli, R. Incretin effects, gastric emptying and insulin responses to low oral glucose loads in patients after gastric bypass and lean and obese controls. Surg. Obes. Relat. Dis. 2016, 12, 1320–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindqvist, A.; Ekelund, M.; Pierzynowski, S.; Groop, L.; Hedenbro, J.; Wierup, N. Gastric bypass in the pig increases GIP levels and decreases active GLP-1 levels. Peptides 2017, 90, 78–82. [Google Scholar] [CrossRef]
- Soty, M.; Gautier-Stein, A.; Rajas, F.; Mithieux, G. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 2017, 25, 1231–1242. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, D.A.; Cota, D.; Seeley, R.J. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu. Rev. Physiol. 2008, 70, 513–535. [Google Scholar] [CrossRef]
- Pal, A.; Rhoads, D.B.; Tavakkoli, A. Effect of portal glucose sensing on systemic glucose levels in SD and ZDF rats. PLoS ONE 2016, 11, e0165592. [Google Scholar] [CrossRef]
- Ionut, V.; Castro, A.V.B.; Woolcott, O.O.; Stefanovski, D.; Iyer, M.S.; Broussard, J.L.; Burch, M.; Elazary, R.; Kolka, C.M.; Mkrtchyan, H.; et al. Hepatic portal vein denervation impairs oral glucose tolerance but not exenatide’s effect on glycemia. Am. J. Physiol. Metab. 2014, 307, E644–E652. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, M.; Nakabayashi, H.; Uehara, K.; Nakagawa, A.; Uchida, K.; Koya, D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am. J. Physiol. Metab. 2013, 305, E376–E387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, O.; Rosenström, U.; Selvaraju, R.K.; Eriksson, B.; Velikyan, I. Species differences in pancreatic binding of DO3A-VS-Cys40-Exendin4. Acta Diabetol. 2017, 54, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malbert, C.-H.; Chauvin, A.; Horowitz, M.; Jones, K.L. Glucose-sensing mediated by portal GLP-1 receptor is markedly impaired in insulin-resistant obese animals. Diabetes 2021, 70, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Malbert, C.-H.; Chauvin, A.; Horowitz, M.; Jones, K.L. Pancreatic GLP-1r binding potential is reduced in insulin-resistant pigs. BMJ Open Diabetes Res. Care 2020, 8, e001540. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, J.; Chauvin, A.; Guerin, S.; Malbert, C.-H. Quantitative Involvement of Duodenal, Portal and Cerebral Nutrient Sensing Towards Food Intake Control; Karger: Paris, France, 2007; Volume 51, (Suppl. 1). [Google Scholar]
- Boubaker, J.; Val-Laillet, D.; Guerin, S.; Malbert, C.-H. Brain processing of duodenal and portal glucose sensing. J. Neuroendocr. 2012, 24, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.; Nichols, T.E.; Knutsson, H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 2016, 113, 7900–7905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gortz, L.; Bjorkman, A.-C.; Andersson, H.; Kral, J. Truncal vagotomy reduces food and liquid intake in man. Physiol. Behav. 1990, 48, 779–781. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul. Pept. 2009, 156, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Kral, J.G.; Paez, W.; Wolfe, B.M. Vagal nerve function in obesity: Therapeutic implications. World J. Surg. 2009, 33, 1995–2006. [Google Scholar] [CrossRef]
- Sarr, M.G.; The EMPOWER Study Group; Billington, C.J.; Brancatisano, R.; Brancatisano, A.; Toouli, J.; Kow, L.; Nguyen, N.T.; Blackstone, R.; Maher, J.W.; et al. The EMPOWER Study: Randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes. Surg. 2012, 22, 1771–1782. [Google Scholar] [CrossRef]
- Bligny, D.; Blat, S.; Chauvin, A.; Guérin, S.; Malbert, C.-H. Reduced mechanosensitivity of duodenal vagal afferent neurons after an acute switch from milk-based to plant-based diets in anaesthetized pigs. J. Physiol. Pharmacol. 2005, 56, 89–100. [Google Scholar] [PubMed]
- De Lartigue, G.; De La Serre, C.B.; Espero, E.; Lee, J.; Raybould, H.E. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E187–E195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, A.J. Vagal afferent dysfunction in obesity: Cause or effect. J. Physiol. 2015, 594, 5–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kentish, S.J.; Vincent, A.D.; Kennaway, D.J.; Wittert, G.; Page, A.J. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J. Neurosci. 2016, 36, 3199–3207. [Google Scholar] [CrossRef] [Green Version]
- de Lartigue, G.; Xu, C. Mechanisms of vagal plasticity influencing feeding behavior. Brain Res. 2018, 1693, 146–150. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, Y.; Shah, S.; Wu, H.; Gautron, L. Levels of Cocaine- and Amphetamine-Regulated Transcript in Vagal Afferents in the Mouse Are Unaltered in Response to Metabolic Challenges. Eneuro 2016. [Google Scholar] [CrossRef] [Green Version]
- Nunez-Salces, M.; Li, H.; Christie, S.; Page, A.J. The Effect of High-Fat Diet-Induced Obesity on the Expression of Nutrient Chemosensors in the Mouse Stomach and the Gastric Ghrelin Cell. Nutrients. 2020, 12, 2493. [Google Scholar] [CrossRef]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013, 207, 275–299. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, D.; Bigiani, A.; Porro, C.A.; Mapelli, J. Inhibitory Plasticity: From Molecules to Computation and Beyond. Int. J. Mol. Sci. 2020, 21, 1805. [Google Scholar] [CrossRef] [Green Version]
- Sobocki, J.; Fourtanier, G.; Estany, J.; Otal, P. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery 2006, 139, 209–216. [Google Scholar] [CrossRef]
- Laskiewicz, J.; Królczyk, G.; Zurowski, G.; Sobocki, J.; Matyja, A.; Thor, P.J. Effects of vagal neuromodulation and vagotomy on control of food intake and body weight in rats. J. Physiol. Pharmacol. 2003, 54, 603–610. [Google Scholar] [PubMed]
- Bugajski, A.J.; Gil, K.; Ziomber, A.; Zurowski, D.; Zaraska, W.; Thor, P.J. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J. Physiol. Pharmacol. 2007, 58, 5–12. [Google Scholar] [PubMed]
- Val-Laillet, D.; Biraben, A.; Randuineau, G.; Malbert, C.-H. Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite 2010, 55, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Dali, M.; Picq, C.; Rossel, O.; Maciejasz, P.; Malbert, C.-H.; Guiraud, D. Comparison of the efficiency of chopped and non-rectangular electrical stimulus waveforms in activating small vagus nerve fibers. J. Neurosci. Methods 2019, 320, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Condamin, M.; Boyer, A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980, 209, 423–431. [Google Scholar] [CrossRef]
- Guiraud, D.; Andreu, D.; Bonnet, S.; Carrault, G.; Couderc, P.; Hagège, A.; Henry, C.; Hernandez, A.; Karam, N.; Le Rolle, V.; et al. Vagus nerve stimulation: State of the art of stimulation and recording strategies to address autonomic function neuromodulation. J. Neural Eng. 2016, 13, 041002. [Google Scholar] [CrossRef]
- Malbert, C.-H. The brain-gut axis: Insights from the obese pig model. Bull. Académie Natl. Médecine 2013, 197, 1683–1694. [Google Scholar] [CrossRef]
- Malbert, C.-H. Could vagus nerve stimulation have a role in the treatment of diabetes? Bioelectron. Med. 2018, 1, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Helmers, S.L.; Begnaud, J.; Cowley, A.; Corwin, H.M.; Edwards, J.C.; Holder, D.L.; Kostov, H.; Larsson, P.G.; Levisohn, P.M.; De Menezes, M.S.; et al. Application of a computational model of vagus nerve stimulation. Acta Neurol. Scand. 2012, 126, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Conway, C.R.; Chibnall, J.T.; Gebara, M.A.; Price, J.L.; Snyder, A.Z.; Mintun, M.A.; Craig, A.B.; Cornell, M.E.; Perantie, D.C.; Giuffra, L.A.; et al. Association of cerebral metabolic activity changes with vagus nerve stimulation antidepressant response in treatment-resistant depression. Brain Stimul. 2013, 6, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Vonck, K.; De Herdt, V.; Bosman, T.; Dedeurwaerdere, S.; Van Laere, K.; Boon, P. Thalamic and limbic involvement in the mechanism of action of vagus nerve stimulation, a SPECT study. Seizure 2008, 17, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lu, K.-H.; Powley, T.L.; Liu, Z. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS ONE 2017, 12, e0189518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malbert, C.-H.; Picq, C.; Divoux, J.-L.; Henry, C.; Horowitz, M. Obesity-associated alterations in glucose metabolism are reversed by chronic bilateral stimulation of the abdominal vagus nerve. Diabetes 2017, 66, 848–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthoud, H.-R.; Münzberg, H.; Morrison, C.D. Blaming the brain for obesity: Integration of hedonic and homeostatic mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.S.; Yang, Y.K.; Yeh, T.L.; Lee, I.-H.; Yao, W.J.; Chiu, N.T.; Lu, R.-B. Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers—A SPECT study. NeuroImage 2008, 40, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B.; Taborsky, G.J. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology 1986, 118, 1551–1557. [Google Scholar] [CrossRef]
- Li, S.; Zhai, X.; Rong, P.; McCabe, M.F.; Wang, X.; Zhao, J.; Ben, H.; Wang, S. Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in zucker fatty rats. PLoS ONE 2014, 9, e112066. [Google Scholar] [CrossRef] [Green Version]
- Kaneto, A.; Miki, E.; Kosaka, K.; Okinaka, S.; Nakao, K. Effects of stimulation of the cingulate gyrus on insulin secretion. Endocrinology 1965, 77, 617–624. [Google Scholar] [CrossRef]
- Daniele, G.; Iozzo, P.; Molina-Carrion, M.; Lancaster, J.; Ciociaro, D.; Cersosimo, E.; Tripathy, D.; Triplitt, C.; Fox, P.; Musi, N.; et al. Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes 2015, 64, 3406–3412. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malbert, C.-H. Vagally Mediated Gut-Brain Relationships in Appetite Control-Insights from Porcine Studies. Nutrients 2021, 13, 467. https://doi.org/10.3390/nu13020467
Malbert C-H. Vagally Mediated Gut-Brain Relationships in Appetite Control-Insights from Porcine Studies. Nutrients. 2021; 13(2):467. https://doi.org/10.3390/nu13020467
Chicago/Turabian StyleMalbert, Charles-Henri. 2021. "Vagally Mediated Gut-Brain Relationships in Appetite Control-Insights from Porcine Studies" Nutrients 13, no. 2: 467. https://doi.org/10.3390/nu13020467
APA StyleMalbert, C.-H. (2021). Vagally Mediated Gut-Brain Relationships in Appetite Control-Insights from Porcine Studies. Nutrients, 13(2), 467. https://doi.org/10.3390/nu13020467




