Monitoring a Calcium Biofortification Workflow in an Orchard of Pyrus communis var. Rocha Applying Precision Agriculture Technology †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofortification Itinerary
2.2. Orchard Characterization and NDVI
2.3. Leaf Gas Exchange
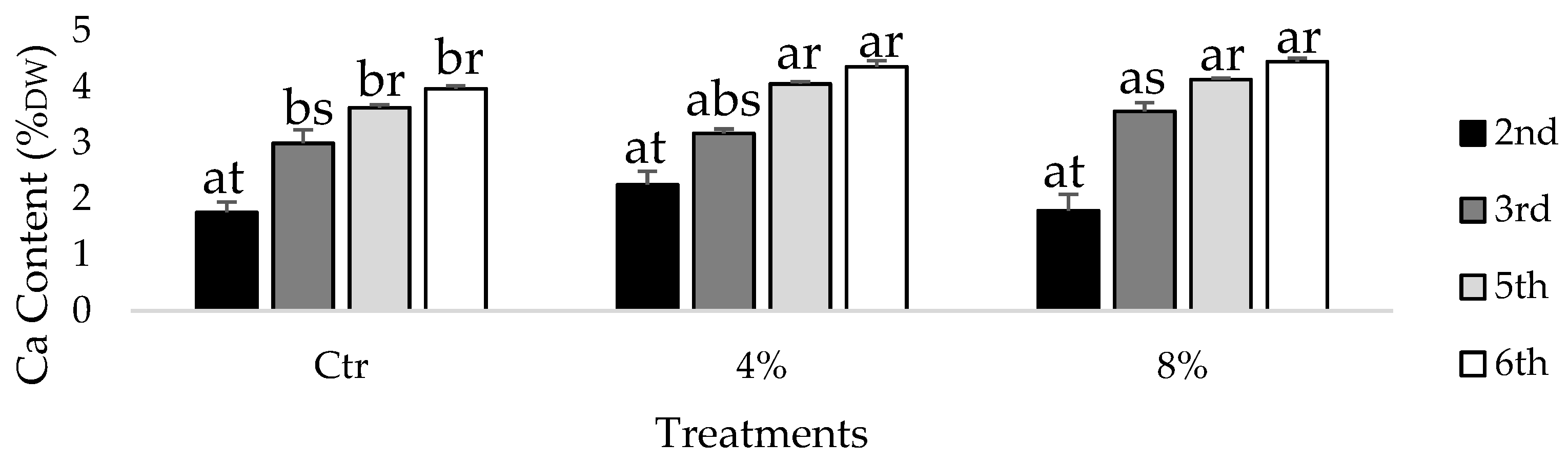
2.4. Calcium Content in Leaves
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 2 January 2021).
- FAO—Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017; pp. 3–16. ISBN 978-92-5-109551-5. [Google Scholar]
- EFSA Panel on NDA. Scientific opinion on dietary reference values for calcium. EFSA J. 2015, 13, 1–82. [Google Scholar] [CrossRef] [Green Version]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loures, L.; Chamizo, A.; Ferreira, P.; Loures, A.; Castanho, R.; Panagopoulos, T. Assessing the Effectiveness of Precision Agriculture Management Systems in Mediterranean Small Farms. Sustainability 2020, 12, 3765. [Google Scholar] [CrossRef]
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Scotti-Campos, P.; Simões, M.; Almeida, A.S.; Legoinha, P.; Pessoa, M.F.; et al. Quantification and Tissue Localization of Selenium in Rice (Oryza sativa L., Poaceae) Grains: A Perspective of Agronomic Biofortification. Plants 2020, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Luís, I.C.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Coelho, A.R.F.; Simões, M.; Patanita, M.; Dôres, J.; Ramalho, J.C.; Silva, M.M.; et al. Zinc Enrichment in Two Contrasting Genotypes of Triticum aestivum L Grains: Interactions between Edaphic Conditions and Foliar Fertilizers. Plants 2021, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Daccak, D.; Pessoa, C.C.; Coelho, A.; Marques, A.; Luís, I.; Silva, M.M.; Guerra, M.; Leitão, R.; Ramalho, J.; Simões, M.; et al. Grapes Enrichment with Zinc for Vinification: Mineral Analysis with Atomic Absorption Spectrophotometry, XRF and Tissue Analysis. In Proceedings of the 1st International Electronic Conference on Plant Science; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Caleiro, J.C.; Simões, M.; Kullberg, J.; Legoinha, P.; Brito, G.; et al. Can Foliar Pulverization with CaCl2 and Ca(NO3)2 Trigger Ca Enrichment in Solanum Tuberosum L. Tubers? Plants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Reboredo, F.; Sánchez, C.; Santos, M.; Ramos, P.; Rodrigues, C.; Ribeiro, V.; Pessoa, M.; Lidon, F. Calcium biofortification of apples: Implications on fruit quality parameters. In COST Action Project FA 0905 Mineral-Improved Crop Production for Healthy Food and Feed, Final Conference Agronomic, Molecular Genetics and Human Nutrition Approaches for Improving the Nutritional Quality and Safety of Food Crops; Ela Quality Resort: Antalya-Belek, Turkey, 2014; pp. 52–53. [Google Scholar]
- Lidon, F.; Ribeiro, V.; Reboredo, F.; Pessoa, M.; Santos, M.; Ramos, P.; Sánchez, C. Calcium biofortification of apples: Interaction with Macronutrients. In COST Action Project FA 0905 Mineral-Improved Crop Production for Healthy Food and Feed, Final Conference Agronomic, Molecular Genetics and Human Nutrition Approaches for Improving the Nutritional Quality and Safety of Food Crops; Ela Quality Resort: Antalya-Belek, Turkey, 2014; pp. 102–103. [Google Scholar]
- Singh, U.; Praharaj, C.; Chaturvedi, S.; Bohra, A. Biofortification: Introduction, approaches, limitations, and challenges. In Biofortification of Food Crops; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New Delhi, India; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; pp. 3–18. [Google Scholar] [CrossRef]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- ANP—Associação Nacional de Produtores de Pera Rocha. Available online: https://perarocha.pt/anp/ (accessed on 9 January 2021).
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Campos, P.S.; Simões, M.; Almeida, A.S.; Pessoa, M.F.; Galhano, G.; et al. Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients. Plants 2021, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; pp. 33–65. [Google Scholar]
- Pessoa, C.C.; Coelho, A.; Marques, A.; Luís, I.; Daccak, D.; Silva, M.M.; Ramalho, J.; Simões, M.; Reboredo, F.; Pessoa, M.; et al. Increase of Calcium in ‘Rocha’ Pear (Pyrus communis L.) for Development of Functional Foods. In Proceedings of the 1st International Electronic Conference on Plant Science; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rebelo, M.C.; Santos, M.E.; Antunes, M.L.; Antonieta, M. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant Soil 1995, 172, 87–96. [Google Scholar] [CrossRef]
- Ramalho, J.D.C.; Nunes, M.A. Photosynthesis impairment in Coffea arabica due to calcium deficiency. Agron. Lusit. 1999, 47, 101–116. [Google Scholar]
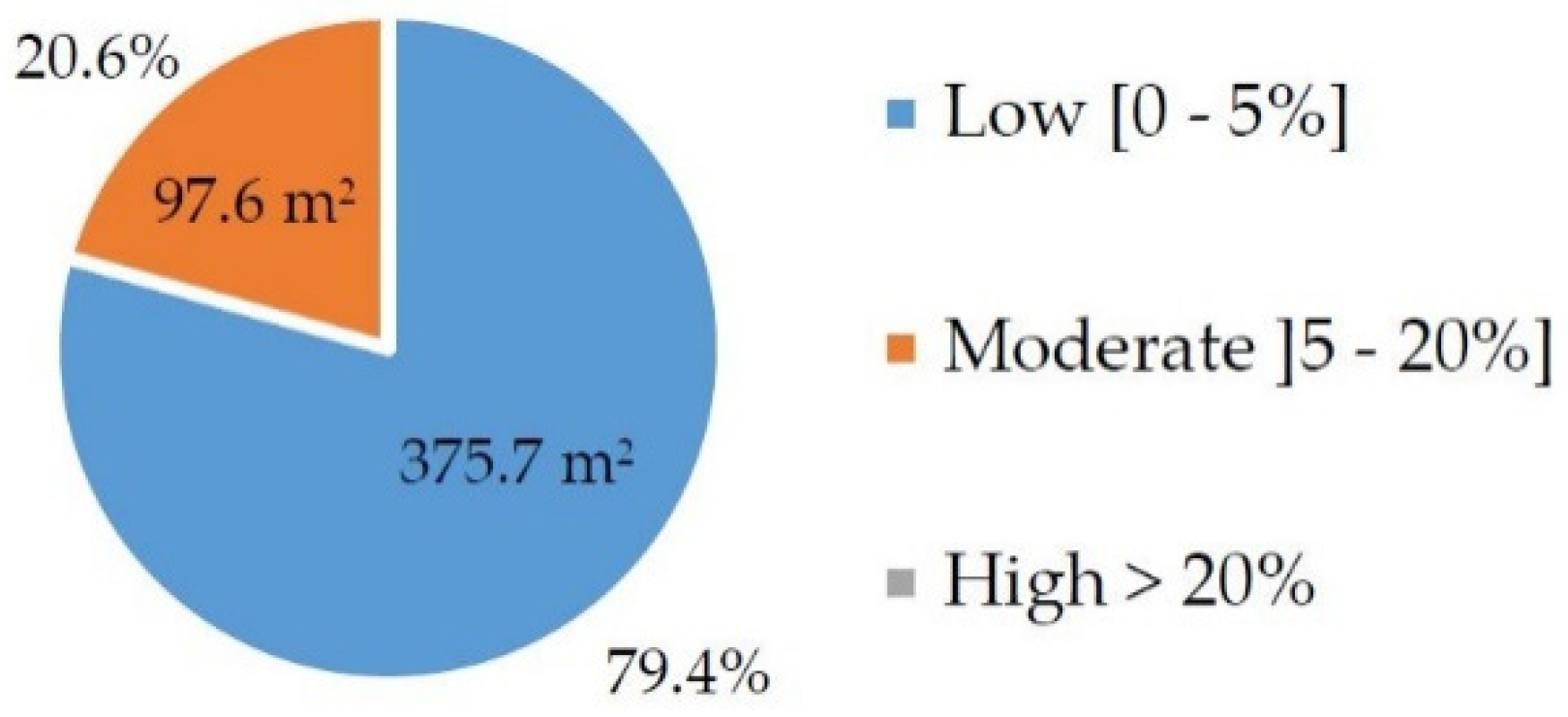
| Treatments | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Ctr | 0.440 | 0.914 | 0.800 ± 0.093 |
| 4% | 0.440 | 0.906 | 0.781 ± 0.111 |
| 8% | 0.420 | 0.914 | 0.797 ± 0.110 |
| Treatments | 12th June | 26th July | 12th September |
|---|---|---|---|
| Pn (µmol CO2 m−2s−1) | |||
| Ctr | 17.69 ± 0.24a,r | 16.09 ± 0.55as | 7.77 ± 0.25at |
| 4% | 17.70 ± 0.13a,r | 15.49 ± 0.23as | 7.47 ± 0.39at |
| 8% | 17.85 ± 0.19a,r | 16.49 ± 0.32as | 8.37 ± 0.33at |
| gs (mmol H2O m−2s−1) | |||
| 0% | 255.6 ± 6.8ar | 199.0 ± 10.9bs | 68.5 ± 3.8at |
| 4% | 252.4 ± 5.1ar | 221.1 ± 4.9bs | 65.5 ± 5.1at |
| 8% | 245.2 ± 3.9ar | 269.0 ± 8.8ar | 76.6 ± 5.5as |
| E (mmol H2O m−2s−1) | |||
| 0% | 3.27 ± 0.06ar | 2.21 ± 0.07bs | 2.03 ± 0.09bs |
| 4% | 3.38 ± 0.04ar | 2.40 ± 0.05bs | 2.17 ± 0.13bs |
| 8% | 3.24 ± 0.03ar | 3.29 ± 0.09ar | 2.53 ± 0.15as |
| iWUE (mmol CO2 mol−1 H2O) | |||
| 0% | 5.43 ± 0.06as | 7.29 ± 0.17ar | 3.95 ± 0.15at |
| 4% | 5.26 ± 0.05as | 6.53 ± 0.17br | 3.51 ± 0.07abt |
| 8% | 5.51 ± 0.06ar | 5.08 ± 0.12cr | 3.41 ± 0.09bs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, C.C.; Daccak, D.; Luís, I.C.; Marques, A.C.; Coelho, A.R.F.; Caleiro, J.; Silva, M.M.; Kullberg, J.C.; Brito, M.G.; Legoinha, P.; et al. Monitoring a Calcium Biofortification Workflow in an Orchard of Pyrus communis var. Rocha Applying Precision Agriculture Technology. Biol. Life Sci. Forum 2021, 3, 3. https://doi.org/10.3390/IECAG2021-09661
Pessoa CC, Daccak D, Luís IC, Marques AC, Coelho ARF, Caleiro J, Silva MM, Kullberg JC, Brito MG, Legoinha P, et al. Monitoring a Calcium Biofortification Workflow in an Orchard of Pyrus communis var. Rocha Applying Precision Agriculture Technology. Biology and Life Sciences Forum. 2021; 3(1):3. https://doi.org/10.3390/IECAG2021-09661
Chicago/Turabian StylePessoa, Cláudia Campos, Diana Daccak, Inês Carmo Luís, Ana C. Marques, Ana Rita F. Coelho, João Caleiro, Maria Manuela Silva, José Carlos Kullberg, Maria Graça Brito, Paulo Legoinha, and et al. 2021. "Monitoring a Calcium Biofortification Workflow in an Orchard of Pyrus communis var. Rocha Applying Precision Agriculture Technology" Biology and Life Sciences Forum 3, no. 1: 3. https://doi.org/10.3390/IECAG2021-09661
APA StylePessoa, C. C., Daccak, D., Luís, I. C., Marques, A. C., Coelho, A. R. F., Caleiro, J., Silva, M. M., Kullberg, J. C., Brito, M. G., Legoinha, P., Simões, M., Pessoa, M. F., Reboredo, F. H., Silva, M. J., Semedo, J. N., Pais, I. P., Rodrigues, A. P., Campos, P. S., Ramalho, J. C., & Lidon, F. C. (2021). Monitoring a Calcium Biofortification Workflow in an Orchard of Pyrus communis var. Rocha Applying Precision Agriculture Technology. Biology and Life Sciences Forum, 3(1), 3. https://doi.org/10.3390/IECAG2021-09661

















