Salivary Exosomes in Health and Disease: Future Prospects in the Eye
Abstract
:1. Introduction
2. Exosomes in Non-Ocular Diseases
2.1. Systemic Autoimmune Diseases
2.1.1. Oral Lichen Planus
2.1.2. Periodontitis
2.1.3. Sjogren’s Syndrome
2.1.4. Inflammatory Bowel Disease
2.2. Neurodegenerative Disease
2.2.1. Alzheimer’s Disease
2.2.2. Parkinson’s Disease
2.3. Malignant Neoplasms
2.3.1. Oral Cancers
2.3.2. Breast Cancer
2.3.3. Colorectal Cancer
2.3.4. Lung Cancer
| Disease | Exosome Type | Biomarkers | Effect | Reference |
|---|---|---|---|---|
| Alzheimer’s Disease | Saliva | Aβ, p-Tau Aβ1-42, P-S396-tau | Disease propagation via Aβ and tau deposition | [14] |
| Blood | [131,132] | |||
| Urine | [136] | |||
| Astrocytes | BACE-1, sAPPβ, Complement C3, p-Tau | [129] | ||
| [128] | ||||
| [135] | ||||
| CSF | Cellular prion protein | Neuroprotective; reduce exosome uptake of Aβ | [140] | |
| MSCs | BACE1, Aβ1-40, Aβ1-42 | Inhibit Aβ deposition | [142] | |
| miR-29a | Increase synaptic plasticity via HDAC4 downregulation | [143,147] | ||
| Parkinson’s Disease | Saliva | α-synuclein oligomers | Disease propagation via α-synuclein oligomerization | [40,181] |
| CSF | [169] | |||
| Microglia | Inhibit lysosomal breakdown of α-synuclein | [170] | ||
| Serum | Uptake of α-synuclein into microglia | [176] | ||
| miR-137 | Increase oxidative stress via OXR1 inhibition | [177] | ||
| Oral Lichen Planus | Saliva | miR-4484, miR-146a, miR-155 | Unknown | [37,38] |
| T cells | MIP-1 alpha/beta | T-cell migration; apoptosis of keratinocytes | [55,56] | |
| Periodontitis | Saliva | miR-140-5p, miR-146a-5p, miR-628-5p LPS, 5mC methylation | Unknown | [43,68] |
| Periodontal ligament stem cells | miR-17-5p | Inhibit inflammation and angiogenesis | [65] | |
| Sjorgen’s Syndrome | Saliva | miR-768-3p, miR-574-3p | Unknown | [77] |
| Anti-Ro/SSA, anti-La/SSB, Sm ribonucleoproteins | Autoantigen presentation to lymphocytes | [79] | ||
| CD44 antigen; NGAL | T cell activation; neutrophil activation | [80] | ||
| T cells | miR-142-3p | Decrease protein production from salivary glands via cAMP inhibition | [73] | |
| Inflammatory Bowel Disease | Saliva | PSMA7 | Proteasome activity and inflammatory response | [39] |
| MSCs | miR-326 | Inhibit ubiquitination via NF-kappaB downregulation | [98] | |
| T regulatory cells | miR-195a-3p | Reduce inflammation | [100] | |
| Macrophages | miR-21a-5p | Exacerbate IBD via E-cadherin inhibition | [45] | |
| Oral squamous cell carcinoma | Saliva | miR-302b-3p, miR-517b-3p, miR-512-3p, miR-412-3p | Unknown | [201] |
| miR-31, miR-29a-3p | Promote M2 subtype macrophage polarization | [203,204] | ||
| MSCs | miR-101-3p | Inhibit cancer progression via COL10A1 downregulation | [196] | |
| Esophageal Cancer | Saliva | GOLM1-NAA35 chimeric RNA | Unknown | [202] |
| Plasma | miR-93-5p; miR-19b-3p | Proliferation of esophageal cancer cells via PTEN inhibition | [41,197] | |
| Head and Neck | Saliva | Human papillomavirus DNA, miR-486-5p, miR-486-3p, miR-10b-5p | Unknown | [199,200] |
| Breast cancer | Saliva | CA6, CSTA, TPT1, IGF2BP1 | Unknown | [224] |
| Urine | MMP-1 | Pro-angiogenic | [217] | |
| MSCs | miRNA-100, miR-148b-3p, miR-381 | Inhibit angiogenesis and cancer cell proliferation | [214,218,220] | |
| Colorectal cancer | Saliva | ANGPTL1 | Blocks metastasis via MMP9 inhibition | [238] |
| miR-21, miR-186-5p, miR-29a-3p, miR-29c-3p, miR-766-3p, miR-491-5p | Unknown | [241,242] | ||
| Lung cancer | Saliva | BPIFA1, CRNN, MuC5B, IQGAP | Unknown | [254] |
| Lung cancer bronchoalveolar lavage fluid | E-cadherin | Promote cancer cell migration and invasion | [252] | |
| Macrophage | miR-3679-5p | Promote aerobic glycolysis and chemoresistance | [253] |
3. Exosomes in Ocular Diseases
3.1. Diabetic Retinopathy
3.2. Retinitis Pigmentosa
3.3. Age-Related Macular Degeneration
3.4. Corneal Diseases
3.5. Autoimmune Uveitis
3.6. Uveal Melanoma
3.7. Retinoblastoma
3.8. Proliferative Vitreoretinopathy
3.9. Glaucoma
| Disease | Exosome Type | Biomarkers | Effect | Reference |
|---|---|---|---|---|
| Diabetic Retinopathy | Retinal cells | Fibroblast growth factor, TNF-α, angiostatin miR-20a-3p, miR-20a-5p, miR-20b, VEGF | Pro-inflammatory, pro-angiogenic | [278,279] |
| Circular RNA-cPWWP2A | Regulates endothelial cell activity via inhibition of miR-579 | [289] | ||
| miR-124-3p | Anti-inflammatory | [284] | ||
| RPE | miR-202-5p | Anti-angiogenic | [286] | |
| Plasma | IgG | Damage to retinal endothelial cells by activating complement | [284] | |
| [280] | ||||
| miR-15a | Insulin production by pancreatic beta-cells, oxidative stress in T2D | [282] | ||
| MSCs | miR-222 | Retinal repair | [287] | |
| Age-Related Macular Degeneration | Serum | miR-19a, miR-126, miR-410 | Pro-angiogenic, retinal cell apoptosis | [308] |
| Retinal astroglial cells | Endostatin, MMP-3 | Anti-angiogenic, inhibit migration of macrophages and endothelial cells | [313] | |
| RPE | C3, CD63, CD81, LAMP2 | Drusen production | [296] | |
| VEGF-2 | Pro-angiogenic, retinal endothelial damage | [306] | ||
| Cathepsin D, cytokeratins 8 and 14 | Reduce oxidative stress | [305] | ||
| Retinitis pigmentosa | Retinal cells | PARP | Photoreceptor degeneration | [294] |
| Retinoblastoma | Retinoblastoma cells | miR-5787, miR-6732-5p, miR-301b-3p, miR-216b-5p, miR-92a-3p | Promote tumor growth and angiogenesis | [341,342] |
| Corneal disease | Corneal stroma, Corneal epithelial cells | Fibronectin, TSP-1, α-SMA | Cell migration, myofibroblast differentiation, wound closure | [316,319] |
| Corneal fibroblasts | MMP14 | Pro-angiogenic, load MMP2 into exosomes | [317] | |
| Limbal stromal cells | Keratin 15 | Limbal epithelial cell proliferation via Akt phosphorylation | [318] | |
| MSCs | Col3a1, Acta2, Fibronectin | Corneal stromal repair | [315,321] | |
| Autoimmune Uveitis | RPE | CD14, CD16 | Anti-inflammatory, proliferation of IL-10 and T regulatory cells | [328] |
| Regulatory B-cells | IL-35 | [331] | ||
| Serum | Retinoid-binding protein R16 | [332] | ||
| Uveal Melanoma | Liver vasculature | Melan-A | Promote tumor growth and metastasis | [339] |
| Uveal melanoma cells | HSP90, HSP70, integrin V | [337] | ||
| Serum | Interferon-gamma, IL-2, IL-11, IL-12, Pentraxin-3 | [338] | ||
| Proliferative Vitreoretinoppathy | RPE | miR-543 | Induce the epithelial–mesenchymal transition (EMT) of recipient RPE cells | [347] |
| miR-4488, miR-1273g-5p | Inhibit TGF-β2-stimulated EMT in RPE cells by downregulating ABCA4 | [264] | ||
| Glaucoma | Aqueous humor, Trabecular meshwork, Non-pigmented ciliary epithelium | Myocilin, miR-182, miR-29b | Blockage of aqueous outflow via trabecular meshwork | [357,358,359,362] |
4. Exosomes as Drug Therapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell. Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocozza, F.; Grisard, E.; Martin-Jaular, L.; Mathieu, M.; Thery, C. SnapShot: Extracellular Vesicles. Cell 2020, 182, 262–262.e1. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Consortium, E.-T.; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, O.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lasser, C.; Szabo, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzas, E.I.; Lotvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef] [Green Version]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113 Pt 19, 3365–3374. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Zhang, H.G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef]
- van den Boorn, J.G.; Dassler, J.; Coch, C.; Schlee, M.; Hartmann, G. Exosomes as nucleic acid nanocarriers. Adv. Drug. Deliv. Rev. 2013, 65, 331–335. [Google Scholar] [CrossRef]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Miura, Y.; Harazono, A.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yamaguchi, T.; Toda, T.; Endo, T.; Tsubuki, M.; et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 2011, 34, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.B.; Xia, M.; Gao, Z.; Zhou, H.; Liu, M.; Huang, S.; Zhen, R.; Wu, J.Y.; Roth, W.W.; Bond, V.C.; et al. Characterization of Exosomes in Plasma of Patients with Breast, Ovarian, Prostate, Hepatic, Gastric, Colon, and Pancreatic Cancers. J. Cancer Ther. 2019, 10, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell. Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, T.; Wong, D.T.W. Saliva-Exosomics in Cancer: Molecular Characterization of Cancer-Derived Exosomes in Saliva. Enzymes 2017, 42, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Rastogi, S.; Vishwakarma, P.; Bharti, P.S.; Sharma, V.; Renu, K.; Modi, G.P.; Vishnu, V.Y.; Chatterjee, P.; Dey, A.B.; et al. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods 2021, 347, 108980. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheinani, A.H.; Vogeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2019, 43, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Cheng, L.; Deng, C.; Huang, L.; Li, J.; Wang, Y.; Li, M.; Yang, Q.; Dong, X.; Su, J.; et al. The genetic source tracking of human urinary exosomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2108876118. [Google Scholar] [CrossRef]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Dixon, C.L.; Sheller-Miller, S.; Saade, G.R.; Fortunato, S.J.; Lai, A.; Palma, C.; Guanzon, D.; Salomon, C.; Menon, R. Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology 2018, 159, 2229–2240. [Google Scholar] [CrossRef] [Green Version]
- Sheller-Miller, S.; Menon, R. Isolation and characterization of human amniotic fluid-derived exosomes. Methods Enzymol. 2020, 645, 181–194. [Google Scholar] [CrossRef]
- Cheng, J.; Nonaka, T.; Wong, D.T.W. Salivary Exosomes as Nanocarriers for Cancer Biomarker Delivery. Materials 2019, 12, 654. [Google Scholar] [CrossRef] [Green Version]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Wren, M.E.; Shirtcliff, E.A.; Drury, S.S. Not all biofluids are created equal: Chewing over salivary diagnostics and the epigenome. Clin. Ther. 2015, 37, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Cecchettini, A.; Finamore, F.; Puxeddu, I.; Ferro, F.; Baldini, C. Salivary extracellular vesicles versus whole saliva: New perspectives for the identification of proteomic biomarkers in Sjogren’s syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 118), 240–248. [Google Scholar]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Helmerhorst, E.J.; Oppenheim, F.G. Saliva: A dynamic proteome. J. Dent. Res. 2007, 86, 680–693. [Google Scholar] [CrossRef]
- Gandhi, V.; O’Brien, M.H.; Yadav, S. High-Quality and High-Yield RNA Extraction Method From Whole Human Saliva. Biomark. Insights 2020, 15, 1177271920929705. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Kim, Y.; Li, F.; Galeev, T.; Kitchen, R.R.; Gerstein, M.; Koyano, K.; Jeong, S.H.; Wang, X.; Elashoff, D.; et al. Novel approaches for bioinformatic analysis of salivary RNA sequencing data for development. Bioinformatics 2018, 34, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bijnsdorp, I.V.; Geldof, A.A.; Lavaei, M.; Piersma, S.R.; van Moorselaar, R.J.; Jimenez, C.R. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles 2013, 2, 22097. [Google Scholar] [CrossRef]
- Chiabotto, G.; Gai, C.; Deregibus, M.C.; Camussi, G. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers 2019, 11, 891. [Google Scholar] [CrossRef] [Green Version]
- van der Lubbe, N.; Jansen, P.M.; Salih, M.; Fenton, R.A.; van den Meiracker, A.H.; Danser, A.H.; Zietse, R.; Hoorn, E.J. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 2012, 60, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.T.; Wang, S.; Huang, S.L.; Zheng, Y.; Wang, C.Q.; Hu, B.Y.; Qin, W.; Zou, T.T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, P.; Perez-Lorenzo, M.J.; Alcazar-Garrido, A.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J. Extracell. Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, J.S.; Hong, S.H.; Choi, J.K.; Jung, J.K.; Lee, H.J. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Yang, H.; Liu, J.; Dan, H.; Zeng, X.; Zhou, Y.; Jiang, L.; Chen, Q. MicroRNAs in oral lichen planus and potential miRNA-mRNA pathogenesis with essential cytokines: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 164–173. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, F.; Zhang, Q.; Liu, Y.; You, P.; Sun, S.; Lin, J.; Chen, N. Salivary exosomal PSMA7: A promising biomarker of inflammatory bowel disease. Protein Cell 2017, 8, 686–695. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Wu, Y.; Liu, G.; Jiang, Y.; Wang, X.; Wang, Z.; Feng, T. alpha-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci. Lett. 2019, 696, 114–120. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhu, Z.; Song, L.; He, Z. Transferred by exosomes-derived MiR-19b-3p targets PTEN to regulate esophageal cancer cell apoptosis, migration and invasion. Biosci. Rep. 2020, 40, BSR20201858. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary Small Extracellular Vesicles Associated miRNAs in Periodontal Status-A Pilot Study. Int. J. Mol. Sci. 2020, 21, 2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, K.; Hong, Y.; Zhu, Q.; Shi, X.; Xiao, F.; Fu, H.; Yin, Q.; Xing, Y.; Wu, X.; Kong, X.; et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjogren’s syndrome by modulating the function of myeloid-derived suppressor cells. Cell. Mol. Immunol. 2021, 18, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, D.; Tan, Y.; Deng, F.; Li, R. M1 Macrophage exosomes MiR-21a-5p aggravates inflammatory bowel disease through decreasing E-cadherin and subsequent ILC2 activation. J. Cell. Mol. Med. 2021, 25, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Jabs, D.A.; Nikolskaia, O.V.; Mimouni, D.; Anhalt, G.J.; Nousari, H.C. Lichen planus and cicatrizing conjunctivitis: Characterization of five cases. Am. J. Ophthalmol. 2003, 136, 239–243. [Google Scholar] [CrossRef]
- Li, C.; He, H.; Wang, J.; Xia, X.; Zhang, M.; Wu, Q. Possible roles of exosomal miRNAs in the pathogenesis of oral lichen planus. Am. J. Transl. Res. 2019, 11, 5313–5323. [Google Scholar]
- Nogueira, P.A.; Carneiro, S.; Ramos-e-Silva, M. Oral lichen planus: An update on its pathogenesis. Int. J. Dermatol. 2015, 54, 1005–1010. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wang, C.M.; Potenziani, S.; Hsu, S. Lichen planus of the eyelids: A case report and review of the literature. Dermatol. Online J. 2017, 23, 13030. [Google Scholar] [CrossRef]
- Rhee, M.K.; Mootha, V.V. Bilateral keratoconjunctivitis associated with lichen planus. Cornea 2004, 23, 100–105. [Google Scholar] [CrossRef]
- Gomez-Elizondo, D.E.; Lopez-Martinez, M.; Ruiz-Lozano, R.E.; Valdez-Garcia, J.E.; Hernandez-Camarena, J.C. Corneal perforation associated with isolated ocular lichen planus: A case report. Eur. J. Ophthalmol. 2021, 31, NP9–NP12. [Google Scholar] [CrossRef]
- Rozas Munoz, E.; Martinez-Escala, M.E.; Juanpere, N.; Armentia, J.; Pujol, R.M.; Herrero-Gonzalez, J.E. Isolated conjunctival lichen planus: A diagnostic challenge. Arch. Dermatol. 2011, 147, 465–467. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.D.; Ekdawi, N.S.; Torgerson, R.R.; Camilleri, M.J.; Bruce, A.J.; Rogers, R.S., 3rd; Maguire, L.J. Lichen planus and cicatricial conjunctivitis: Disease course and response to therapy of 11 patients. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 100–104. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, J.; Zhou, G. Differentially circulating exosomal microRNAs expression profiling in oral lichen planus. Am. J. Transl. Res. 2018, 10, 2848–2858. [Google Scholar]
- Yang, J.Y.; Tan, Y.Q.; Zhou, G. T cell-derived exosomes containing cytokines induced keratinocytes apoptosis in oral lichen planus. Oral Dis. 2021, 28, 682–690. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, J.; Lu, R.; Tan, Y.Q.; Du, G.F.; Zhou, G. T cell-derived exosomes induced macrophage inflammatory protein-1alpha/beta drive the trafficking of CD8+ T cells in oral lichen planus. J. Cell. Mol. Med. 2020, 24, 14086–14098. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, J.; Zhou, G. Circulating exosomes regulate T-cell-mediated inflammatory response in oral lichen planus. J. Oral Pathol. Med. 2019, 48, 143–150. [Google Scholar] [CrossRef]
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.T.; Shen, T.C.; Chen, S.C.; Chang, C.L.; Li, C.H.; Li, X.; Palanisamy, K.; Hsia, N.Y.; Chang, W.S.; Tsai, C.W.; et al. Periodontitis and the subsequent risk of glaucoma: Results from the real-world practice. Sci. Rep. 2020, 10, 17568. [Google Scholar] [CrossRef]
- Yeh, L.J.; Shen, T.C.; Sun, K.T.; Lin, C.L.; Hsia, N.Y. Periodontitis and Subsequent Risk of Cataract: Results From Real-World Practice. Front. Med. 2022, 9, 721119. [Google Scholar] [CrossRef]
- Tang, Y.L.; Shentu, X.C.; Zhao, S.J.; Tang, X.J.; He, L.; Ping, F.Y. Ocular findings in syndromic gingival fibromatosis: A case study and electronic microscopic investigation of lens. Int. J. Ophthalmol. 2014, 7, 574–576. [Google Scholar] [CrossRef]
- Guncu, G.N.; Caglayan, F. Resolution of anterior scleritis after periodontal therapy. Eur. J. Dent. 2011, 5, 337–339. [Google Scholar] [CrossRef] [Green Version]
- Karesvuo, P.; Gursoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Huumonen, S.; Vesti, E.; Kononen, E. Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2013, 84, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wagley, S.; Marra, K.V.; Salhi, R.A.; Gautam, S.; Campo, R.; Veale, P.; Veale, J.; Arroyo, J.G. Periodontal disease and age-related macular degeneration: Results from the National Health and Nutrition Examination Survey III. Retina 2015, 35, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shuai, Y.; Zhou, F.; Yin, J.; Hu, J.; Guo, S.; Wang, Y.; Liu, W. PDLSCs Regulate Angiogenesis of Periodontal Ligaments via VEGF Transferred by Exosomes in Periodontitis. Int. J. Med. Sci. 2020, 17, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.; Xiong, X.; Li, K.; Yao, Z.; Dong, H.; Jiang, Z.; Yu, D.; Yeung, S.J.; Zhang, H. Detection of Exosomal PD-L1 RNA in Saliva of Patients with Periodontitis. Front. Genet. 2019, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary Outer Membrane Vesicles and DNA Methylation of Small Extracellular Vesicles as Biomarkers for Periodontal Status: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 2423. [Google Scholar] [CrossRef]
- Baldini, C.; Ferro, F.; Luciano, N.; Bombardieri, S.; Grossi, E. Artificial neural networks help to identify disease subsets and to predict lymphoma in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 137–144. [Google Scholar]
- Goules, A.V.; Tzioufas, A.G. Lymphomagenesis in Sjogren’s syndrome: Predictive biomarkers towards precision medicine. Autoimmun. Rev. 2019, 18, 137–143. [Google Scholar] [CrossRef]
- Akpek, E.K.; Mathews, P.; Hahn, S.; Hessen, M.; Kim, J.; Grader-Beck, T.; Birnbaum, J.; Baer, A.N. Ocular and systemic morbidity in a longitudinal cohort of Sjogren’s syndrome. Ophthalmology 2015, 122, 56–61. [Google Scholar] [CrossRef]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine Sjogren’s syndrome by modulating the balance of Treg and Th17 cells. Stem Cell Res. Ther. 2021, 12, 478. [Google Scholar] [CrossRef]
- Cortes-Troncoso, J.; Jang, S.I.; Perez, P.; Hidalgo, J.; Ikeuchi, T.; Greenwell-Wild, T.; Warner, B.M.; Moutsopoulos, N.M.; Alevizos, I. T cell exosome-derived miR-142-3p impairs glandular cell function in Sjogren’s syndrome. JCI Insight 2020, 5, e133497. [Google Scholar] [CrossRef]
- Li, N.; Zhao, L.; Wei, Y.; Ea, V.L.; Nian, H.; Wei, R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res. Ther. 2019, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, C.; Wang, S.; Guo, J.; Guo, J.; Fu, J.; Ren, L.; An, Y.; He, J.; Li, Z. Human umbilical cord mesenchymal stem cells confer potent immunosuppressive effects in Sjogren’s syndrome by inducing regulatory T cells. Mod. Rheumatol. 2021, 31, 186–196. [Google Scholar] [CrossRef]
- Gallo, A.; Jang, S.I.; Ong, H.L.; Perez, P.; Tandon, M.; Ambudkar, I.; Illei, G.; Alevizos, I. Targeting the Ca2+ Sensor STIM1 by Exosomal Transfer of Ebv-miR-BART13-3p is Associated with Sjogren’s Syndrome. EBioMedicine 2016, 10, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Alevizos, I.; Alexander, S.; Turner, R.J.; Illei, G.G. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjogren’s syndrome. Arthritis Rheum. 2011, 63, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjogren’s syndrome. Arthritis Rheum. 2007, 56, 3588–3600. [Google Scholar] [CrossRef] [Green Version]
- Kapsogeorgou, E.K.; Abu-Helu, R.F.; Moutsopoulos, H.M.; Manoussakis, M.N. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005, 52, 1517–1521. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Ovstebo, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjogren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef] [Green Version]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease (1). N. Engl. J. Med. 1991, 325, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Soukiasian, S.H.; Foster, C.S.; Raizman, M.B. Treatment strategies for scleritis and uveitis associated with inflammatory bowel disease. Am. J. Ophthalmol. 1994, 118, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Pablo, L. Managing IBD outside the gut: Ocular manifestations. Dig. Dis. 2013, 31, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, L.L.; Biancardi, A.L.; de Moraes, H.V., Jr.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836–5848. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef]
- Mady, R.; Grover, W.; Butrus, S. Ocular complications of inflammatory bowel disease. Sci. World J. 2015, 2015, 438402. [Google Scholar] [CrossRef] [Green Version]
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718. [Google Scholar] [CrossRef]
- Lakatos, L.; Pandur, T.; David, G.; Balogh, Z.; Kuronya, P.; Tollas, A.; Lakatos, P.L. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: Results of a 25-year follow-up study. World J. Gastroenterol. 2003, 9, 2300–2307. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, W.; Xu, X.; Kang, J.; Wang, J.; Wen, Y.; Tang, X.; Yan, Y.; Qian, H.; Zhang, X.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am. J. Transl. Res. 2018, 10, 2026–2036. [Google Scholar]
- Yang, S.; Liang, X.; Song, J.; Li, C.; Liu, A.; Luo, Y.; Ma, H.; Tan, Y.; Zhang, X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res. Ther. 2021, 12, 315. [Google Scholar] [CrossRef]
- Yu, H.; Yang, X.; Xiao, X.; Xu, M.; Yang, Y.; Xue, C.; Li, X.; Wang, S.; Zhao, R.C. Human Adipose Mesenchymal Stem Cell-derived Exosomes Protect Mice from DSS-Induced Inflammatory Bowel Disease by Promoting Intestinal-stem-cell and Epithelial Regeneration. Aging Dis. 2021, 12, 1423–1437. [Google Scholar] [CrossRef]
- Mao, F.; Wu, Y.; Tang, X.; Kang, J.; Zhang, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed. Res. Int. 2017, 2017, 5356760. [Google Scholar] [CrossRef] [Green Version]
- Cleynen, I.; Vazeille, E.; Artieda, M.; Verspaget, H.W.; Szczypiorska, M.; Bringer, M.A.; Lakatos, P.L.; Seibold, F.; Parnell, K.; Weersma, R.K.; et al. Genetic and microbial factors modulating the ubiquitin proteasome system in inflammatory bowel disease. Gut 2014, 63, 1265–1274. [Google Scholar] [CrossRef]
- Hetzenecker, A.M.; Seidl, M.C.; Kosovac, K.; Herfarth, H.; Kellermeier, S.; Obermeier, F.; Falk, W.; Schoelmerich, J.; Hausmann, M.; Rogler, G. Downregulation of the ubiquitin-proteasome system in normal colonic macrophages and reinduction in inflammatory bowel disease. Digestion 2012, 86, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yuan, J.; Cai, X.; Xu, Z.; Wang, J.; Ocansey, D.K.W.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin. Transl. Med. 2020, 10, e113. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lee, M.M.; Chan, B.D.; Kam, R.K.; Zhang, G.; Lu, A.P.; Tai, W.C. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics 2016, 16, 1131–1145. [Google Scholar] [CrossRef]
- Liao, F.; Lu, X.; Dong, W. Exosomes derived from T regulatory cells relieve inflammatory bowel disease by transferring miR-195a-3p. IUBMB Life 2020, 72, 2591–2600. [Google Scholar] [CrossRef]
- Apodaca, L.A.; Baddour, A.A.D.; Garcia, C., Jr.; Alikhani, L.; Giedzinski, E.; Ru, N.; Agrawal, A.; Acharya, M.M.; Baulch, J.E. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 57. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Wei, L.; Yu, F.; Yu, B.; Xu, A. Long Noncoding RNA POU3F3 and alpha-Synuclein in Plasma L1CAM Exosomes Combined with beta-Glucocerebrosidase Activity: Potential Predictors of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 703. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Hill, J.M.; Clement, C.; Sambamurti, K.; Dua, P.; Lukiw, W.J. Beta-Amyloid Precursor Protein (betaAPP) Processing in Alzheimer’s Disease (AD) and Age-Related Macular Degeneration (AMD). Mol. Neurobiol. 2015, 52, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.D.; Lin, J.; Mace, B.E.; Herrmann, R.; Sullivan, P.; Bowes Rickman, C. Targeting age-related macular degeneration with Alzheimer’s disease based immunotherapies: Anti-amyloid-beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vis. Res. 2008, 48, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Ukalovic, K.; Cao, S.; Lee, S.; Tang, Q.; Beg, M.F.; Sarunic, M.V.; Hsiung, G.R.; Mackenzie, I.R.; Hirsch-Reinshagen, V.; Cui, J.Z.; et al. Drusen in the Peripheral Retina of the Alzheimer’s Eye. Curr. Alzheimer Res. 2018, 15, 743–750. [Google Scholar] [CrossRef]
- Tsai, D.C.; Chen, S.J.; Huang, C.C.; Yuan, M.K.; Leu, H.B. Age-Related Macular Degeneration and Risk of Degenerative Dementia among the Elderly in Taiwan: A Population-Based Cohort Study. Ophthalmology 2015, 122, 2327–2335.e2. [Google Scholar] [CrossRef]
- Parisi, V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin. Ophthalmol. 2003, 18, 50–57. [Google Scholar] [CrossRef]
- Cipollini, V.; Abdolrahimzadeh, S.; Troili, F.; De Carolis, A.; Calafiore, S.; Scuderi, L.; Giubilei, F.; Scuderi, G. Neurocognitive Assessment and Retinal Thickness Alterations in Alzheimer Disease: Is There a Correlation? J. Neuroophthalmol. 2020, 40, 370–377. [Google Scholar] [CrossRef]
- Iseri, P.K.; Altinas, O.; Tokay, T.; Yuksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol. 2006, 26, 18–24. [Google Scholar] [CrossRef]
- Kirbas, S.; Turkyilmaz, K.; Anlar, O.; Tufekci, A.; Durmus, M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuroophthalmol. 2013, 33, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Risacher, S.L.; Wudunn, D.; Pepin, S.M.; MaGee, T.R.; McDonald, B.C.; Flashman, L.A.; Wishart, H.A.; Pixley, H.S.; Rabin, L.A.; Pare, N.; et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol. Aging 2013, 34, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Risacher, S.L.; WuDunn, D.; Tallman, E.F.; West, J.D.; Gao, S.; Farlow, M.R.; Brosch, J.R.; Apostolova, L.G.; Saykin, A.J. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020, 2, fcaa019. [Google Scholar] [CrossRef]
- Gilmore, G.C.; Groth, K.E.; Thomas, C.W. Stimulus contrast and word reading speed in Alzheimer’s disease. Exp. Aging Res. 2005, 31, 15–33. [Google Scholar] [CrossRef]
- Fernandez, G.; Mandolesi, P.; Rotstein, N.P.; Colombo, O.; Agamennoni, O.; Politi, L.E. Eye movement alterations during reading in patients with early Alzheimer disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8345–8352. [Google Scholar] [CrossRef] [Green Version]
- Garbutt, S.; Matlin, A.; Hellmuth, J.; Schenk, A.K.; Johnson, J.K.; Rosen, H.; Dean, D.; Kramer, J.; Neuhaus, J.; Miller, B.L.; et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain 2008, 131, 1268–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq-Antonacci, R.; Maruff, P.; Masters, C.; Currie, J. Spectrum of saccade system function in Alzheimer disease. Arch. Neurol. 2003, 60, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.S.; Park, S.W.; Lee, J.H.; Yi, D.; Jeon, S.Y.; Choi, H.J.; Joung, H.; Ghim, U.H.; Park, U.C.; Kim, Y.K.; et al. Association of Retinal Changes With Alzheimer Disease Neuroimaging Biomarkers in Cognitively Normal Individuals. JAMA Ophthalmol. 2021, 139, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Apte, R.S. Retinal Biomarkers of Alzheimer Disease. Am. J. Ophthalmol. 2020, 218, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Blazes, M.S.; Lee, C.S. Imaging Amyloid and Tau in the Retina: Current Research and Future Directions. J. Neuroophthalmol. 2023. [Google Scholar] [CrossRef]
- Winston, C.N.; Goetzl, E.J.; Schwartz, J.B.; Elahi, F.M.; Rissman, R.A. Complement protein levels in plasma astrocyte-derived exosomes are abnormal in conversion from mild cognitive impairment to Alzheimer’s disease dementia. Alzheimers Dement. 2019, 11, 61–66. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef] [Green Version]
- Saman, S.; Lee, N.C.; Inoyo, I.; Jin, J.; Li, Z.; Doyle, T.; McKee, A.C.; Hall, G.F. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J. Alzheimers Dis. 2014, 40 (Suppl. 1), S47–S70. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Hernandez, H.; Cuevas, E.; Raymick, J.B.; Robinson, B.L.; Ali, S.F.; Hanig, J.; Sarkar, S. Characterization of Serum Exosomes from a Transgenic Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 388–395. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Vassar, R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarini, A.; Armato, U.; Gardenal, E.; Gui, L.; Dal Pra, I. Amyloid beta-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143-Further Implications for Alzheimer’s Therapy. Front. Neurosci. 2017, 11, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Wang, H.; Shi, Y.; Gao, D.; Sun, Z.; Chen, Z.; Jiang, H.; Zhang, J. A Pilot Study of Urinary Exosomes in Alzheimer’s Disease. Neurodegener. Dis. 2019, 19, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Aulston, B.; Liu, Q.; Mante, M.; Florio, J.; Rissman, R.A.; Yuan, S.H. Extracellular Vesicles Isolated from Familial Alzheimer’s Disease Neuronal Cultures Induce Aberrant Tau Phosphorylation in the Wild-Type Mouse Brain. J. Alzheimers Dis. 2019, 72, 575–585. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Usuki, S.; Sakai, S.; Hanamatsu, H.; Mioka, T.; Kimura, N.; Okada, M.; Tahara, H.; Furukawa, J.; et al. A potential function for neuronal exosomes: Sequestering intracerebral amyloid-beta peptide. FEBS Lett. 2015, 589, 84–88. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef] [Green Version]
- An, K.; Klyubin, I.; Kim, Y.; Jung, J.H.; Mably, A.J.; O’Dowd, S.T.; Lynch, T.; Kanmert, D.; Lemere, C.A.; Finan, G.M.; et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol. Brain 2013, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhdanova, D.Y.; Poltavtseva, R.A.; Svirshchevskaya, E.V.; Bobkova, N.V. Effect of Intranasal Administration of Multipotent Mesenchymal Stromal Cell Exosomes on Memory of Mice in Alzheimer’s Disease Model. Bull. Exp. Biol. Med. 2021, 170, 575–582. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G. Bone marrow mesenchymal stem cells-derived exosomes reduce Abeta deposition and improve cognitive function recovery in mice with Alzheimer’s disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell. Biol. Int. 2021, 45, 775–784. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Xie, Z.H.; Liu, Z.; Zhang, X.R.; Yang, H.; Wei, L.F.; Wang, Y.; Xu, S.L.; Sun, L.; Lai, C.; Bi, J.Z.; et al. Wharton’s Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-beta deposition in an APP/PS1 transgenic mouse model. Clin. Exp. Med. 2016, 16, 89–98. [Google Scholar] [CrossRef]
- Ding, M.; Shen, Y.; Wang, P.; Xie, Z.; Xu, S.; Zhu, Z.; Wang, Y.; Lyu, Y.; Wang, D.; Xu, L.; et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 2165–2177. [Google Scholar] [CrossRef]
- Portelius, E.; Dean, R.A.; Andreasson, U.; Mattsson, N.; Westerlund, A.; Olsson, M.; Demattos, R.B.; Racke, M.M.; Zetterberg, H.; May, P.C.; et al. beta-site amyloid precursor protein-cleaving enzyme 1(BACE1) inhibitor treatment induces Abeta5-X peptides through alternative amyloid precursor protein cleavage. Alzheimers Res. Ther. 2014, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.W.; Chen, J.; Wang, M.; Mast, N.; Pikuleva, I.A.; Turko, I.V. Quantification of histone deacetylase isoforms in human frontal cortex, human retina, and mouse brain. PLoS ONE 2015, 10, e0126592. [Google Scholar] [CrossRef] [Green Version]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef]
- Devic, I.; Hwang, H.; Edgar, J.S.; Izutsu, K.; Presland, R.; Pan, C.; Goodlett, D.R.; Wang, Y.; Armaly, J.; Tumas, V.; et al. Salivary alpha-synuclein and DJ-1: Potential biomarkers for Parkinson’s disease. Brain 2011, 134, e178. [Google Scholar] [CrossRef] [Green Version]
- Greenland, J.C.; Barker, R.A. The Differential Diagnosis of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Exon Publications: Brisbane, Australia, 2018. [Google Scholar] [CrossRef]
- Massano, J.; Bhatia, K.P. Clinical approach to Parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012, 2, a008870. [Google Scholar] [CrossRef]
- Corin, M.S.; Elizan, T.S.; Bender, M.B. Oculomotor function in patients with Parkinson’s disease. J. Neurol. Sci. 1972, 15, 251–265. [Google Scholar] [CrossRef]
- Shibasaki, H.; Tsuji, S.; Kuroiwa, Y. Oculomotor abnormalities in Parkinson’s disease. Arch. Neurol. 1979, 36, 360–364. [Google Scholar] [CrossRef]
- Almer, Z.; Klein, K.S.; Marsh, L.; Gerstenhaber, M.; Repka, M.X. Ocular motor and sensory function in Parkinson’s disease. Ophthalmology 2012, 119, 178–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuska, J.; Bonnet, C.; Rusz, J.; Sieger, T.; Jech, R.; Rivaud-Pechoux, S.; Vidailhet, M.; Gaymard, B.; Ruzicka, E. Fast vergence eye movements are disrupted in Parkinson’s disease: A video-oculography study. Park. Relat. Disord. 2015, 21, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, B.; Lubinski, W.; Honczarenko, K.; Potemkowski, A.; Safranow, K. Ophthalmological features of Parkinson disease. Med. Sci. Monit. 2014, 20, 2243–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archibald, N.K.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. Visual symptoms in Parkinson’s disease and Parkinson’s disease dementia. Mov. Disord. 2011, 26, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Davidsdottir, S.; Cronin-Golomb, A.; Lee, A. Visual and spatial symptoms in Parkinson’s disease. Vis. Res. 2005, 45, 1285–1296. [Google Scholar] [CrossRef] [Green Version]
- Nebe, A.; Ebersbach, G. Selective diplopia in Parkinson’s disease: A special subtype of visual hallucination? Mov. Disord. 2007, 22, 1175–1178. [Google Scholar] [CrossRef]
- Repka, M.X.; Claro, M.C.; Loupe, D.N.; Reich, S.G. Ocular motility in Parkinson’s disease. J. Pediatr. Ophthalmol. Strabismus 1996, 33, 144–147. [Google Scholar] [CrossRef]
- Tamer, C.; Melek, I.M.; Duman, T.; Oksuz, H. Tear film tests in Parkinson’s disease patients. Ophthalmology 2005, 112, 1795. [Google Scholar] [CrossRef]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef]
- Borm, C.; Smilowska, K.; de Vries, N.M.; Bloem, B.R.; Theelen, T. How I do it: The Neuro-Ophthalmological Assessment in Parkinson’s Disease. J. Park. Dis. 2019, 9, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Bayer, A.U.; Keller, O.N.; Ferrari, F.; Maag, K.P. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am. J. Ophthalmol. 2002, 133, 135–137. [Google Scholar] [CrossRef]
- Tsironi, E.E.; Dastiridou, A.; Katsanos, A.; Dardiotis, E.; Veliki, S.; Patramani, G.; Zacharaki, F.; Ralli, S.; Hadjigeorgiou, G.M. Perimetric and retinal nerve fiber layer findings in patients with Parkinson’s disease. BMC Ophthalmol. 2012, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of alpha-synuclein aggregation by exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Majbour, N.K.; Aasly, J.O.; Hustad, E.; Thomas, M.A.; Vaikath, N.N.; Elkum, N.; van de Berg, W.D.J.; Tokuda, T.; Mollenhauer, B.; Berendse, H.W.; et al. CSF total and oligomeric alpha-Synuclein along with TNF-alpha as risk biomarkers for Parkinson’s disease: A study in LRRK2 mutation carriers. Transl. Neurodegener. 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Stuendl, A.; Kunadt, M.; Kruse, N.; Bartels, C.; Moebius, W.; Danzer, K.M.; Mollenhauer, B.; Schneider, A. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 2016, 139, 481–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate alpha-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Dilsizoglu Senol, A.; Samarani, M.; Syan, S.; Guardia, C.M.; Nonaka, T.; Liv, N.; Latour-Lambert, P.; Hasegawa, M.; Klumperman, J.; Bonifacino, J.S.; et al. alpha-Synuclein fibrils subvert lysosome structure and function for the propagation of protein misfolding between cells through tunneling nanotubes. PLoS Biol. 2021, 19, e3001287. [Google Scholar] [CrossRef]
- Stykel, M.G.; Humphries, K.M.; Kamski-Hennekam, E.; Buchner-Duby, B.; Porte-Trachsel, N.; Ryan, T.; Coackley, C.L.; Bamm, V.V.; Harauz, G.; Ryan, S.D. Alpha-Synuclein mutation impairs processing of endomembrane compartments and promotes exocytosis and seeding of alpha-synuclein pathology. Cell Rep. 2021, 35, 109099. [Google Scholar] [CrossRef]
- Si, X.L.; Fang, Y.J.; Li, L.F.; Gu, L.Y.; Yin, X.Z.; Jun, T.; Yan, Y.P.; Pu, J.L.; Zhang, B.R. From inflammasome to Parkinson’s disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson’s disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and alpha-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 438. [Google Scholar] [CrossRef]
- Niu, M.; Li, Y.; Li, G.; Zhou, L.; Luo, N.; Yao, M.; Kang, W.; Liu, J. A longitudinal study on alpha-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur. J. Neurol. 2020, 27, 967–974. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, J.; Chen, L.; Jin, Y.; Zhang, G.; Lin, Z.; Du, S.; Fu, Z.; Chen, T.; Qin, Y.; et al. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res. 2019, 1722, 146331. [Google Scholar] [CrossRef]
- Li, N.; Pan, X.; Zhang, J.; Ma, A.; Yang, S.; Ma, J.; Xie, A. Plasma levels of miR-137 and miR-124 are associated with Parkinson’s disease but not with Parkinson’s disease with depression. Neurol. Sci. 2017, 38, 761–767. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef]
- Sun, T.; Ding, Z.X.; Luo, X.; Liu, Q.S.; Cheng, Y. Blood Exosomes Have Neuroprotective Effects in a Mouse Model of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 3807476. [Google Scholar] [CrossRef]
- Rani, K.; Mukherjee, R.; Singh, E.; Kumar, S.; Sharma, V.; Vishwakarma, P.; Bharti, P.S.; Nikolajeff, F.; Dinda, A.K.; Goyal, V.; et al. Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Park. Relat. Disord. 2019, 67, 21–23. [Google Scholar] [CrossRef]
- Nonaka, T.; Wong, D.T.W. Liquid Biopsy in Head and Neck Cancer: Promises and Challenges. J. Dent. Res. 2018, 97, 701–708. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Ludwig, S. Exosomen: Potenzielle Flussigbiopsie bei Kopf-Hals-Karzinomen. [Exosomes: Potential liquid biopsy in head and neck cancer]. HNO 2020, 68, 106–110. [Google Scholar] [CrossRef]
- Mouliere, F.; Thierry, A.R. The importance of examining the proportion of circulating DNA originating from tumor, microenvironment and normal cells in colorectal cancer patients. Expert Opin. Biol. Ther. 2012, 12 (Suppl. 1), S209–S215. [Google Scholar] [CrossRef] [PubMed]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Georgaki, M.; Theofilou, V.I.; Pettas, E.; Stoufi, E.; Younis, R.H.; Kolokotronis, A.; Sauk, J.J.; Nikitakis, N.G. Understanding the complex pathogenesis of oral cancer: A comprehensive review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 566–579. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.; Mohammad, S. Oral cancer: Risk factors and molecular pathogenesis. J. Maxillofac. Oral Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Sciubba, J.J. Oral cancer. The importance of early diagnosis and treatment. Am. J. Clin. Dermatol. 2001, 2, 239–251. [Google Scholar] [CrossRef]
- Westgaard, K.L.; Hynne, H.; Amdal, C.D.; Young, A.; Singh, P.B.; Chen, X.; Rykke, M.; Hove, L.H.; Aqrawi, L.A.; Utheim, T.P.; et al. Oral and ocular late effects in head and neck cancer patients treated with radiotherapy. Sci. Rep. 2021, 11, 4026. [Google Scholar] [CrossRef]
- Marioni, G.; Doro, D.; Marino, F.; Verdecchia, P.; Staffieri, C.; Staffieri, A. Skin and eye: Uncommon sites of distant metastasis from tongue base squamous cell carcinoma. Acta Otolaryngol. 2003, 123, 1110–1114. [Google Scholar] [CrossRef]
- Binkley, E.M.; Sampson, A.D.; Syed, N.A.; Boldt, H.C. Metastatic Squamous Cell Carcinoma of the Tonsil Mimicking Choroidal Melanoma. Ocul. Oncol. Pathol. 2020, 6, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Du, L.Y.; Guo, F.; Li, X.; Cheng, B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell. Biochem. 2019, 458, 11–26. [Google Scholar] [CrossRef]
- Liu, M.X.; Liao, J.; Xie, M.; Gao, Z.K.; Wang, X.H.; Zhang, Y.; Shang, M.H.; Yin, L.H.; Pu, Y.P.; Liu, R. miR-93-5p Transferred by Exosomes Promotes the Proliferation of Esophageal Cancer Cells via Intercellular Communication by Targeting PTEN. Biomed. Environ. Sci. 2018, 31, 171–185. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef] [Green Version]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.R.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef] [Green Version]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Dong, H.; Deng, W.; Lin, W.; Li, K.; Xiong, X.; Guo, Y.; Zhou, F.; Ma, C.; Chen, Y.; et al. Evaluation of Salivary Exosomal Chimeric GOLM1-NAA35 RNA as a Potential Biomarker in Esophageal Carcinoma. Clin. Cancer Res. 2019, 25, 3035–3045. [Google Scholar] [CrossRef]
- Cai, J.; Qiao, B.; Gao, N.; Lin, N.; He, W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Cell Physiol. 2019, 316, C731–C740. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Freedman, M.I.; Folk, J.C. Metastatic tumors to the eye and orbit. Patient survival and clinical characteristics. Arch. Ophthalmol. 1987, 105, 1215–1219. [Google Scholar] [CrossRef]
- Weiss, L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin. Exp. Metastasis 1992, 10, 191–199. [Google Scholar] [CrossRef]
- McCartney, A. Intraocular metastasis. Br. J. Ophthalmol. 1993, 77, 133. [Google Scholar] [CrossRef] [Green Version]
- Konstantinidis, L.; Rospond-Kubiak, I.; Zeolite, I.; Heimann, H.; Groenewald, C.; Coupland, S.E.; Damato, B. Management of patients with uveal metastases at the Liverpool Ocular Oncology Centre. Br. J. Ophthalmol. 2014, 98, 92–98. [Google Scholar] [CrossRef]
- Kanthan, G.L.; Jayamohan, J.; Yip, D.; Conway, R.M. Management of metastatic carcinoma of the uveal tract: An evidence-based analysis. Clin. Exp. Ophthalmol. 2007, 35, 553–565. [Google Scholar] [CrossRef]
- Shtam, T.; Naryzhny, S.; Samsonov, R.; Karasik, D.; Mizgirev, I.; Kopylov, A.; Petrenko, E.; Zabrodskaya, Y.; Kamyshinsky, R.; Nikitin, D.; et al. Plasma exosomes stimulate breast cancer metastasis through surface interactions and activation of FAK signaling. Breast Cancer Res. Treat. 2019, 174, 129–141. [Google Scholar] [CrossRef]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Sharifi, K.; Salehi, M.; Mohammadi-Yeganeh, S. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev. Rep. 2021, 17, 1027–1038. [Google Scholar] [CrossRef]
- Sun, L.; He, M.; Xu, N.; Xu, D.H.; Ben-David, Y.; Yang, Z.Y.; Li, Y.J. Regulation of RAB22A by mir-193b inhibits breast cancer growth and metastasis mediated by exosomes. Int. J. Oncol. 2018, 53, 2705–2714. [Google Scholar] [CrossRef] [Green Version]
- Inubushi, S.; Kawaguchi, H.; Mizumoto, S.; Kunihisa, T.; Baba, M.; Kitayama, Y.; Takeuchi, T.; Hoffman, R.M.; Tanino, H.; Sasaki, R. Oncogenic miRNAs Identified in Tear Exosomes From Metastatic Breast Cancer Patients. Anticancer Res. 2020, 40, 3091–3096. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef] [Green Version]
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell. Oncol. 2017, 40, 457–470. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, S.R.; Jung, B.K.; Jeon, Y.K.; Lee, Y.S.; Kim, M.K.; Kim, Y.G.; Jang, J.Y.; Kim, C.W. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Liu, Y.; Qu, Y.; Liu, L.; Li, H. Exosomes Derived From MicroRNA-148b-3p-Overexpressing Human Umbilical Cord Mesenchymal Stem Cells Restrain Breast Cancer Progression. Front. Oncol. 2019, 9, 1076. [Google Scholar] [CrossRef] [Green Version]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Boo, L.; Ky, H.; Satharasinghe, D.A.; Tan, S.W.; Cheong, S.K.; Huang, H.D.; Lan, K.C.; et al. Adipose MSCs Suppress MCF7 and MDA-MB-231 Breast Cancer Metastasis and EMT Pathways Leading to Dormancy via Exosomal-miRNAs Following Co-Culture Interaction. Pharmaceuticals 2020, 14, 8. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Guo, X.; Zhu, Z.; Cai, H.; Kong, X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J. Hematol. Oncol. 2018, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.S.; Wong, D.T. Breast cancer exosome-like microvesicles and salivary gland cells interplay alters salivary gland cell-derived exosome-like microvesicles in vitro. PLoS ONE 2012, 7, e33037. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F.; Mayorga-Wark, O.; Arreola, D.; Edwards, C.; Bigler, L.; Dubinsky, W.P. Breast cancer related proteins are present in saliva and are modulated secondary to ductal carcinoma in situ of the breast. Cancer Investig. 2008, 26, 159–167. [Google Scholar] [CrossRef]
- Zamanova, S.; Shabana, A.M.; Mondal, U.K.; Ilies, M.A. Carbonic anhydrases as disease markers. Expert Opin. Ther. Pat. 2019, 29, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.B.; Lee, J.H.; Park, M.S.; Byun, H.J.; Kang, S.; Seo, S.S.; Kim, J.Y.; Park, S.Y. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011, 585, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Cruzado-Sanchez, D.; Saavedra-Mejia, L.A.; Tellez, W.A.; Maquera-Torres, G.; Serpa-Frias, S. Metastatic Intraocular Tumor Due to Colorectal Adenocarcinoma: Case Report and Literature Review. J. Ophthalmic Vis. Res. 2020, 15, 565–570. [Google Scholar] [CrossRef]
- Khawaja, M.R.; Minturn, J.T.; Spittler, A.J.; Chiorean, E.G. Ocular metastasis of colorectal cancer: An uncommon presentation of a common malignancy. Hematol. Oncol. Stem Cell Ther. 2015, 8, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Chen, H.; Fang, Y.; Chen, L.; Zhong, C.; Bu, T.; Dai, S.; Pan, X.; Fu, D.; Qian, Y.; et al. Exosomal ANGPTL1 attenuates colorectal cancer liver metastasis by regulating Kupffer cell secretion pattern and impeding MMP9 induced vascular leakiness. J. Exp. Clin. Cancer Res. 2021, 40, 21. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Yuan, Y.; Liu, B.; Pan, S.; Liu, Q.; Qi, X.; Zhou, H.; Dong, W.; Jia, L. The potential of exosomes derived from colorectal cancer as a biomarker. Clin. Chim. Acta 2019, 490, 186–193. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [Green Version]
- Sazanov, A.A.; Kiselyova, E.V.; Zakharenko, A.A.; Romanov, M.N.; Zaraysky, M.I. Plasma and saliva miR-21 expression in colorectal cancer patients. J. Appl. Genet. 2017, 58, 231–237. [Google Scholar] [CrossRef]
- Rapado-Gonzalez, O.; Majem, B.; Alvarez-Castro, A.; Diaz-Pena, R.; Abalo, A.; Suarez-Cabrera, L.; Gil-Moreno, A.; Santamaria, A.; Lopez-Lopez, R.; Muinelo-Romay, L.; et al. A Novel Saliva-Based miRNA Signature for Colorectal Cancer Diagnosis. J. Clin. Med. 2019, 8, 2029. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, X.; Xiao, J.; Xu, Y.; Cai, Y.; Sun, C.; Ma, K. Lung squamous cell carcinoma with solitary ocular metastasis and its successful treatment with thoracic surgery and chemotherapy: An interesting and rare case report. BMC Cancer 2018, 18, 1004. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Liu, J.; Zhu, L.; Zhou, Q. A Comprehensive Understanding of Choroidal Metastasis from Lung Cancer. Onco Targets Ther. 2021, 14, 4451–4465. [Google Scholar] [CrossRef]
- Chen, H.F.; Wang, W.X.; Li, X.F.; Wu, L.X.; Zhu, Y.C.; Du, K.Q.; Xu, C.W. Eye metastasis in lung adenocarcinoma mimicking anterior scleritis: A case report. World J. Clin. Cases 2020, 8, 410–414. [Google Scholar] [CrossRef]
- Lampaki, S.; Kioumis, I.; Pitsiou, G.; Lazaridis, G.; Syrigos, K.; Trakada, G.; Kakolyris, S.; Zarogoulidis, K.; Mpoukovinas, I.; Rapti, A.; et al. Lung cancer and eye metastases. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 40–44. [Google Scholar]
- Liu, S.L.; Nie, Y.H.; He, T.; Yan, X.X.; Xing, Y.Q. Iris metastasis as the first sign of small cell lung cancer: A case report. Oncol. Lett. 2017, 13, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Sakellakis, M.; Peroukides, S.; Iconomou, G.; Kalofonos, H. Iris Metastasis in a Patient With Small Cell Lung Cancer: A Case Report. Iran. Red. Crescent Med. J. 2016, 18, e21522. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Frank, J.H.; Shields, C.L. Non-small cell lung cancer with iris metastasis controlled with osimertinib and monthly intravitreal bevacizumab. Am. J. Ophthalmol. Case Rep. 2022, 25, 101269. [Google Scholar] [CrossRef]
- Rahman, M.A.; Barger, J.F.; Lovat, F.; Gao, M.; Otterson, G.A.; Nana-Sinkam, P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget 2016, 7, 54852–54866. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, S.; Li, S.; Wu, J.; Ma, R.; Cao, H.; Zhu, Y.; Feng, J. Exosomes: Decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS ONE 2014, 9, e89534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Z.; Li, S.; Wang, M.; Dai, D.; Jing, H.; Liu, L. Upregulation of E-cadherin in bronchoalveolar lavage fluid-derived exosomes in patients with lung cancer. Thorac. Cancer 2020, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Pan, H.; Wang, Y.; Shi, M.; Yu, H.; Wang, C.; Pan, X.; Chen, Z. Exosomes Derived From Macrophages Enhance Aerobic Glycolysis and Chemoresistance in Lung Cancer by Stabilizing c-Myc via the Inhibition of NEDD4L. Front. Cell Dev. Biol. 2020, 8, 620603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, C.; Qiao, Z.; Shang, Z.; Uzzaman, A.; Liu, S.; Jiang, X.; Fan, L.Y.; Ji, L.; Guan, X.; et al. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J. Proteome Res. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Bian, B.; Zhao, C.; He, X.; Gong, Y.; Ren, C.; Ge, L.; Zeng, Y.; Li, Q.; Chen, M.; Weng, C.; et al. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J. Extracell. Vesicles 2020, 9, 1748931. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef]
- Dong, H.; Wang, M.; Li, Q. Exosomal miR-4488 and miR-1273g-5p inhibit the epithelial-mesenchymal transition of transforming growth factor beta2-mediated retinal pigment epithelial cells by targeting ATP-binding cassette A4. Bioengineered 2021, 12, 9693–9706. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, W.; Pang, Q.; Xue, C.; Yan, X. Single-particle analysis of tear fluid reveals abundant presence of tissue factor-exposing extracellular vesicles with strong coagulation activity. Talanta 2022, 239, 123089. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Ophthalmologica 2020, 243, 9–20. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Aguilar, E.; Friedlander, M.; Gariano, R.F. Endothelial proliferation in diabetic retinal microaneurysms. Arch. Ophthalmol. 2003, 121, 740–741. [Google Scholar] [CrossRef]
- Cherian, S.; Roy, S.; Pinheiro, A.; Roy, S. Tight glycemic control regulates fibronectin expression and basement membrane thickening in retinal and glomerular capillaries of diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Hsieh, S.T.; Chiang, H.Y. Fibronectin inhibitor pUR4 attenuates tumor necrosis factor alpha-induced endothelial hyperpermeability by modulating beta1 integrin activation. J. Biomed. Sci. 2019, 26, 37. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Newman, D.K. Surgical management of the late complications of proliferative diabetic retinopathy. Eye 2010, 24, 441–449. [Google Scholar] [CrossRef]
- Gunduz, K.; Bakri, S.J. Management of proliferative diabetic retinopathy. Compr. Ophthalmol. Update 2007, 8, 245–256. [Google Scholar]
- Li, D.Q.; Choudhry, N. Tractional Retinal Detachment Secondary to Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, e183507. [Google Scholar] [CrossRef]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged with High Glucose. Front. Pharmacol. 2020, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, A.; Szuscik, I.; Kusnierz-Cabala, B.; Kapusta, M.; Konkolewska, M.; Zurakowski, A.; Georgescu, A.; Stepien, E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Crac. 2015, 55, 35–48. [Google Scholar]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Busik, J.V. Extracellular Vesicle-Induced Classical Complement Activation Leads to Retinal Endothelial Cell Damage via MAC Deposition. Int. J. Mol. Sci. 2020, 21, 1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Dong, X.; Wang, T.; Kong, Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp. Eye Res. 2019, 189, 107813. [Google Scholar] [CrossRef] [PubMed]
- Kamalden, T.A.; Macgregor-Das, A.M.; Kannan, S.M.; Dunkerly-Eyring, B.; Khaliddin, N.; Xu, Z.; Fusco, A.P.; Yazib, S.A.; Chow, R.C.; Duh, E.J.; et al. Exosomal MicroRNA-15a Transfer from the Pancreas Augments Diabetic Complications by Inducing Oxidative Stress. Antioxid. Redox Signal. 2017, 27, 913–930. [Google Scholar] [CrossRef]
- Katome, T.; Namekata, K.; Mitamura, Y.; Semba, K.; Egawa, M.; Naito, T.; Harada, C.; Harada, T. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J. Diabetes Complicat. 2015, 29, 275–281. [Google Scholar] [CrossRef]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef] [Green Version]
- Wooff, Y.; Cioanca, A.V.; Chu-Tan, J.A.; Aggio-Bruce, R.; Schumann, U.; Natoli, R. Small-Medium Extracellular Vesicles and Their miRNA Cargo in Retinal Health and Degeneration: Mediators of Homeostasis, and Vehicles for Targeted Gene Therapy. Front. Cell. Neurosci. 2020, 14, 160. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Y.; Zou, J.; Wang, W.; Wei, T.; Wang, X.; Zhu, L.; Zhang, M.; Zhu, J.; Xie, T.; et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp. Eye Res. 2020, 201, 108271. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [Green Version]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ge, H.M.; Liu, B.H.; Dong, R.; Shan, K.; Chen, X.; Yao, M.D.; Li, X.M.; Yao, J.; Zhou, R.M.; et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 7455–7464. [Google Scholar] [CrossRef] [Green Version]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Fishman, G.A. A historical perspective on the early treatment of night blindness and the use of dubious and unproven treatment strategies for patients with retinitis pigmentosa. Surv. Ophthalmol. 2013, 58, 652–663. [Google Scholar] [CrossRef]
- Sahaboglu, A.; Tanimoto, N.; Kaur, J.; Sancho-Pelluz, J.; Huber, G.; Fahl, E.; Arango-Gonzalez, B.; Zrenner, E.; Ekstrom, P.; Lowenheim, H.; et al. PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PLoS ONE 2010, 5, e15495. [Google Scholar] [CrossRef] [Green Version]
- Arango-Gonzalez, B.; Trifunovic, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef]
- Vidal-Gil, L.; Sancho-Pelluz, J.; Zrenner, E.; Oltra, M.; Sahaboglu, A. Poly ADP ribosylation and extracellular vesicle activity in rod photoreceptor degeneration. Sci. Rep. 2019, 9, 3758. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.; Carneiro, A.; Vieira, M.; Tenreiro, S.; Seabra, M.C. Age-Related Macular Degeneration: Pathophysiology, Management, and Future Perspectives. Ophthalmologica 2021, 244, 495–511. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef] [Green Version]
- Bird, A.C. Therapeutic targets in age-related macular disease. J. Clin. Investig. 2010, 120, 3033–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasiak, J. Senescence in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 2020, 77, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Choudhary, M.M.; Schachat, A.P. Neovascular Age-Related Macular Degeneration. Dev. Ophthalmol. 2016, 55, 125–136. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Chong, N.V. The complement system and age-related macular degeneration. Eye 2006, 20, 867–872. [Google Scholar] [CrossRef]
- Scholl, H.P.; Charbel Issa, P.; Walier, M.; Janzer, S.; Pollok-Kopp, B.; Borncke, F.; Fritsche, L.G.; Chong, N.V.; Fimmers, R.; Wienker, T.; et al. Systemic complement activation in age-related macular degeneration. PLoS ONE 2008, 3, e2593. [Google Scholar] [CrossRef]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, Y.L.; Wang, Y.X.; Zhao, P.Q.; Wang, Z.Y. Retinal pigment epithelium cell-derived exosomes: Possible relevance to CNV in wet-age related macular degeneration. Med. Hypotheses 2016, 97, 98–101. [Google Scholar] [CrossRef]
- ElShelmani, H.; Wride, M.A.; Saad, T.; Rani, S.; Kelly, D.J.; Keegan, D. The Role of Deregulated MicroRNAs in Age-Related Macular Degeneration Pathology. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; Hu, Y.; Cui, B.; Li, W.; Xu, G.; Liu, L.; Liu, S. MicroRNA-410 reduces the expression of vascular endothelial growth factor and inhibits oxygen-induced retinal neovascularization. PLoS ONE 2014, 9, e95665. [Google Scholar] [CrossRef]
- Wang, L.; Lee, A.Y.; Wigg, J.P.; Peshavariya, H.; Liu, P.; Zhang, H. miR-126 Regulation of Angiogenesis in Age-Related Macular Degeneration in CNV Mouse Model. Int. J. Mol. Sci. 2016, 17, 895. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Peng, Y.; Mo, J.; Yao, Y. MiR-126 enhances VEGF expression in induced pluripotent stem cell-derived retinal neural stem cells by targeting spred-1. Int. J. Clin. Exp. Pathol. 2018, 11, 1023–1030. [Google Scholar]
- Mak, H.K.; Yung, J.S.Y.; Weinreb, R.N.; Ng, S.H.; Cao, X.; Ho, T.Y.C.; Ng, T.K.; Chu, W.K.; Yung, W.H.; Choy, K.W.; et al. MicroRNA-19a-PTEN Axis Is Involved in the Developmental Decline of Axon Regenerative Capacity in Retinal Ganglion Cells. Mol. Ther. Nucleic Acids 2020, 21, 251–263. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef] [Green Version]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [Green Version]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef] [Green Version]
- Han, K.Y.; Tran, J.A.; Chang, J.H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [Green Version]
- Han, K.Y.; Dugas-Ford, J.; Seiki, M.; Chang, J.H.; Azar, D.T. Evidence for the Involvement of MMP14 in MMP2 Processing and Recruitment in Exosomes of Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5323–5329. [Google Scholar] [CrossRef] [Green Version]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Karamichos, D.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D. Corneal Epithelial-Stromal Fibroblast Constructs to Study Cell-Cell Communication in Vitro. Bioengineering 2019, 6, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 2019, 5738510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Zheng, Q.Q.; Shen, J.; Li, Q.S.; Song, X.H.; Luo, H.B.; Hong, C.Y.; Yao, K. Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. 2018, 131, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef]
- Escandon, P.; Liu, A.; Nicholas, S.E.; Khan, A.; Riaz, K.M.; Karamichos, D. Unravelling Novel Roles of Salivary Exosomes in the Regulation of Human Corneal Stromal Cell Migration and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4330. [Google Scholar] [CrossRef]
- Leibowitz, J.A.; Woods, A.T.; Kesselman, M.M.; Mayi, B.S. Uveitis as a Predictor of Predisposition to Autoimmunity. Cureus 2020, 12, e7451. [Google Scholar] [CrossRef] [Green Version]
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Prete, M.; Dammacco, R.; Fatone, M.C.; Racanelli, V. Autoimmune uveitis: Clinical, pathogenetic, and therapeutic features. Clin. Exp. Med. 2016, 16, 125–136. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Liu, B.; Arakelyan, A.; Zicari, S.; Hannes, S.; Chen, P.; Li, Z.; Grivel, J.C.; Chaigne-Delalande, B.; Sen, H.N.; et al. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4101–4107. [Google Scholar] [CrossRef] [Green Version]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 2017, 7, 4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Choi, J.K.; Jittayasothorn, Y.; Egwuagu, C.E. Interleukin 35-Producing Exosomes Suppress Neuroinflammation and Autoimmune Uveitis. Front. Immunol. 2020, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yun, J.; Kaplan, H.J.; Zhao, Y.; Sun, D.; Shao, H. Vaccination with circulating exosomes in autoimmune uveitis prevents recurrent intraocular inflammation. Clin. Exp. Ophthalmol. 2021, 49, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.N.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef]
- Materin, M.A.; Faries, M.; Kluger, H.M. Molecular alternations in uveal melanoma. Curr. Probl. Cancer 2011, 35, 211–224. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Tsering, T.; Laskaris, A.; Abdouh, M.; Bustamante, P.; Parent, S.; Jin, E.; Ferrier, S.T.; Arena, G.; Burnier, J.V. Uveal Melanoma-Derived Extracellular Vesicles Display Transforming Potential and Carry Protein Cargo Involved in Metastatic Niche Preparation. Cancers 2020, 12, 2923. [Google Scholar] [CrossRef]
- Wroblewska, J.P.; Lach, M.S.; Kulcenty, K.; Galus, L.; Suchorska, W.M.; Rosel, D.; Brabek, J.; Marszalek, A. The Analysis of Inflammation-Related Proteins in a Cargo of Exosomes Derived from the Serum of Uveal Melanoma Patients Reveals Potential Biomarkers of Disease Progression. Cancers 2021, 13, 3334. [Google Scholar] [CrossRef]
- Eldh, M.; Olofsson Bagge, R.; Lasser, C.; Svanvik, J.; Sjostrand, M.; Mattsson, J.; Lindner, P.; Choi, D.S.; Gho, Y.S.; Lotvall, J. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer 2014, 14, 962. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Gombos, D.S.; Vats, T.S. Retinoblastoma: An overview. Indian J. Pediatr. 2012, 79, 916–921. [Google Scholar] [CrossRef]
- Castro-Magdonel, B.E.; Orjuela, M.; Alvarez-Suarez, D.E.; Camacho, J.; Cabrera-Munoz, L.; Sadowinski-Pine, S.; Medina-Sanson, A.; Lara-Molina, C.; Garcia-Vega, D.; Vazquez, Y.; et al. Circulating miRNome detection analysis reveals 537 miRNAS in plasma, 625 in extracellular vesicles and a discriminant plasma signature of 19 miRNAs in children with retinoblastoma from which 14 are also detected in corresponding primary tumors. PLoS ONE 2020, 15, e0231394. [Google Scholar] [CrossRef] [Green Version]
- Ravishankar, H.; Mangani, A.S.; Phoebe Moses, G.L.; Mani, S.P.; Parameswaran, S.; Khetan, V.; Ganesan, S.; Krishnakumar, S. Serum exosomal miRNA as biomarkers for Retinoblastoma. Exp. Eye Res. 2020, 199, 108184. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Qiu, J.; Chen, P.; Han, X.; Wu, Y.; Zhuang, J.; Yang, M.; Wu, C.; Wu, N.; et al. Exosomes derived from retinoblastoma cells enhance tumour deterioration by infiltrating the microenvironment. Oncol. Rep. 2021, 45, 278–290. [Google Scholar] [CrossRef]
- Pastor, J.C.; Rojas, J.; Pastor-Idoate, S.; Di Lauro, S.; Gonzalez-Buendia, L.; Delgado-Tirado, S. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog. Retin. Eye Res. 2016, 51, 125–155. [Google Scholar] [CrossRef]
- Claes, C.; Lafeta, A.P. Proliferative vitreoretinopathy. Dev. Ophthalmol. 2014, 54, 188–195. [Google Scholar] [CrossRef]
- Pastor, J.C.; de la Rua, E.R.; Martin, F. Proliferative vitreoretinopathy: Risk factors and pathobiology. Prog. Retin. Eye Res. 2002, 21, 127–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Pan, J.; Yang, S.; Yao, H.; Li, M.; Li, H.; Lei, H.; Jin, H.; Wang, F. Exosomes mediate an epithelial-mesenchymal transition cascade in retinal pigment epithelial cells: Implications for proliferative vitreoretinopathy. J. Cell. Mol. Med. 2020, 24, 13324–13335. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Dong, H. Proteomic evidence that ABCA4 is vital for traumatic proliferative vitreoretinopathy formation and development. Exp. Eye Res. 2019, 181, 232–239. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Asrani, S.; Freedman, S.F.; El-Dairi, M.; Bhatti, M.T. Differentiating glaucomatous from non-glaucomatous optic nerve cupping by optical coherence tomography. Open Neurol. J. 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Huang, H.B.; Wei, S.H. Clinical characteristics of nonglaucomatous optic disc cupping. Exp. Ther. Med. 2014, 7, 995–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fard, M.A.; Moghimi, S.; Sahraian, A.; Ritch, R. Optic nerve head cupping in glaucomatous and non-glaucomatous optic neuropathy. Br. J. Ophthalmol. 2019, 103, 374–378. [Google Scholar] [CrossRef]
- Killer, H.E.; Pircher, A. What is the optimal glaucoma treatment: Reducing aqueous humour production or facilitating its outflow? Eye 2020, 34, 1719–1721. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.A.; Perkumas, K.M.; Highstrom, L.M.; Stamer, W.D. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Lerner, N.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicle-mediated crosstalk between NPCE cells and TM cells result in modulation of Wnt signalling pathway and ECM remodelling. J. Cell. Mol. Med. 2020, 24, 4646–4658. [Google Scholar] [CrossRef]
- Lerner, N.; Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate signaling between the aqueous humor producing and draining cells in the ocular system. PLoS ONE 2017, 12, e0171153. [Google Scholar] [CrossRef] [Green Version]
- Stamer, W.D.; Hoffman, E.A.; Luther, J.M.; Hachey, D.L.; Schey, K.L. Protein profile of exosomes from trabecular meshwork cells. J. Proteomics 2011, 74, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Jayaram, H.; Phillips, J.I.; Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Gattey, D.M.; Saugstad, J.A.; Keller, K.E. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2884–2890. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bailey, J.C.; Helwa, I.; Dismuke, W.M.; Cai, J.; Drewry, M.; Brilliant, M.H.; Budenz, D.L.; Christen, W.G.; Chasman, D.I.; et al. A Common Variant in MIR182 Is Associated with Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4528–4535. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Chang, X.; Xu, M.; Zhang, M.; Zhang, S.; Wang, Y.; Luo, X.; Xu, J.; Yang, X.; Sun, X. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J. Chem. Neuroanat. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Sugali, C.K.; Rayana, N.P.; Dai, J.; Peng, M.; Harris, S.L.; Webber, H.C.; Liu, S.; Dixon, S.G.; Parekh, P.H.; Martin, E.A.; et al. The Canonical Wnt Signaling Pathway Inhibits the Glucocorticoid Receptor Signaling Pathway in the Trabecular Meshwork. Am. J. Pathol. 2021, 191, 1020–1035. [Google Scholar] [CrossRef]
- Villarreal, G., Jr.; Chatterjee, A.; Oh, S.S.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7433–7440. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.M., Jr. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6972. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.H.; McNatt, L.G.; Pang, I.H.; Millar, J.C.; Hellberg, P.E.; Hellberg, M.H.; Steely, H.T.; Rubin, J.S.; Fingert, J.H.; Sheffield, V.C.; et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Investig. 2008, 118, 1056–1064. [Google Scholar] [CrossRef] [Green Version]
- Tabak, S.; Feinshtein, V.; Schreiber-Avissar, S.; Beit-Yannai, E. Non-Pigmented Ciliary Epithelium-Derived Extracellular Vesicles Loaded with SMAD7 siRNA Attenuate Wnt Signaling in Trabecular Meshwork Cells In Vitro. Pharmaceuticals 2021, 14, 858. [Google Scholar] [CrossRef]
- McDonnell, F.; O’Brien, C.; Wallace, D. The role of epigenetics in the fibrotic processes associated with glaucoma. J. Ophthalmol. 2014, 2014, 750459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trelford, C.B.; Denstedt, J.T.; Armstrong, J.J.; Hutnik, C.M.L. The Pro-Fibrotic Behavior of Human Tenon’s Capsule Fibroblasts in Medically Treated Glaucoma Patients. Clin. Ophthalmol. 2020, 14, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Aires, I.D.; Santiago, A.R. Microglial exosomes in retinal neuroinflammation: Focus in glaucoma. Neural Regen. Res. 2021, 16, 1801–1802. [Google Scholar] [CrossRef]
- Ran, N.; Gao, X.; Dong, X.; Li, J.; Lin, C.; Geng, M.; Yin, H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 2020, 236, 119826. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jiang, C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm. Sin. B 2020, 10, 979–986. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjug. Chem. 2019, 30, 994–1005. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, H.; Cho, H.; Choi, J.; Hwang, K.Y.; Choi, Y.; Kim, S.H.; Yang, Y. Recent Advances in Exosome-Based Drug Delivery for Cancer Therapy. Cancers 2021, 13, 4435. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2021, 12, 2385–2402. [Google Scholar] [CrossRef]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. Daru 2019, 27, 533–539. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Kase, Y.; Uzawa, K.; Wagai, S.; Yoshimura, S.; Yamamoto, J.I.; Toeda, Y.; Okubo, M.; Eizuka, K.; Ando, T.; Nobuchi, T.; et al. Engineered exosomes delivering specific tumor-suppressive RNAi attenuate oral cancer progression. Sci. Rep. 2021, 11, 5897. [Google Scholar] [CrossRef]
- Sayyed, A.A.; Gondaliya, P.; Mali, M.; Pawar, A.; Bhat, P.; Khairnar, A.; Arya, N.; Kalia, K. MiR-155 Inhibitor-Laden Exosomes Reverse Resistance to Cisplatin in a 3D Tumor Spheroid and Xenograft Model of Oral Cancer. Mol. Pharm. 2021, 18, 3010–3025. [Google Scholar] [CrossRef]
- Cohen, O.; Betzer, O.; Elmaliach-Pnini, N.; Motiei, M.; Sadan, T.; Cohen-Berkman, M.; Dagan, O.; Popovtzer, A.; Yosepovich, A.; Barhom, H.; et al. ‘Golden’ exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: In vivo tracking in a model for head and neck cancer. Biomater. Sci. 2021, 9, 2103–2114. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Kalhor, N.; Sheikholeslami, A.; Dolati, M.; Amini, E.; Fazaeli, H. Exosomes of Mesenchymal Stem Cells as a Proper Vehicle for Transfecting miR-145 into the Breast Cancer Cell Line and Its Effect on Metastasis. Biomed. Res. Int. 2021, 2021, 5516078. [Google Scholar] [CrossRef]
- Ogawa, Y.; Akimoto, Y.; Ikemoto, M.; Goto, Y.; Ishikawa, A.; Ohta, S.; Takase, Y.; Kawakami, H.; Tsujimoto, M.; Yanoshita, R. Stability of human salivary extracellular vesicles containing dipeptidyl peptidase IV under simulated gastrointestinal tract conditions. Biochem. Biophys. Rep. 2021, 27, 101034. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yim, H.; Park, C.; Ahn, S.H.; Ahn, Y.; Lee, A.; Yang, H.; Choi, C. Targeted Delivery of Exosomes Armed with Anti-Cancer Therapeutics. Membranes 2022, 12, 85. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
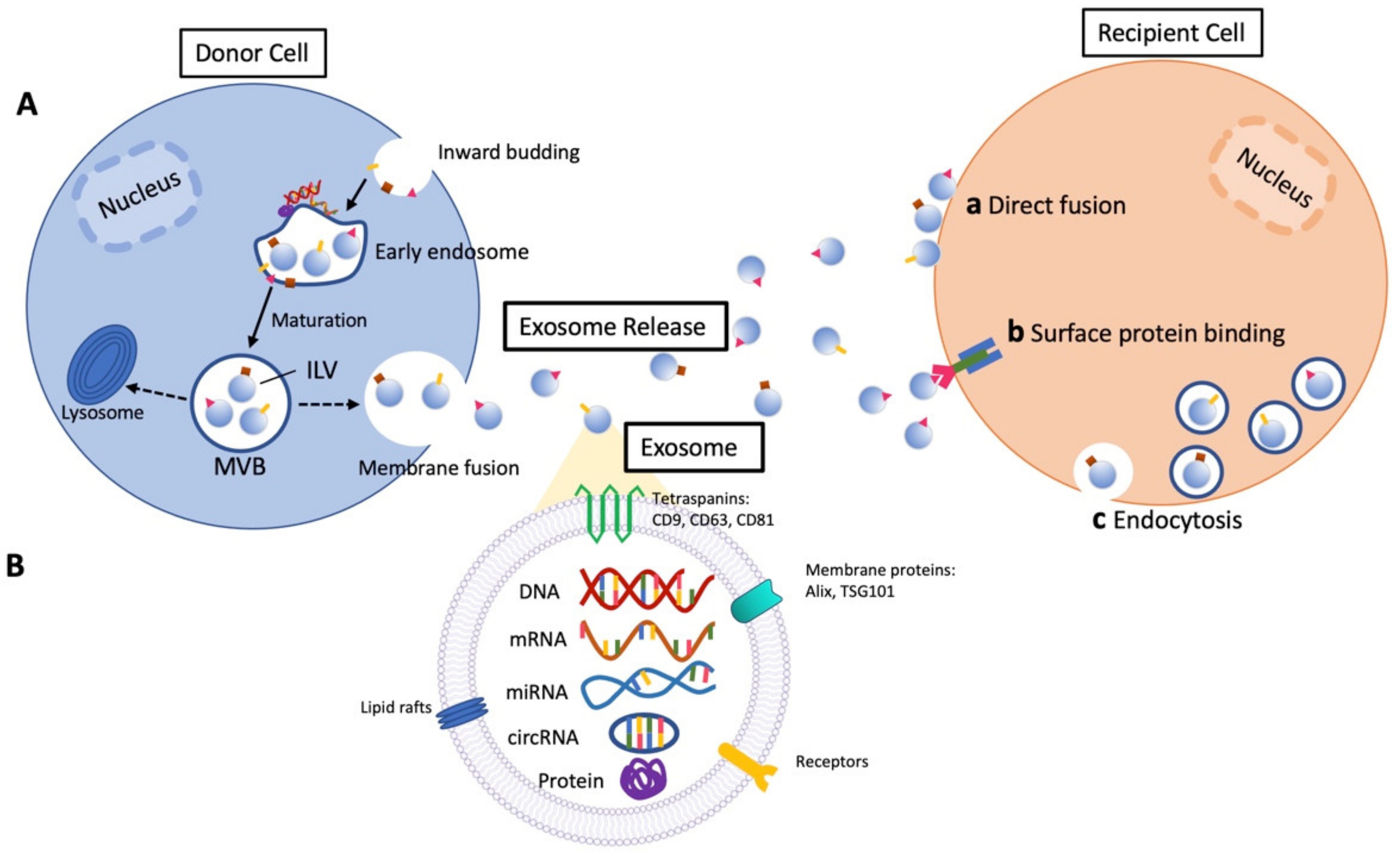
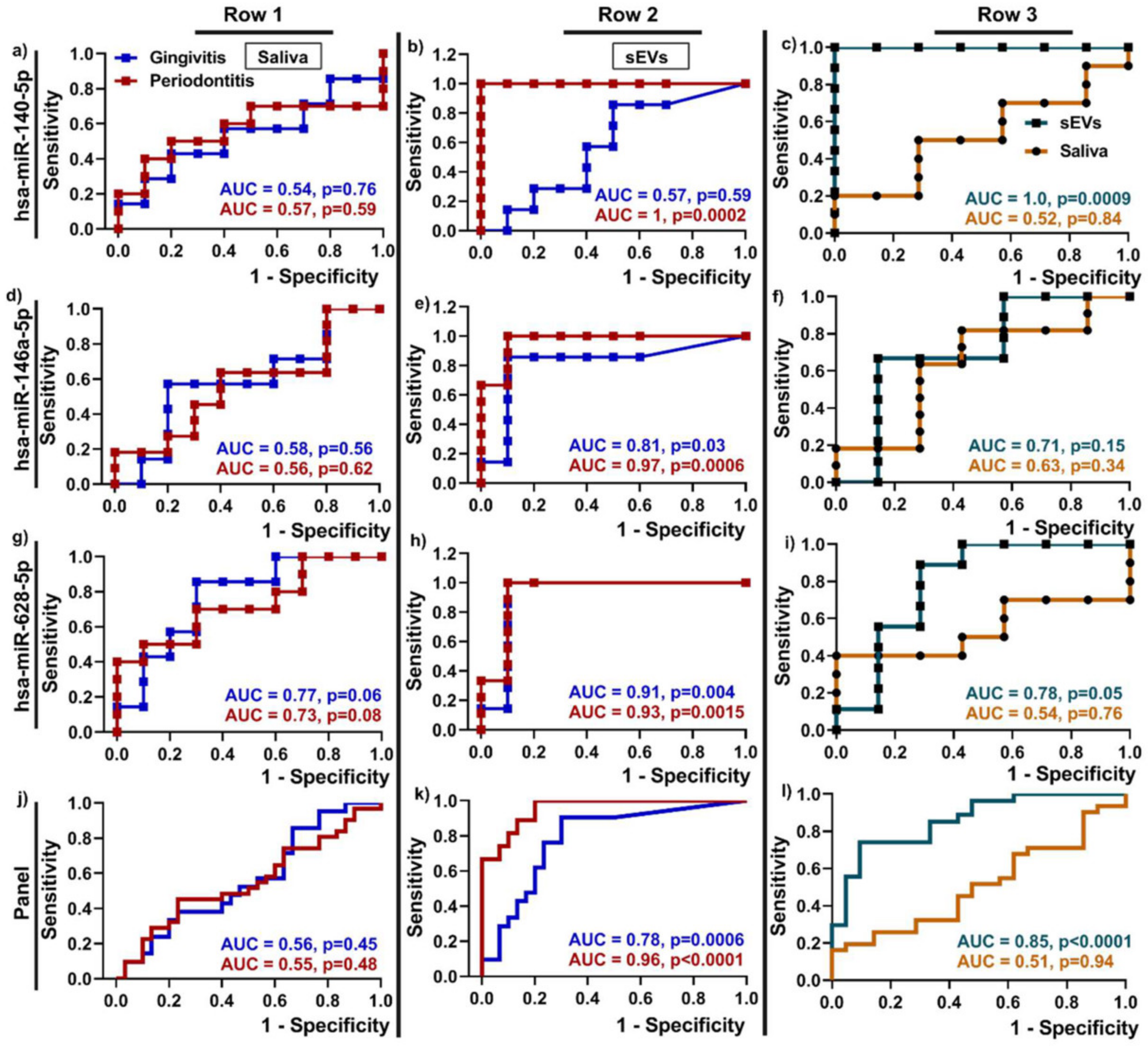
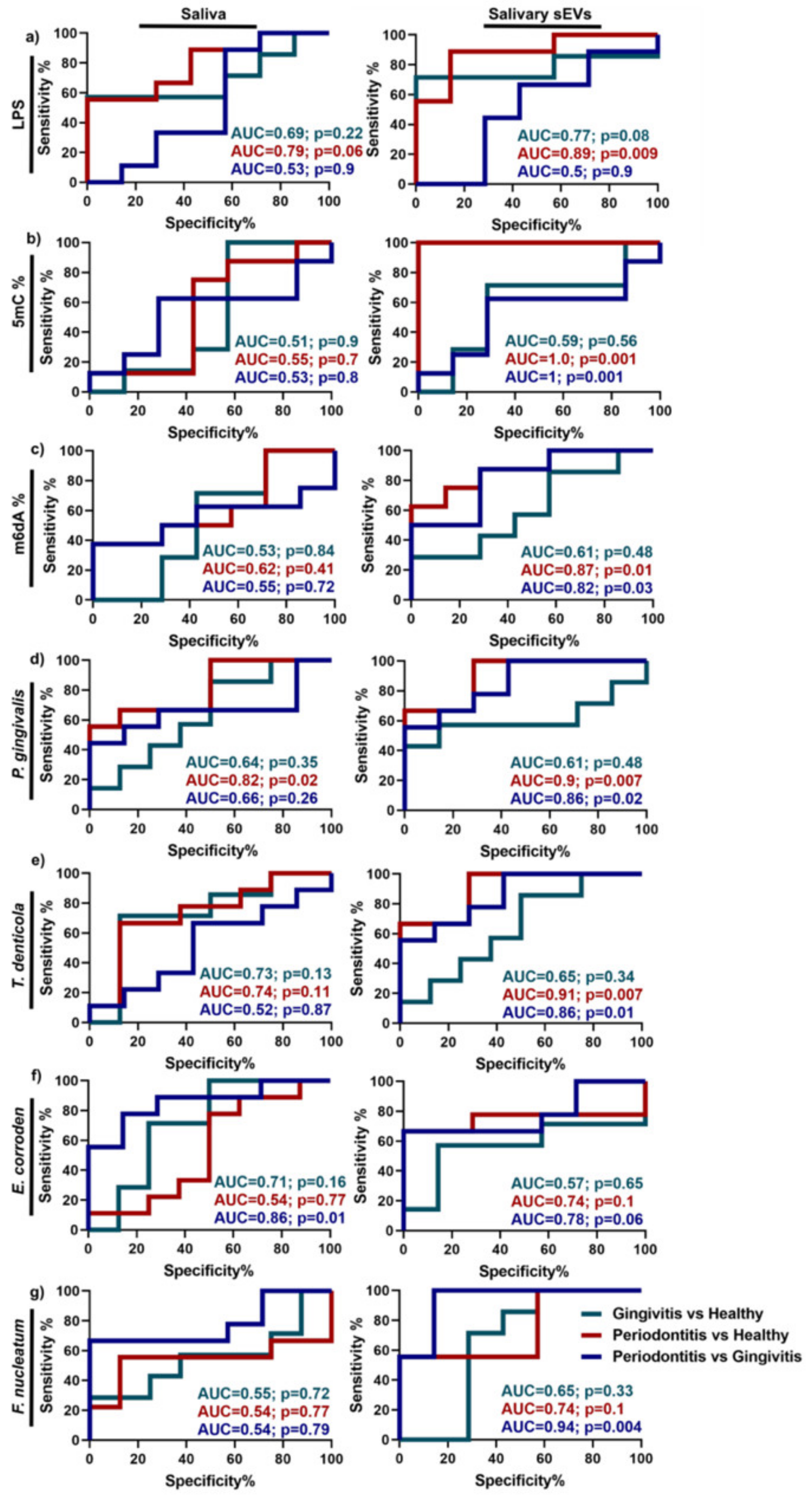
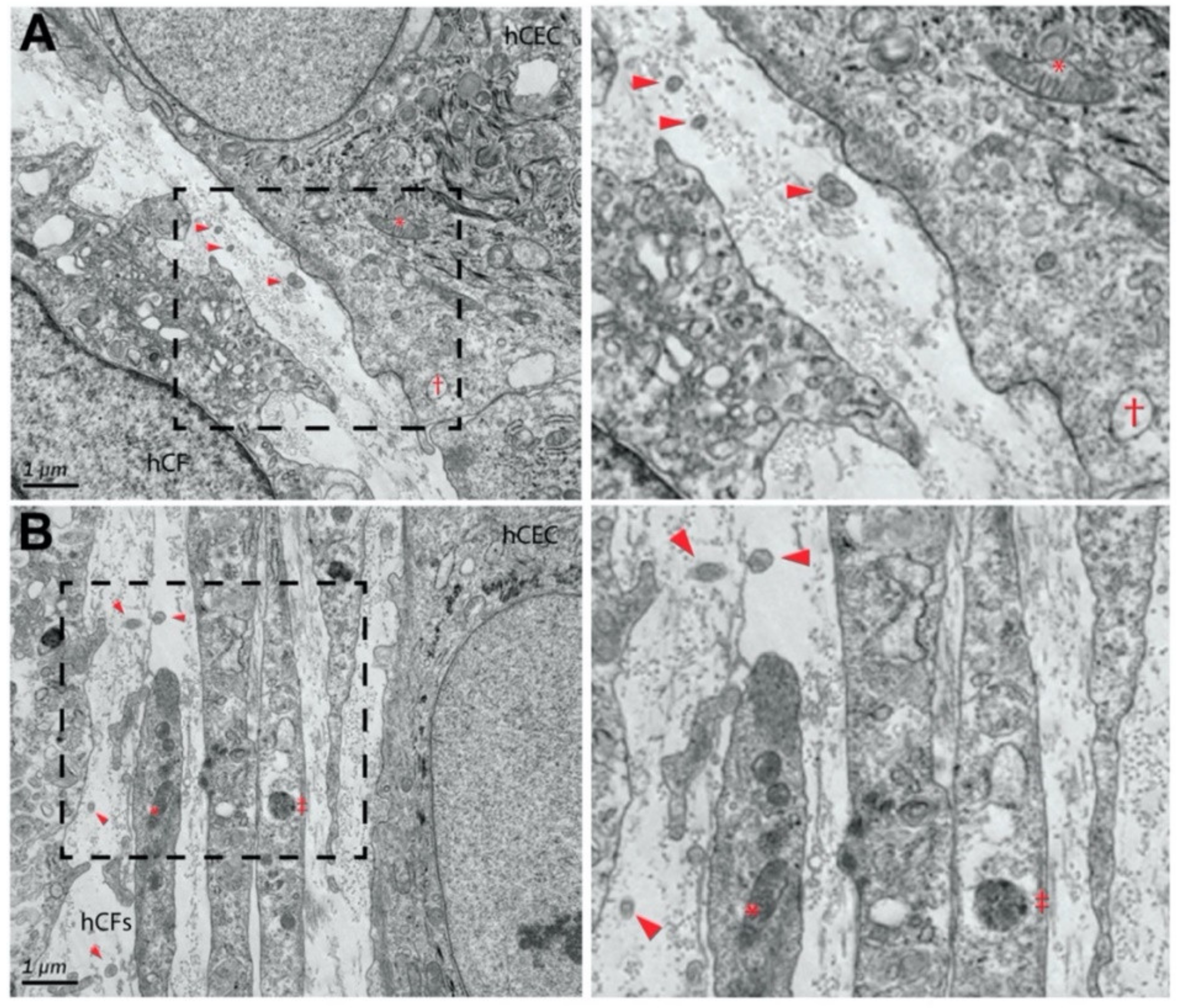

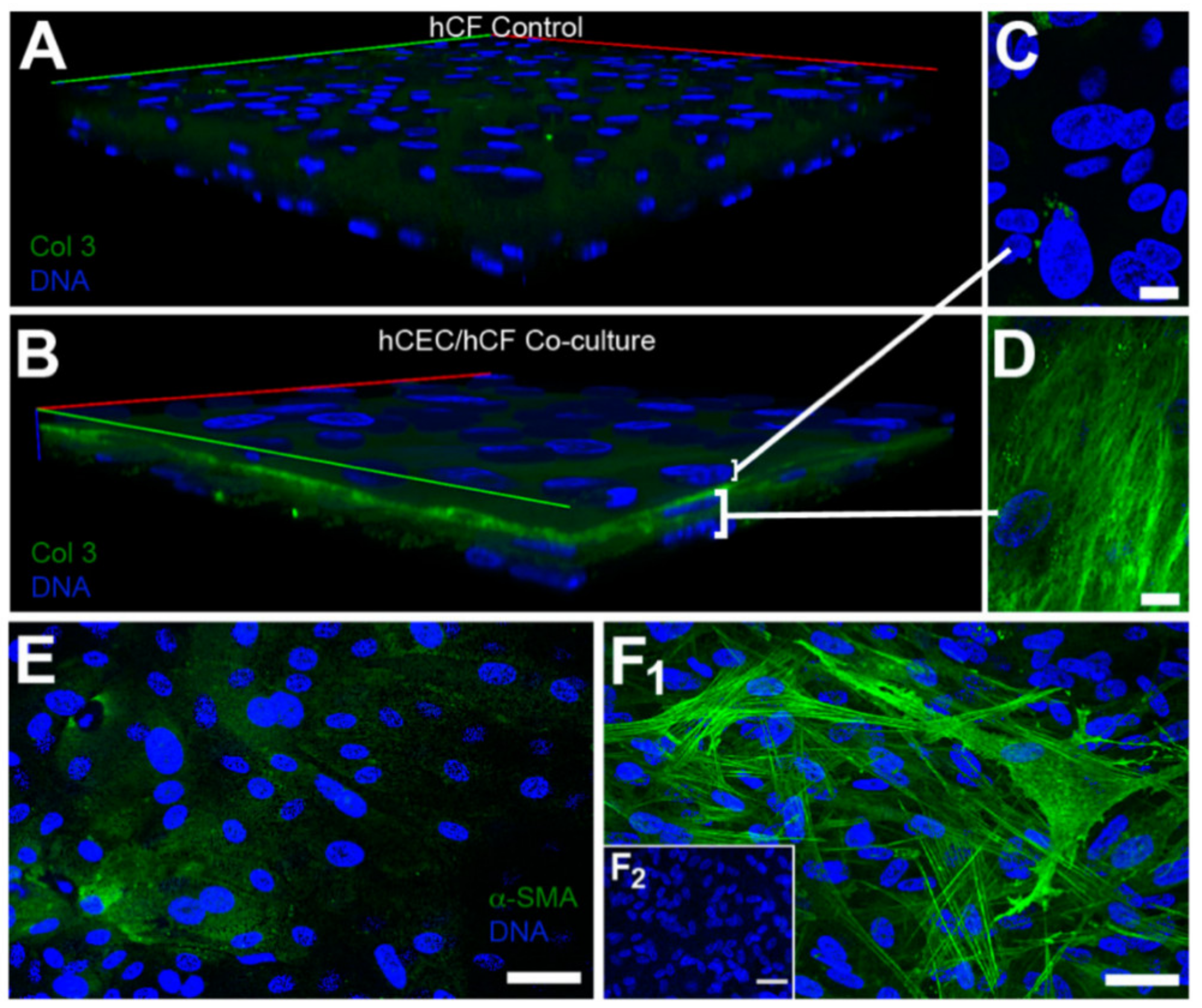
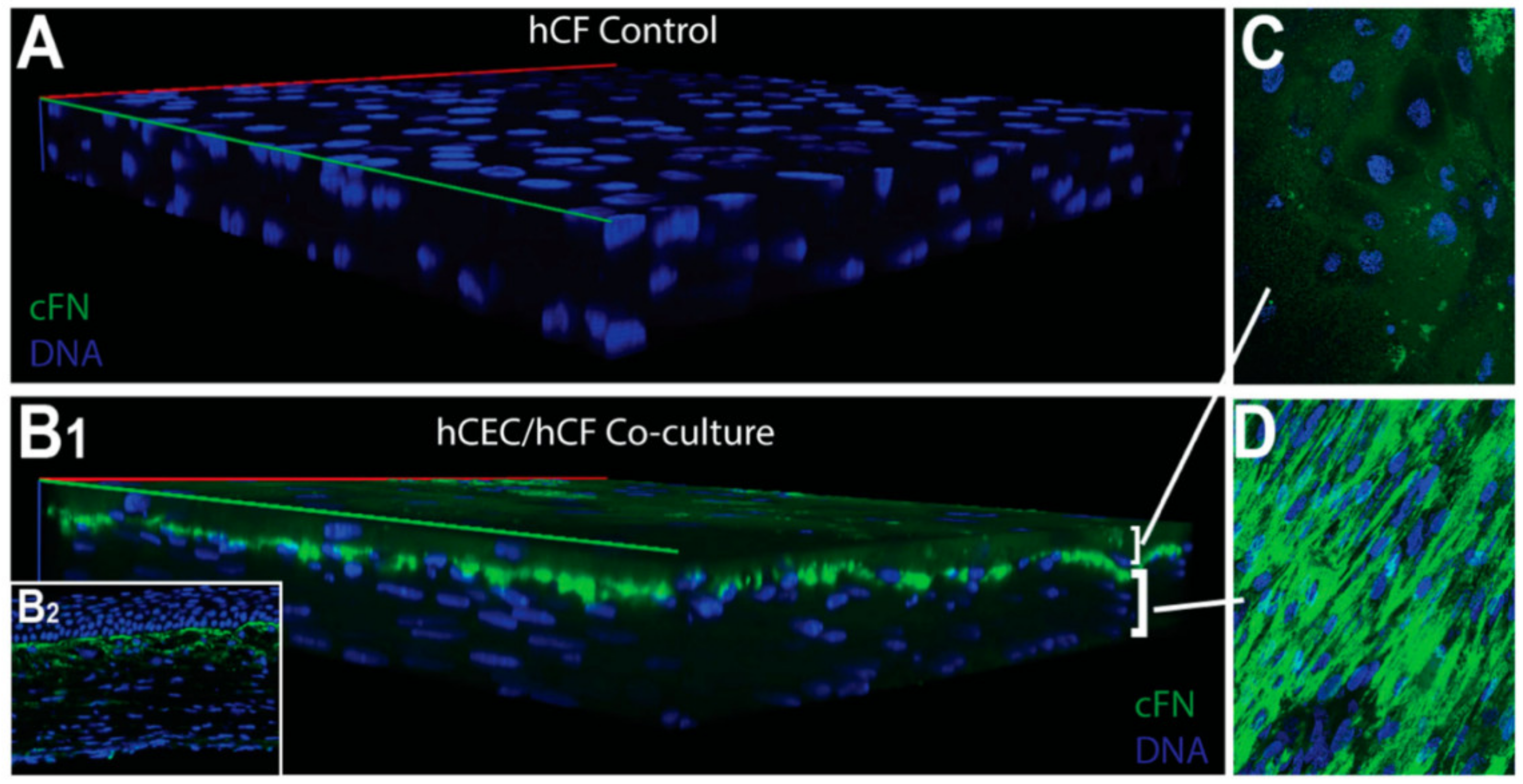
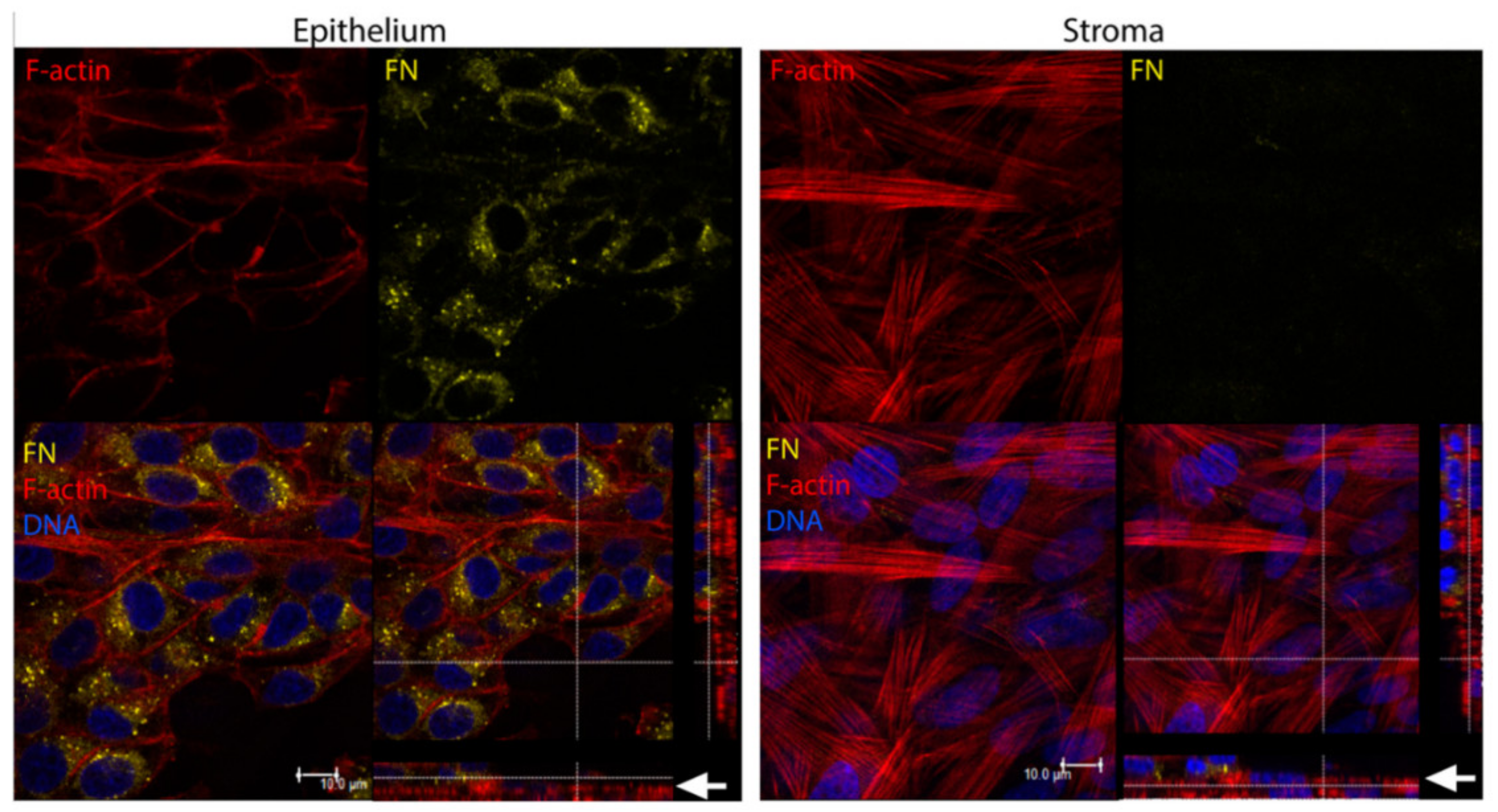
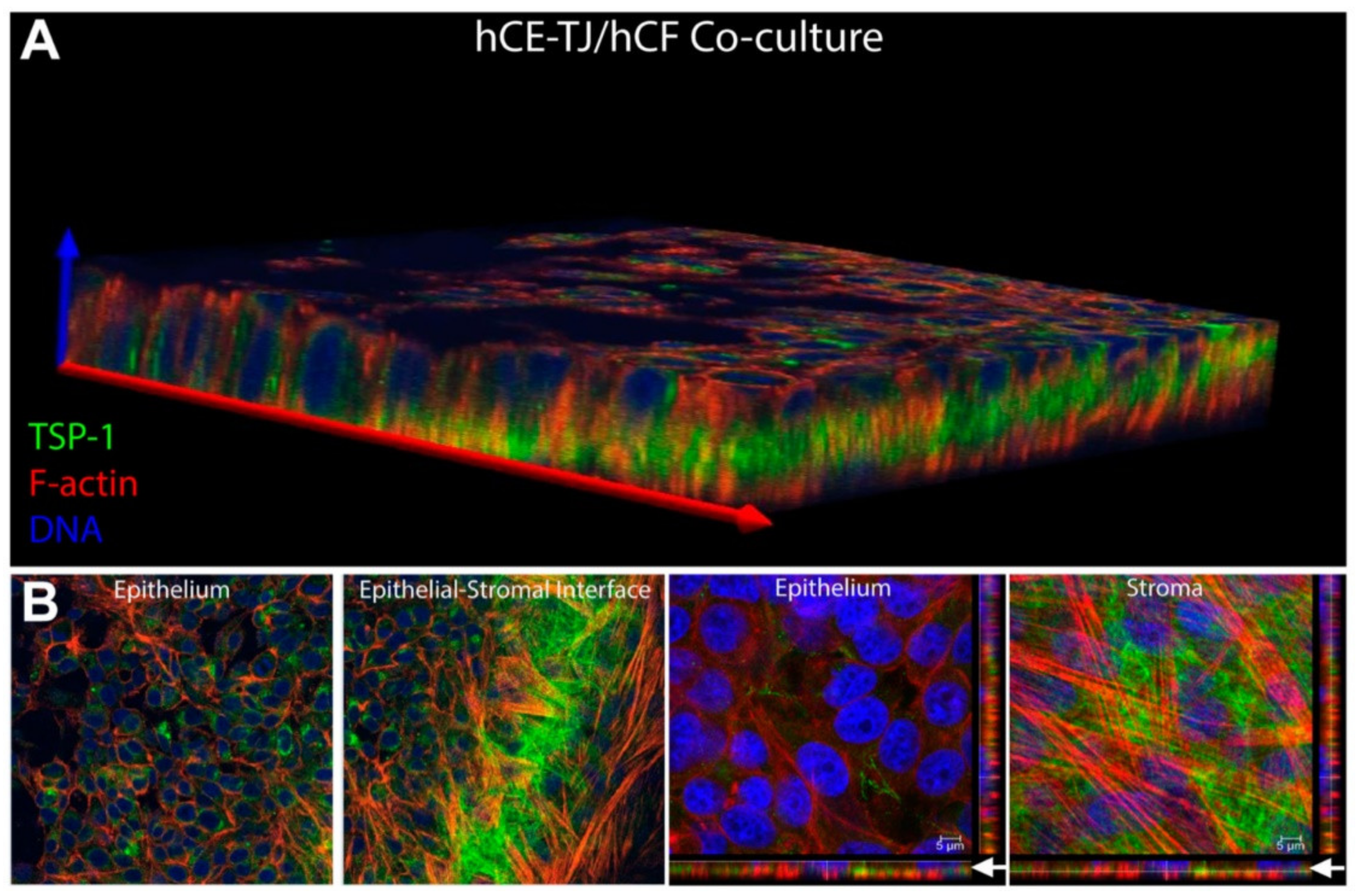

| Whole Saliva or Type of Non-SE | Disadvantage of Whole Saliva/Non-SE | Advantage of SEs | Shared Disadvantages of SEs and Non-SEs |
|---|---|---|---|
| Whole Saliva | Harbors contaminating elements and higher amylase enzyme levels | SE have a lipid bilayer that protects their cargo from contamination and degradation | |
| Proteins from whole saliva are susceptible to degradation when removed from their natural environment | |||
| Serum-derived exosome | Collection requires trained personnel and is often more invasive | SE collection is easy and non-invasive, improving patient compliance | Lack of methodologies to quantify contents of exosomes |
| Coagulation poses challenges in handling these exosomes | Saliva does not coagulate | ||
| Urinary exosome | Applications limited to kidney and prostate pathologies | Wide-ranging applications of SE, including systemic autoimmune disease, neurodegenerative disease, neoplasms, and ocular disease | |
| MSC-derived exosome | Rapid clearance from blood after administration in vivo | Stable in biological fluids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Hefley, B.; Escandon, P.; Nicholas, S.E.; Karamichos, D. Salivary Exosomes in Health and Disease: Future Prospects in the Eye. Int. J. Mol. Sci. 2023, 24, 6363. https://doi.org/10.3390/ijms24076363
Liu A, Hefley B, Escandon P, Nicholas SE, Karamichos D. Salivary Exosomes in Health and Disease: Future Prospects in the Eye. International Journal of Molecular Sciences. 2023; 24(7):6363. https://doi.org/10.3390/ijms24076363
Chicago/Turabian StyleLiu, Angela, Brenna Hefley, Paulina Escandon, Sarah E. Nicholas, and Dimitrios Karamichos. 2023. "Salivary Exosomes in Health and Disease: Future Prospects in the Eye" International Journal of Molecular Sciences 24, no. 7: 6363. https://doi.org/10.3390/ijms24076363
APA StyleLiu, A., Hefley, B., Escandon, P., Nicholas, S. E., & Karamichos, D. (2023). Salivary Exosomes in Health and Disease: Future Prospects in the Eye. International Journal of Molecular Sciences, 24(7), 6363. https://doi.org/10.3390/ijms24076363







