Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Material
2.2. Chemicals
2.3. Extraction Techniques
2.3.1. Atmospheric Solid–Liquid Extraction (ASLE)
2.3.2. Ultrasound-Assisted Extraction (USAE)
2.4. Resin Purification (RP) Process
2.5. Chemical Analyses
2.5.1. Total Polyphenol Content (TPC)
2.5.2. Total Phlorotannin Content (TPhC)
2.5.3. Antioxidant Capacity (AC)/Oxygen Radical Absorbance Capacity Assay (ORAC)
2.5.4. Antioxidant Capacity (AC)/DPPH Assay
2.5.5. Fourier Transform Infrared Spectroscopy Analysis of USAE and RP Streams
2.5.6. Mannitol Content Determination
2.5.7. Tentative Identification of Phlorotannins Using a UHPLC Coupled with a QToF Detector
Sample Injection and Chromatograph and Detector Conditions
Identification of Phlorotannins
Use of Areas
2.6. Statistical Analysis
2.7. Relative Mass Balance and Selective Separation Performance Parameters
3. Results and Discussion
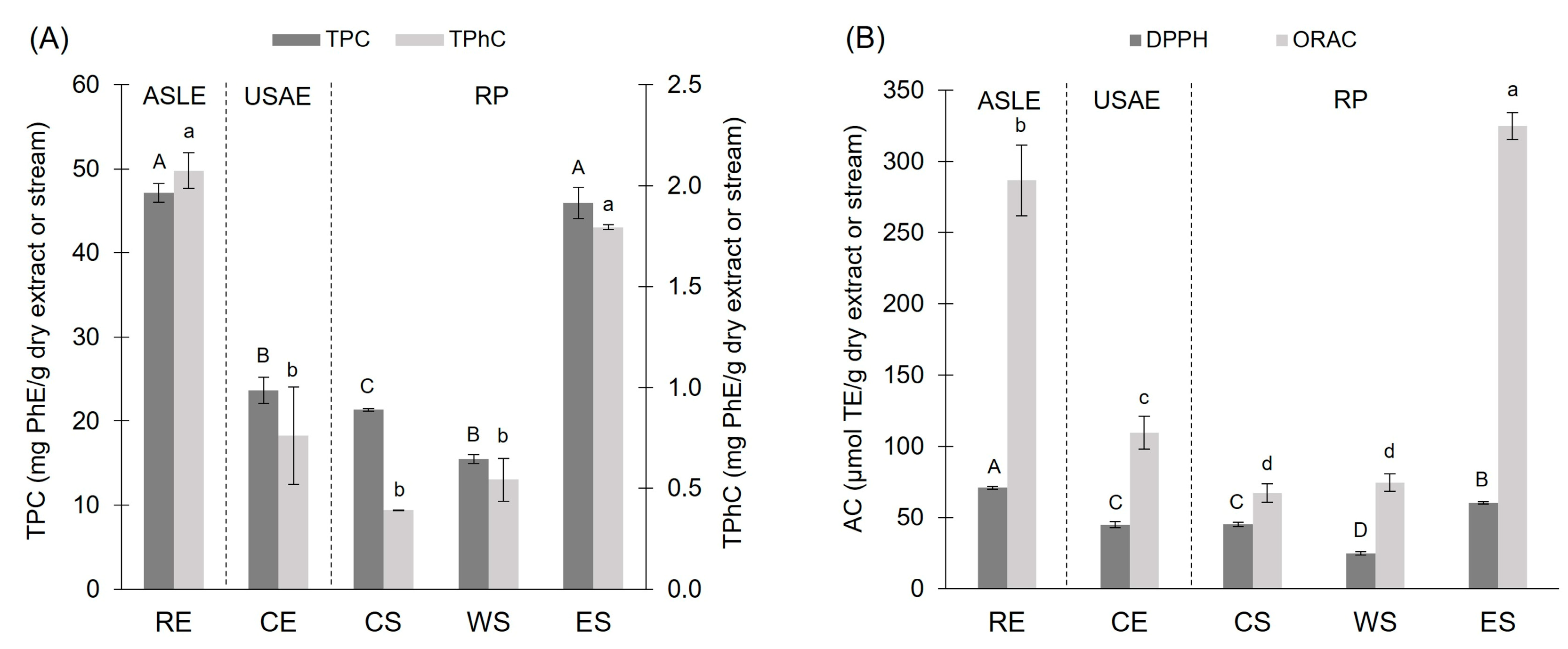
3.1. Spectrophotometric Characterization and Quantification: TPC, TPhC, ACORAC, and ACDPPH
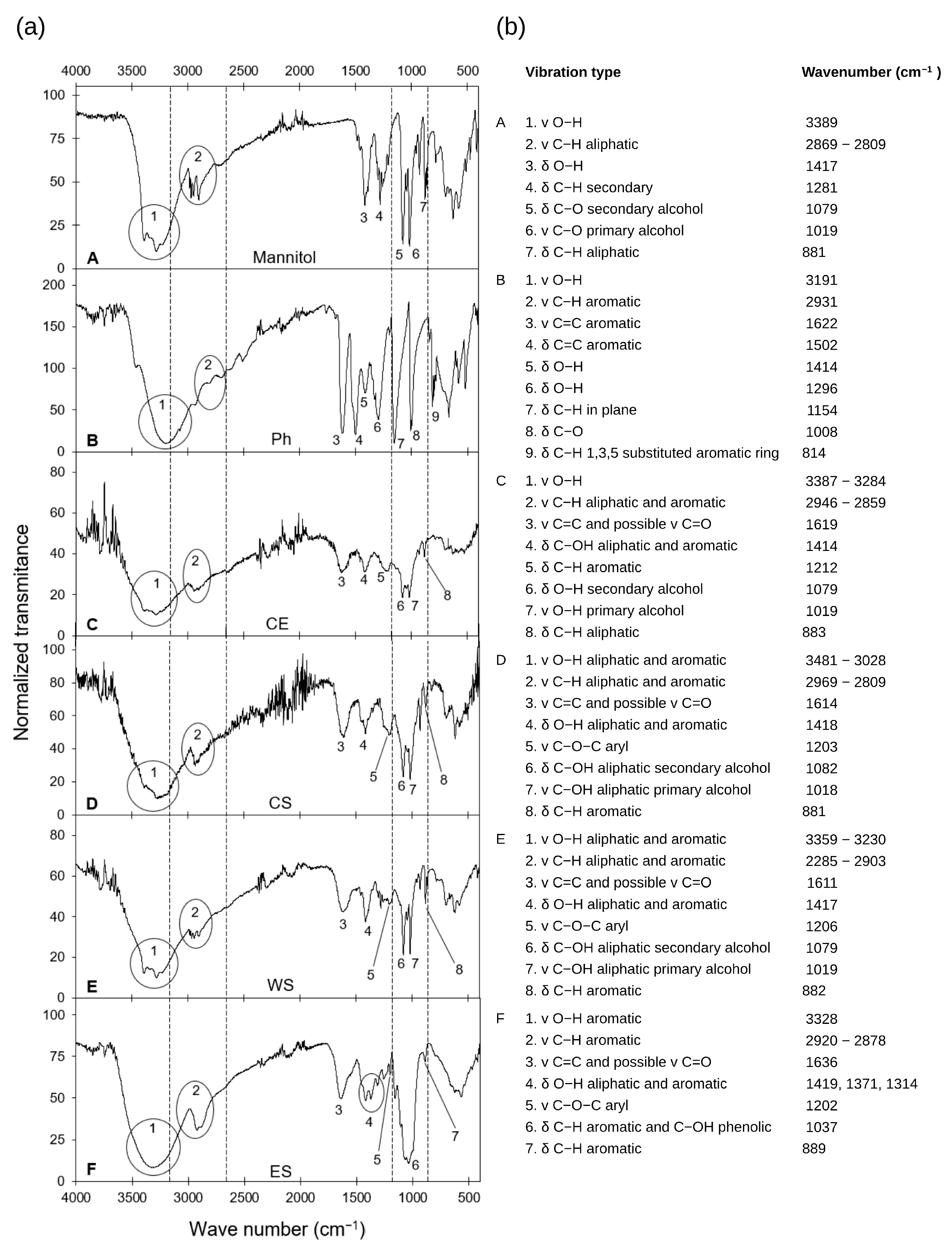
3.2. Fourier Transform Infrared Spectroscopy Analysis
3.3. Mannitol Content
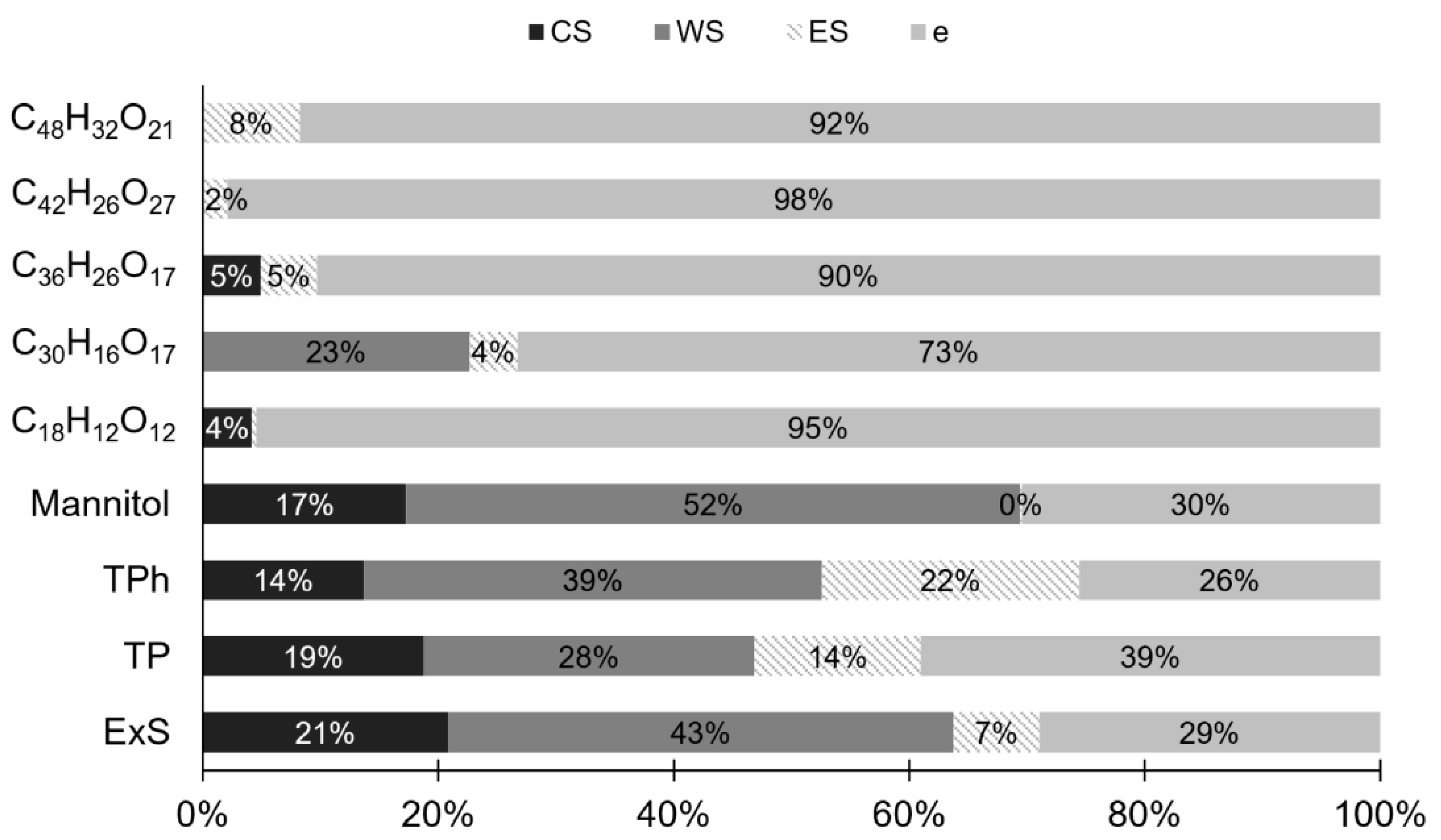
3.4. Tentative Identification of Phlorotannins and Determination of Their Relative Abundances by UHPLC-QToF Mass Spectrometry
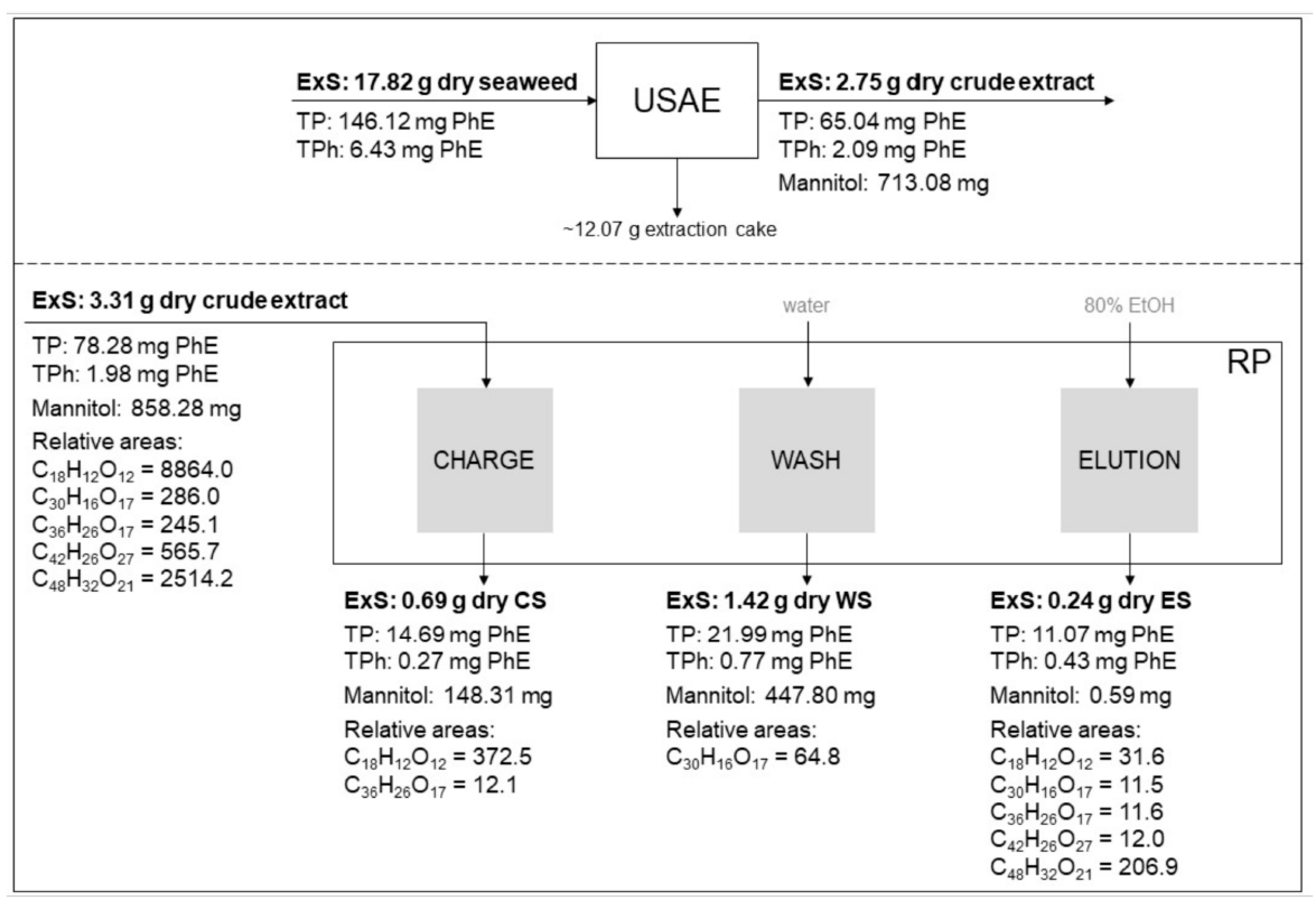
3.5. Process Evaluation: Quality Parameters and RP Mass Balance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soquetta, M.B.; Terra, L.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Perez-Correa, J.R. Polyphenols of Carménère Grapes. Mini Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from brown algae: A review on their antioxidant mechanisms and applications in oxidative stress-mediated diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Simón, L.; Arazo-Rusindo, M.; Quest, A.F.G.; Mariotti-Celis, M.S. Phlorotannins: Novel Orally Administrated Bioactive Compounds That Induce Mitochondrial Dysfunction and Oxidative Stress in Cancer. Antioxidants 2023, 12, 1734. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Mariotti-Celis, M.S.; Parada, J.; Pedreschi, F.; Pérez-Correa, J.R. Pressurized Hot Liquid Extraction with 15% v/v Glycerol-Water as An Effective Environment-Friendly Process to Obtain Durvillaea incurvata and Lessonia spicata Phlorotannin Extracts with Antioxidant and Antihyperglycemic Potential. Antioxidants 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kwon, O.I.; Hwang, H.J.; Shin, H.-C.; Yang, S. Therapeutic effects of phlorotannins in the treatment of neurodegenerative disorders. Front. Mol. Neurosci. 2023, 16, 1193590. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Smyth, T.J.; Ahmed, A.M.; Sweeney, T. A cold water extract of Fucus vesiculosus inhibits lipopolysaccharide (LPS) induced pro-inflammatory responses in the porcine colon ex-vivo model. Innov. Food Sci. Emerg. Technol. 2016, 37, 229–236. [Google Scholar] [CrossRef]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. MicroRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of low glycemic response foods using functional ingredients from seaweed. J. Funct. Foods. 2019, 56, 33–39. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Noticias Ambientales. Chile Faces the Disappearance of 80 Beaches: A Concerning Call to Action. Available online: https://noticiasambientales.com/environment-en/chile-faces-the-disappearance-of-80-beaches-a-concerning-call-to-action/ (accessed on 2 February 2025).
- Food and Agriculture Organization (FAO). Living Climate Change on the Coastline of Chile. Available online: https://www.fao.org/newsroom/story/Living-climate-change-on-the-coastline-of-Chile/en (accessed on 2 February 2025).
- López, B.A.; Macaya, E.C.; Jeldres, R.; Valdivia, N.; Bonta, C.C.; Tala, F.; Thiel, M. Spatio-temporal variability of strandings of the southern bull kelp Durvillaea antarctica (Fucales, Phaeophyceae) on beaches along the coast of Chile—Linked to local storms. J. Appl. Phycol. 2019, 31, 2159–2173. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC—Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Ferreira-Anta, T.; Torres, M.D.; Vilarino, J.M.; Dominguez, H.; Flórez-Fernández, N. Green Extraction of Antioxidant Fractions from Humulus lupulus Varieties and Microparticle Production via Spray-Drying. Foods 2023, 12, 3881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Yang, R.; Zhang, Y.; Chen, Y.; Jiang, C.; Li, X. Synergistic Therapeutic Effects of D-Mannitol–Cerium–Quercetin (Rutin) Coordination Polymer Nanoparticles on Acute Lung Injury. Molecules 2024, 29, 2819. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Villain, F. Free radical scavenging properties of mannitol and its role as a constituent of hyaluronic acid fillers: A literature review. Int. J. Cosmet. Sci. 2017, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Camilo, C.; Mateos, R.; Pérez-Correa, J.R. A macroporous resin purification process to obtain food-grade phlorotannin-rich extracts with α-glucosidase inhibitory activity from Chilean brown seaweeds: An UHPLC-MSn profiling. Food Chem. 2023, 402, 134472. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Dittrich, M. Fourier Transform Infrared Spectroscopy for Molecular Analysis of Microbial Cells. In Microbial Systems Biology. Methods in Molecular Biology 881; Navid, A., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 187–211. [Google Scholar] [CrossRef]
- Gheda, S.; Hamouda, R.A.; Naby, M.A.; Mohamed, T.M.; Al-Shaikh, T.M.; Khamis, A. Potent Effect of Phlorotannins Derived from Sargassum linifolium as Antioxidant and Antidiabetic in a Streptozotocin-Induced Diabetic Rats Model. Appl. Sci. 2023, 13, 4711. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food. Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, A.L.; Jarratt, G.; Haritos, V.S. Analysis of the fate of valuable bioactive polyphenols during commercial winemaking and their partitioning into wastes for valorization. Clean Waste Syst. 2023, 5, 100104. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The impact of temperature and ethanol concentration on the global recovery of specific polyphenols in an integrated HPLE/RP process on Carménère pomace extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2020. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.-J.; Cho, S. Enrichment and purification of marine polyphenol phlorotannins using macroporous adsorption resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Lienqueo, M.E. Purification of phlorotannins from Macrocystis pyrifera using macroporous resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mariotti-Celis, M.S.; Rivera-Tovar, P.R.; Huamán-Castilla, N.L.; Pérez-Correa, J.R. Purification and fractionation of crude seaweed extracts by adsorption-desorption processes. In Marine Phenolic Compounds: Science and Engineering; Pérez-Correa, J.R., Mateos, R., Domínguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 187–215. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ismail, M.M.; El Zokm, G.M.; El Sikaily, A.M.; Selim, A.I.; Ismail, G.A. Chemodiversity and bioactivity assessment of phlorotannins from some Phaeophyta species from the Red Sea. J. Appl. Phycol. 2023, 35, 1769–1788. [Google Scholar] [CrossRef]
- Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal. Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Maqui (Aristotelia chilensis (Mol.) Stuntz) and murta (Ugni molinae Turcz): Native Chilean sources of polyphenol compounds. Mini Rev. Org. Chem. 2019, 16, 261–276. [Google Scholar] [CrossRef]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. Potential of Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound Assisted Extraction against Aging Related Diseases. Foods 2024, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Tovar, P.R.; Torres, M.D.; Camilo, C.; Mariotti-Celis, M.S.; Domínguez, H.; Pérez-Correa, J.R. Multi-response optimal hot pressurized liquid recovery of extractable polyphenols from leaves of maqui (Aristotelia chilensis [Mol.] Stuntz). Food Chem. 2021, 357, 129729. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, J.H.; Han, J.S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm. Biol. 2017, 55, 1149–1154. [Google Scholar] [CrossRef]
- Han, E.; Kim, H.-S.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Jeon, Y.-J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.-Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.; Pozharitskaya, O.; Shevyrin, V.; Kovaleva, E.; Flisyuk, E.; Shikov, A. Optimization of Extraction of Phlorotannins from the Arctic Fucus vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Yeo, M.; Jung, W.-K.; Kim, G. Fabrication, characterisation and biological activity of phlorotannin-conjugated PCL/β-TCP composite scaffolds for bone tissue regeneration. J. Mater. Chem. 2012, 22, 3568. [Google Scholar] [CrossRef]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of mannitol extracts from brown macro algae by thermophilic Clostridia. Front. Microbiol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll. 2021, 112, 106332. [Google Scholar] [CrossRef]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of Sugar Alcohols as Antidiabetic Supplements: A Review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Milman, B.L. General principles of identification by mass spectrometry. TrAC—Trends Anal. Chem. 2015, 69, 24–33. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Castellanos, L.; Ottens, M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Barba, F.; Lorenzo, J. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Hamid, S.S.; Wakayama, M.; Ichihara, K.; Sakurai, K.; Ashino, Y.; Kadowaki, R.; Soga, T.; Tomita, M. Metabolome profiling of various seaweed species discriminates between brown, red, and green algae. Planta 2019, 249, 1921–1947. [Google Scholar] [CrossRef] [PubMed]


| Polymerization Degree | Molecular Formula | Theoretical [M − H]− (m/z) | Observed [M − H]− (m/z) | Error (ppm) | MS1 (m/z) | MS2 (m/z) |
|---|---|---|---|---|---|---|
| Trimer | C18H11O12 (Carmalol) | 419.0251 | 419.0241 | 2.3 | 389 (−30), 243 (−PGU + H2O + 2H+), 116 (PGU + H+) | N.D. |
| Pentamer | C30H15O17 (Carmalol) | 647.0309 | 647.0368 | −9.1 | 535 (111), 389 (PGU + H2O + 2H+), 243 (PGU + H2O + 2H+), 116 (PGU + H+) | 116, 100 (–O) |
| Hexamer | C36H25O17 (Fucol) | 729.1092 | 729.1088 | 0.5 | 681 (47), 535 (−PGU + H2O + 2H+), 389 (−PGU + H2O + 2H+), 243 (−PGU + H2O + 2H+), 116 (PGU + H+) | A) 116, 100 (–O) |
| Heptamer | C42H25O27 (Eckol) | 961.0583 | 961.0578 | 0.6 | 815 (145), 681 (134), 535 (−PGU + H2O + 2H+), 389 (−PGU + H2O + 2H+), 243 (−PGU + H2O + 2H+), 116 (PGU + H+) | N.D. |
| Octamer | C48H31O21 (Eckol) | 943.1358 | 943.1305 | 5.6 | 791 (151), 501 (−2PGU + H2O + 2H+), 389 (112), 243 (−PGU + H2O + 2H+), 116 (PGU + H+) | A) 791, 225 (566), 167 (60), 81 (84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Tovar, P.R.; Contreras-Contreras, G.; Rivas-Reyes, P.I.; Pérez-Jiménez, J.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S. Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants 2025, 14, 250. https://doi.org/10.3390/antiox14030250
Rivera-Tovar PR, Contreras-Contreras G, Rivas-Reyes PI, Pérez-Jiménez J, Martínez-Cifuentes M, Pérez-Correa JR, Mariotti-Celis MS. Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants. 2025; 14(3):250. https://doi.org/10.3390/antiox14030250
Chicago/Turabian StyleRivera-Tovar, Pamela Raquel, Gabriela Contreras-Contreras, Paulina Isabel Rivas-Reyes, Jara Pérez-Jiménez, Maximiliano Martínez-Cifuentes, José Ricardo Pérez-Correa, and María Salomé Mariotti-Celis. 2025. "Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization" Antioxidants 14, no. 3: 250. https://doi.org/10.3390/antiox14030250
APA StyleRivera-Tovar, P. R., Contreras-Contreras, G., Rivas-Reyes, P. I., Pérez-Jiménez, J., Martínez-Cifuentes, M., Pérez-Correa, J. R., & Mariotti-Celis, M. S. (2025). Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants, 14(3), 250. https://doi.org/10.3390/antiox14030250








