Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children
Abstract
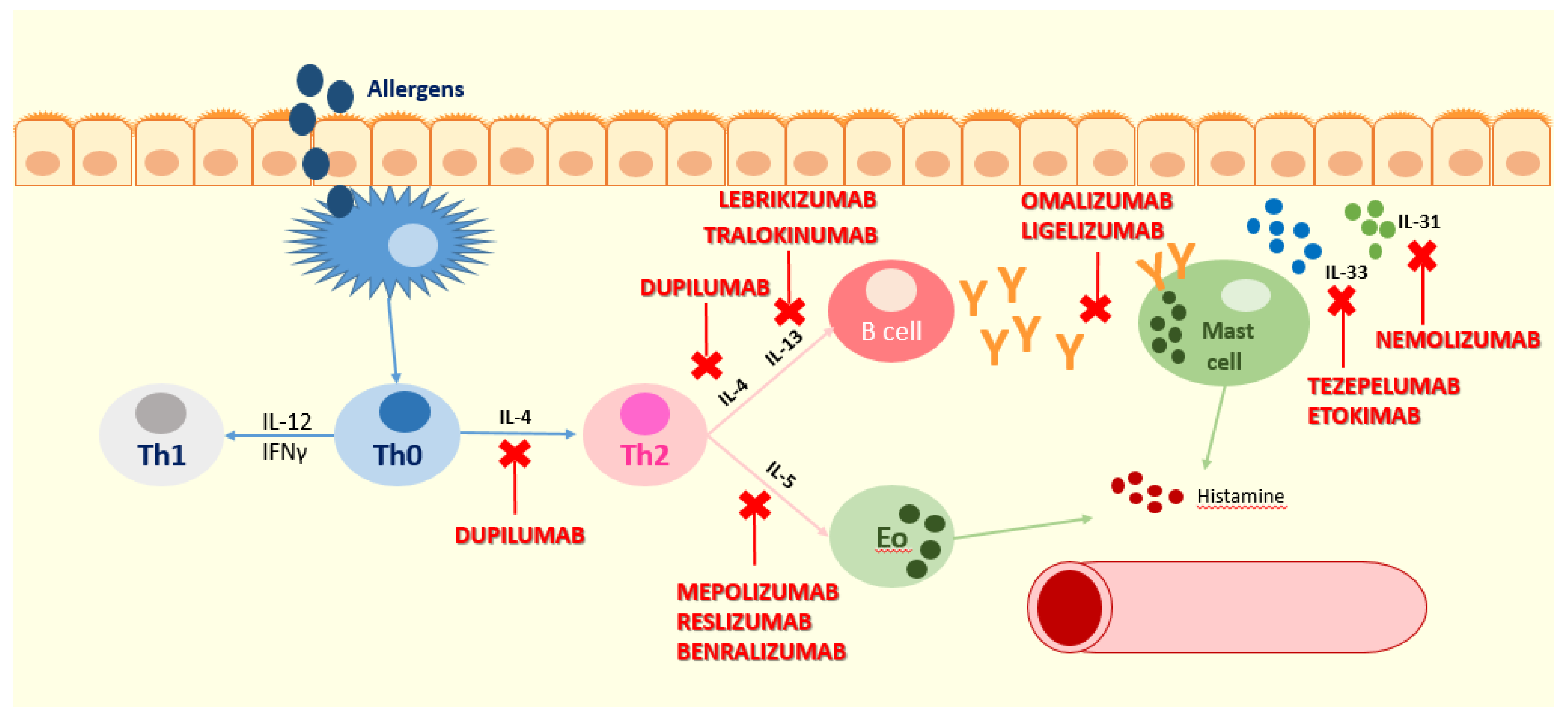
:1. Introduction
2. Atopic Dermatitis
3. Biologics in Atopic Dermatitis
3.1. Omalizumab
3.2. Dupilumab
3.3. Mepolizumab
3.4. Tezepelumab and Etokimab
3.5. Nemolizumab
3.6. Lebrikizumab and Tralokinumab
3.7. OX40 Inhibitors
3.8. Fezakinumab
3.9. JAK Inhibitors
4. Conclusions Regarding the Use of Biologic Drugs for Atopic Dermatitis in Children
5. Chronic Urticaria
6. Biologics in Chronic Spontaneous Urticaria
7. Conclusions Regarding the Use of Biologic Drugs for Chronic Urticaria in Children
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, D.; Di Filippo, P.; Attanasi, M.; Lizzi, M.; Di Pillo, S.; Chiarelli, F. Biologic therapy and severe asthma in children. Biomedicines 2021, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Williams, H.C.; Clayton, T.O.; Robertson, C.F.; Asher, M.I. Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J. Allergy Clin. Immunol. 2009, 124, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef]
- Stefanovic, N.; Flohr, C.; Irvine, A.D. The exposome in atopic dermatitis. Allergy 2020, 75, 63–74. [Google Scholar] [CrossRef]
- Williams, H.C. (Ed.) Atopic Dermatitis; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521570756. [Google Scholar]
- Flohr, C.; Yeo, L. Atopic dermatitis and the hygiene hypothesis revisited. Curr. Probl. Dermatol. 2011, 41, 1–34. [Google Scholar] [CrossRef]
- Strachan, D.P. Family size, infection and atopy: The first decade of the “hygiene hypothesis”. Thorax 2000, 55, S2–S10. [Google Scholar] [CrossRef] [Green Version]
- Abuabara, K.; Hoffstad, O.; Troxel, A.B.; Gelfand, J.M.; McCulloch, C.E.; Margolis, D.J. Patterns and predictors of atopic dermatitis disease control past childhood: An observational cohort study. J. Allergy Clin. Immunol. 2018, 141, 778–780. [Google Scholar] [CrossRef] [Green Version]
- Abuabara, K.; Yu, A.M.; Okhovat, J.-P.; Allen, I.E.; Langan, S.M. The prevalence of atopic dermatitis beyond childhood: A systematic review and meta-analysis of longitudinal studies. Allergy 2018, 73, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Abuabara, K.; Ye, M.; McCulloch, C.E.; Sullivan, A.; Margolis, D.J.; Strachan, D.P.; Paternoster, L.; Yew, Y.W.; Williams, H.C.; Langan, S.M. Clinical onset of atopic eczema: Results from 2 nationally representative British birth cohorts followed through midlife. J. Allergy Clin. Immunol. 2019, 144, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Bylund, S.; Kobyletzki, L.; Svalstedt, M. Prevalence and incidence of atopic dermatitis: A systematic review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef]
- Kobyletzki, L.; Bornehag, C.; Breeze, E.; Larsson, M.; Lindström, C.; Svensson, Å. Factors associated with remission of eczema in children: A population-based follow-up study. Acta Derm. Venereol. 2014, 94, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paternoster, L.; Savenije, O.E.M.; Heron, J.; Evans, D.M.; Vonk, J.M.; Brunekreef, B.; Wijga, A.H.; Henderson, A.J.; Koppelman, G.H.; Brown, S.J. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J. Allergy Clin. Immunol. 2018, 141, 964–971. [Google Scholar] [CrossRef] [Green Version]
- Roduit, C.; Frei, R.; Depner, M.; Karvonen, A.M.; Renz, H.; Braun-Fahrländer, C.; Schmausser-Hechfellner, E.; Pekkanen, J.; Riedler, J.; Dalphin, J.-C.; et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr. 2017, 171, 655. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Terron-Kwiatkowski, A.; Hull, P.R.; O’Regan, G.M.; Clayton, T.H.; Watson, R.M.; Carrick, T.; Evans, A.T.; Liao, H.; Zhao, Y.; et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007, 39, 650–654. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus—And TH2-associated cytokine IL–31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, S.S.; Bautista, D.M. Basophils add fuel to the flame of eczema itch. Cell 2021, 21, 294–296. [Google Scholar] [CrossRef]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomon, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part II. JEADV 2018, 32, 850–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B.; Brehler, A.C.; Novak, N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, S.; Heul, A.V.; Kim, B.S. New and emerging treatments for inflammatory itch. Ann. Allergy Asthma Immunol. 2021, 126, 13–20. [Google Scholar] [CrossRef]
- Boguniewicz, M. Biologic therapy for atopic dermatitis: Moving beyond the practice parameter and guidelines. J. Allergy Clin. Immunol. Pract. 2019, 5, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.Y.G. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Fernández-Antón Martínez, M.C.; Leis-Dosil, V.; Alfageme-Roldán, F.; Paravisini, A.; Sánchez-Ramón, S.; Suárez Fernández, R. Omalizumab en el tratamiento de la dermatitis atópica. Actas Dermosifiliogr. 2012, 103, 624–628. [Google Scholar] [CrossRef]
- Lieberman, P. The unusual suspects: A surprise regarding reactions to omalizumab. Allergy Asthma Proc. 2007, 28, 259–261. [Google Scholar] [CrossRef]
- Campbell, J.D.; Spackman, D.E.; Sullivan, S. Revisiting the costeffectivenes of omalizumab. Allergy 2007, 62, 1469. [Google Scholar] [CrossRef]
- Rohner, M.H.; Thormann, K.; Cazzaniga, S.; Yousefi, S.; Simon, H.; Schlapbach, C.; Simon, D. Dupilumab reduces inflammation and restores the skin barrier in patients with atopic dermatitis. Allergy 2021, 76, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Manti, S.; Marseglia, A.; De Filippo, M.; De Sando, E.; Foiadelli, T.; Marseglia, G.L. Biologics in Children with Allergic Diseases. Curr. Pediatr. Rev. 2020, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate–to–severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Hamilton, J.D.; Suárez-Fariñas, M.; Dhingra, N.; Cardinale, I.; Li, X.; Kostic, A.; Ming, J.E.; Radin, A.R.; Krueger, J.G.; Graham, N.; et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Tsianakas, A.; Luger, T.A.; Radin, A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: Results from a randomized, placebo-controlled clinical trial. Br. J. Dermatol. 2018, 178, 406–414. [Google Scholar] [CrossRef]
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebocontrolled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double–blinded, placebo–controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Deleuran, M.; Thaçi, D.; Beck, L.A.; de Bruin-Weller, M.; Blauvelt, A.; Forman, S.; Bissonnette, R.; Reich, K.; Soong, W.; Hussain, I.; et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J. Am. Acad. Dermatol. 2020, 82, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Bjerre, R.D.; Bandier, J.; Skov, L.; Engstrand, L.; Johansen, J.D. The role of the skin microbiome in atopic dermatitis: A systematic review. Br. J. Dermatol. 2017, 177, 1272–1278. [Google Scholar] [CrossRef]
- Callewaert, C.; Nakatsuji, T.; Knight, R.; Kosciolek, T.; Vrbanac, A.; Kotol, P.; Ardeleanu, M.; Hultsch, T.; Guttman-Yassky, E.; Bissonnette, R.; et al. IL-4Rα blockade by dupilumab decreases staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J. Investig. Dermatol. 2020, 140, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A.; et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: A phase 3 randomized clinical trial. JAMA Dermatol. 2020, 156, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Cork, M.J.; Thac, D. Dupilumab provides favourable long-term safety and efficacy in children aged ≥6 to <12 years with uncontrolled severe atopic dermatitis: Results from an open-label phase IIa study and subsequent phase III open–label extension study. Br. J. Dermatol. 2020, 184, 857–870. [Google Scholar] [CrossRef]
- Paller, A.S.; Siegfried, E.C.; Thac, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef]
- Paller, A.S.; Siegfried, E.C.; Simpson, E.L.; Cork, M.J.; Lockshin, B.; Kosloski, M.P.; Kamal, M.A.; Davis, J.D.; Sun, X.; Pirozzi, G.; et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: Pharmacokinetics, safety and efficacy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 464–475. [Google Scholar] [CrossRef]
- Dupixent® (Dupilumab) Pivotal Trial Meets All Primary and Secondary Endpoints Becoming First Biologic Medicine to Significantly Reduce Signs and Symptoms of Moderate-to-Severe atopic Dermatitis in Children as Young as 6 Months. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-08-30-07-00-00-2288011 (accessed on 4 September 2021).
- Oldhoff, J.M.; Darsow, U.; Werfel, T.; Katzer, K.; Wulf, A.; Laifaoui, J.; Hijnen, D.J.; Plötz, S.; Knol, E.F.; Kapp, A.; et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 2005, 60, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Oldhoff, J.M.; Darsow, U.; Werfel, T.; Bihari, I.C.; Katzer, K.; Laifaoui, J.; Plötz, S.; Kapp, A.; Knol, E.F.; Bruijnzeel-Koomen, C.A.; et al. No effect of anti–interleukin-5 therapy (mepolizumab) on the atopy patch test in atopic dermatitis patients. Int. Arch. Allergy Immunol. 2006, 141, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Oh, M.-H.; Yu, J.; Liu, Y.J.; Zheng, T. The role of TSLP in IL-13-induced atopic march. Sci. Rep. 2011, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, 515. [Google Scholar] [CrossRef]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp. Dermatol. 2018, 27, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of chronic pruritus in atopic dermatitis: Getting the itch out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti-interleukin-31 receptor a antibody for atopic dermatitis. NEJM 2017, 376, 826. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. JAMA Dermatol. 2020, 156, 411. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.-P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate–to–severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Pavel, A.B.; Zhou, L.; Estrada, Y.D.; Zhang, N.; Xu, H.; Peng, X.; Wen, H.-C.; Govas, P.; Gudi, G.; et al. GBR 830, an anti–OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 482–493.e7. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; Jim On, S.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019, 143, 142–154. [Google Scholar] [CrossRef]
- Katoh, N. Emerging treatments for atopic dermatitis. J. Dermatol. 2021, 48, 152–157. [Google Scholar] [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging topical and systemic jak inhibitors in dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Lacour, J.-P.; Spelman, L.; Galimberti, R.; Eichenfield, L.F.; Bissonnette, R.; King, B.A.; Thyssen, J.P.; Silverberg, J.I.; Bieber, T.; et al. Baricitinib in patients with moderate–to–severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef]
- Olumiant (Baricitinib). EU Summary of Product Characteristics: Olumiant. 2018. Available online: https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf (accessed on 4 September 2021).
- Biggioggero, M.; Becciolini, A.; Crotti, C.; Agape, E. Upadacitinib and filgotinib: The role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.-E.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, M.; Ruggiero, A.; Fontanella, G.; Fabbrocini, G.; Patruno, C. New emergent therapies for atopic dermatitis: A review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol. Ther. 2021, 34. [Google Scholar] [CrossRef]
- Miyano, T.; Irvine, A.D.; Tanaka, R.J. A mathematical model to identify optimal combinations of drug targets for dupilumab poor responders in atopic dermatitis. Allergy 2021, all.14870. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Latiff, A.A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Bedrikow, B.R.; et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A.; Lang, D.M.; Khan, D.A.; Craig, T.; Dreyfus, D.; Hsieh, F.; Sheikh, J.; Weldon, D.; Zuraw, B.; Bernstein, D.I.; et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J. Allergy Clin. Immunol. 2014, 133, 1270–1277.e66. [Google Scholar] [CrossRef]
- Kanani, A.; Betschel, S.D.; Warrington, R. Urticaria and angioedema. Allergy Asthma Clin. Immunol. 2018, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Haynes, B.; Soderberg KA, F.A. The Immune System in Health and Disease. Harrison’s Principles of Internal Medicine; McGraw Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Magen, E.; Zueva, E.; Mishal, J.; Schlesinger, M. The clinical and laboratory characteristics of acute spontaneous urticaria and its progression to chronic spontaneous urticaria. Allergy Asthma Proc. 2016, 37, 394–399. [Google Scholar] [CrossRef]
- Zuberbier, T.; Balke, M.; Worm, M.; Edenharter, G.; Maurer, M. Epidemiology of urticaria: A representative cross-sectional population survey. Clin. Exp. Dermatol. 2010, 35, 869–873. [Google Scholar] [CrossRef]
- Lee, S.J.; Ha, E.K.; Jee, H.M.; Lee, K.S.; Lee, S.W.; Kim, M.A.; Kim, D.H.; Jung, Y.-H.; Sheen, Y.H.; Sung, M.S.; et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy. Asthma Immunol. Res. 2017, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Cantarutti, A.; Donà, D.; Visentin, F.; Borgia, E.; Scamarcia, A.; Cantarutti, L.; Peruzzi, E.; Egan, C.G.; Villa, M.; Giaquinto, C. Epidemiology of frequently occurring skin diseases in italian children from 2006 to 2012: A retrospective, population-based study. Pediatr. Dermatol. 2015, 32, 668–678. [Google Scholar] [CrossRef]
- Powell, R.J.; Leech, S.C.; Till, S.; Huber, P.A.J.; Nasser, S.M.; Clark, A.T. BSACI guideline for the management of chronic urticaria and angioedema. Clin. Exp. Allergy 2015, 45, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Altrichter, S.; Borzova, E.; Giménez-Arnau, A.; Grattan, C.E.H.; Lawlor, F.; Mathelier-Fusade, P.; Meshkova, R.Y.; Zuberbier, T.; Metz, M.; et al. The definition, diagnostic testing, and management of chronic inducible urticarias—The EAACI/GA 2 LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy 2016, 71, 780–802. [Google Scholar] [CrossRef] [Green Version]
- Church, M.K.; Weller, K.; Stock, P.; Maurer, M. Chronic spontaneous urticaria in children: Itching for insight. Pediatr. Allergy Immunol. 2011, 22, 1–8. [Google Scholar] [CrossRef]
- Ying, S.; Kikuchi, Y.; Meng, Q.; Kay, A.B.; Kaplan, A.P. Th1/Th2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: Comparison with the allergen-induced late-phase cutaneous reaction. J. Allergy Clin. Immunol. 2002, 109, 694–700. [Google Scholar] [CrossRef]
- Jirapongsananuruk, O.; Pongpreuksa, S.; Sangacharoenkit, P.; Visitsunthorn, N.; Vichyanond, P. Identification of the etiologies of chronic urticaria in children: A prospective study of 94 patients. Pediatr. Allergy Immunol. 2009, 21, 508–514. [Google Scholar] [CrossRef]
- Du Toit, G.; Prescott, R.; Lawrence, P.; Johar, A.; Brown, G.; Weinberg, E.G.; Motala, C.; Potter, P.C. Autoantibodies to the high-affinity IgE receptor in children with chronic urticaria. Ann. Allergy Asthma Immunol. 2006, 96, 341–344. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Civelek, E.; Tuncer, A.; Yavuz, S.T.; Karabulut, E.; Sackesen, C.; Sekerel, B.E. Chronic urticaria: Etiology and natural course in children. Int. Arch. Allergy Immunol. 2011, 156, 224–230. [Google Scholar] [CrossRef]
- Chansakulporn, S.; Pongpreuksa, S.; Sangacharoenkit, P.; Pacharn, P.; Visitsunthorn, N.; Vichyanond, P.; Jirapongsananuruk, O. The natural history of chronic urticaria in childhood: A prospective study. J. Am. Acad. Dermatol. 2014, 71, 663–668. [Google Scholar] [CrossRef]
- Eser, I.; Yologlu, N.; Baydemir, C.; Aydogan, M. The predictive factors for remission of chronic spontaneous urticaria in childhood: Outcome from a prospective study. Allergol. Immunopathol. 2016, 44, 537–541. [Google Scholar] [CrossRef]
- Arik Yilmaz, E.; Karaatmaca, B.; Cetinkaya, P.G.; Soyer, O.; Sekerel, B.E.; Sahiner, U.M. The persistence of chronic spontaneous urticaria in childhood is associated with the urticaria activity score. Allergy Asthma Proc. 2017, 38, 136–142. [Google Scholar] [CrossRef]
- Netchiporouk, E.; Sasseville, D.; Moreau, L.; Habel, Y.; Rahme, E.; Ben-Shoshan, M. Evaluating comorbidities, natural history, and predictors of early resolution in a cohort of children with chronic urticaria. JAMA Dermatol. 2017, 153, 1236. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.; Kinet, J.-P. Signalling through the high-affinity IgE receptor FcεRI. Nature 1999, 402, 24–30. [Google Scholar] [CrossRef]
- Grattan, C.E.H.; Wallington, T.B.; Warin, R.P.; Kennedy, C.T.C.; Lbradfield, J.W. A serological mediator in chronic idiopathic urticaria—A clinical, immunological and histological evaluation. Br. J. Dermatol. 1986, 114, 583–590. [Google Scholar] [CrossRef]
- Bracken, S.J.; Abraham, S.; MacLeod, A.S. autoimmune theories of chronic spontaneous urticaria. Front. Immunol. 2019, 10, 627. [Google Scholar] [CrossRef]
- Baram, D.; Vaday, G.G.; Salamon, P.; Drucker, I.; Hershkoviz, R.; Mekori, Y.A. Human mast cells release metalloproteinase-9 on contact with activated t cells: Juxtacrine regulation by TNF-α. J. Immunol. 2001, 167, 4008–4016. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, A.; Asero, R.; Lorini, M.; Marzano, A.V.; Cugno, M. Plasma levels of matrix metalloproteinase-9 in chronic urticaria patients correlate with disease severity and C-reactive protein but not with circulating histamine-releasing factors. Clin. Exp. Allergy 2010, 40, 875–881. [Google Scholar] [CrossRef]
- Vasagar, K.; Vonakis, B.M.; Gober, L.M.; Viksman, A.; Gibbons, S.P.; Saini, S.S. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin. Exp. Allergy 2006, 36, 770–776. [Google Scholar] [CrossRef]
- Ulambayar, B.; Chen, Y.-H.; Ban, G.-Y.; Lee, J.-H.; Jung, C.-G.; Yang, E.-M.; Park, H.-S.; Ye, Y.-M. Detection of circulating IgG autoantibody to FcεRIα in sera from chronic spontaneous urticaria patients. J. Microbiol. Immunol. Infect. 2020, 53, 141–147. [Google Scholar] [CrossRef]
- Fiebiger, E.; Hammerschmid, F.; Stingl, G.; Maurer, D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J. Clin. Investig. 1998, 101, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, P.; Mittag, D.; Hodgkin, P.D.; Harrison, L.C. Autoreactive T cells in chronic spontaneous urticaria target the IgE Fc receptor Iα subunit. J. Allergy Clin. Immunol. 2016, 138, 761–768.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarelli, C.; Paravati, F.; El Hachem, M.; Duse, M.; Bergamini, M.; Simeone, G.; Barbagallo, M.; Bernardini, R.; Bottau, P.; Bugliaro, F.; et al. Management of chronic urticaria in children: A clinical guideline. Ital. J. Pediatr. 2019, 45, 101. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Giménez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.Y.; Chung, W.H.; et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef] [Green Version]
- Cornillier, H.; Giraudeau, B.; Munck, S.; Hacard, F.; Jonville-Bera, A.P.; d’Acremont, G.; Pham, B.N.; Maruani, A. Chronic spontaneous urticaria in children a systematic review on interventions and comorbidities. Pediatr. Allergy Immunol. 2018, 29, 303–310. [Google Scholar] [CrossRef]
- Johal, K.J.; Saini, S. Current and emerging treatments for chronic spontaneous urticaria. Ann. Allergy Asthma Immunol. 2020, 125, 380–387. [Google Scholar] [CrossRef]
- Agache, I.; Rocha, C.; Pereira, A.; Song, Y.; Alonso-Coello, P.; Solà, I.; Beltran, J.; Posso, M.; Akdis, C.A.; Akdis, M.; et al. Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: A systematic review for the EAACI biological guidelines. Allergy 2021, 76, 59–70. [Google Scholar] [CrossRef]
- Kolkhir, P.; Altrichter, S.; Munoz, M.; Hawro, T.; Maurer, M. New treatments for chronic urticaria. Ann Allergy. Asthma Immunol 2020, 124, 2–12. [Google Scholar] [CrossRef]
- Wang, E.A.; Chan, S.K. Chronic urticaria in children: An update on diagnosis and treatment. Curr. Allergy Asthma Rep. 2020, 20, 31. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Fierro, M.T.; Dapavo, P.; Crimi, N.; Ferrucci, S.; Pepe, P.; Liberati, S.; Pigatto, P.D.; et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: A study of 470 patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 918–924. [Google Scholar] [CrossRef]
- Arm, J.P.; Bottoli, I.; Skerjanec, A.; Floch, D.; Groenewegen, A.; Maahs, S.; Owen, C.E.; Jones, I.; Lowe, P. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy 2014, 44, 1371–1385. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, J.A.; Singh, U.; Rao, M.B.; Berendts, K.; Zhang, X.; Mutasim, D. Benralizumab for chronic spontaneous urticaria. N. Engl. J. Med. 2020, 383, 1389–1391. [Google Scholar] [CrossRef]
- Maul, J.-T.; Distler, M.; Kolios, A.; Maul, L.V.; Guillet, C.; Graf, N.; Imhof, L.; Lang, C.; Navarini, A.A.; Schmid–Grendelmeier, P. Canakinumab lacks efficacy in treating adult patients with moderate to severe chronic spontaneous urticaria in a phase II randomized double–blind placebo-controlled single-center study. J. Allergy Clin. Immunol. Pract. 2021, 9, 463–468.e3. [Google Scholar] [CrossRef]

| Name | Mechanism Of Action | Age (Years) | Indications | Dosage | References |
|---|---|---|---|---|---|
| Omalizumab | Anti-IgE | ≥12 | CSU | 300 mg SC every 4 weeks | [99,100] |
| Dupilumab | Anti-IL-4Rα | ≥6 | Moderate-to-severe AD | Weight < 60 kg 6–11 years old: 300 mg SC at day 1, at day 15 and successively every 4 weeks >12 years old: 400-mg loading dose SC followed by 200 mg every 2 weeks Weight ≥ 60 kg: 600 mg loading dose SC followed by 300 mg SC every 2 weeks | [18,27,38] |
| Mepolizumab | Anti-IL-5 | / | AD: not approved | Phase I RCT | [43,44] |
| Tezepelumab | Anti-TSLP | / | AD: under study | Phase IIA RCT | [46] |
| Etokimab | Anti-IL-33 | / | AD: under study | Phase I RCT | [47] |
| Nemolizumab | Anti-IL-31R | / | AD: under study | Phase IIB RCT | [51] |
| Lebrikizumab | Anti-IL-13 | / | AD: under study | Phase IIB RCT | [53] |
| Tralokinumab | Anti-IL-13 | / | AD: under study | Phase III RCTs | [55] |
| GBR 830 | Anti-OX40 | / | AD: under study | Phase II RCT | [56] |
| Fezakinumab | Anti-IL-22 | / | AD: under study | Phase IIA RCTs | [57,58] |
| Baricitinib | Anti-JAK1 & 2 | / | AD: under study | Phase III RCT | [60,61] |
| Upadacitinib | Anti-JAK1 | / | AD: under study | Phase IIB RCT | [63,64] |
| Abrocitinib | Anti-JAK1 | / | AD: under study | Phase IIB RCT | [65] |
| Ligelizumab | Anti-IgE | / | CSU: under study | Phase I RCT | [96,102] |
| Benralizumab | Anti-IL-5Rα | / | CSU: under study | Phase I RCT | [103] |
| Canakinumab | Anti-IL-1β | / | CAPS + CSU: under study | Phase II RCT | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, P.; Russo, D.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children. Biomedicines 2021, 9, 1615. https://doi.org/10.3390/biomedicines9111615
Di Filippo P, Russo D, Attanasi M, Di Pillo S, Chiarelli F. Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children. Biomedicines. 2021; 9(11):1615. https://doi.org/10.3390/biomedicines9111615
Chicago/Turabian StyleDi Filippo, Paola, Daniele Russo, Marina Attanasi, Sabrina Di Pillo, and Francesco Chiarelli. 2021. "Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children" Biomedicines 9, no. 11: 1615. https://doi.org/10.3390/biomedicines9111615
APA StyleDi Filippo, P., Russo, D., Attanasi, M., Di Pillo, S., & Chiarelli, F. (2021). Immunological Targets of Biologic Drugs in Allergic Skin Diseases in Children. Biomedicines, 9(11), 1615. https://doi.org/10.3390/biomedicines9111615








