IL-6 Does Not Influence the Expression of SLC41A1 and Other Mg-Homeostatic Factors
Abstract
:1. Introduction
2. Results
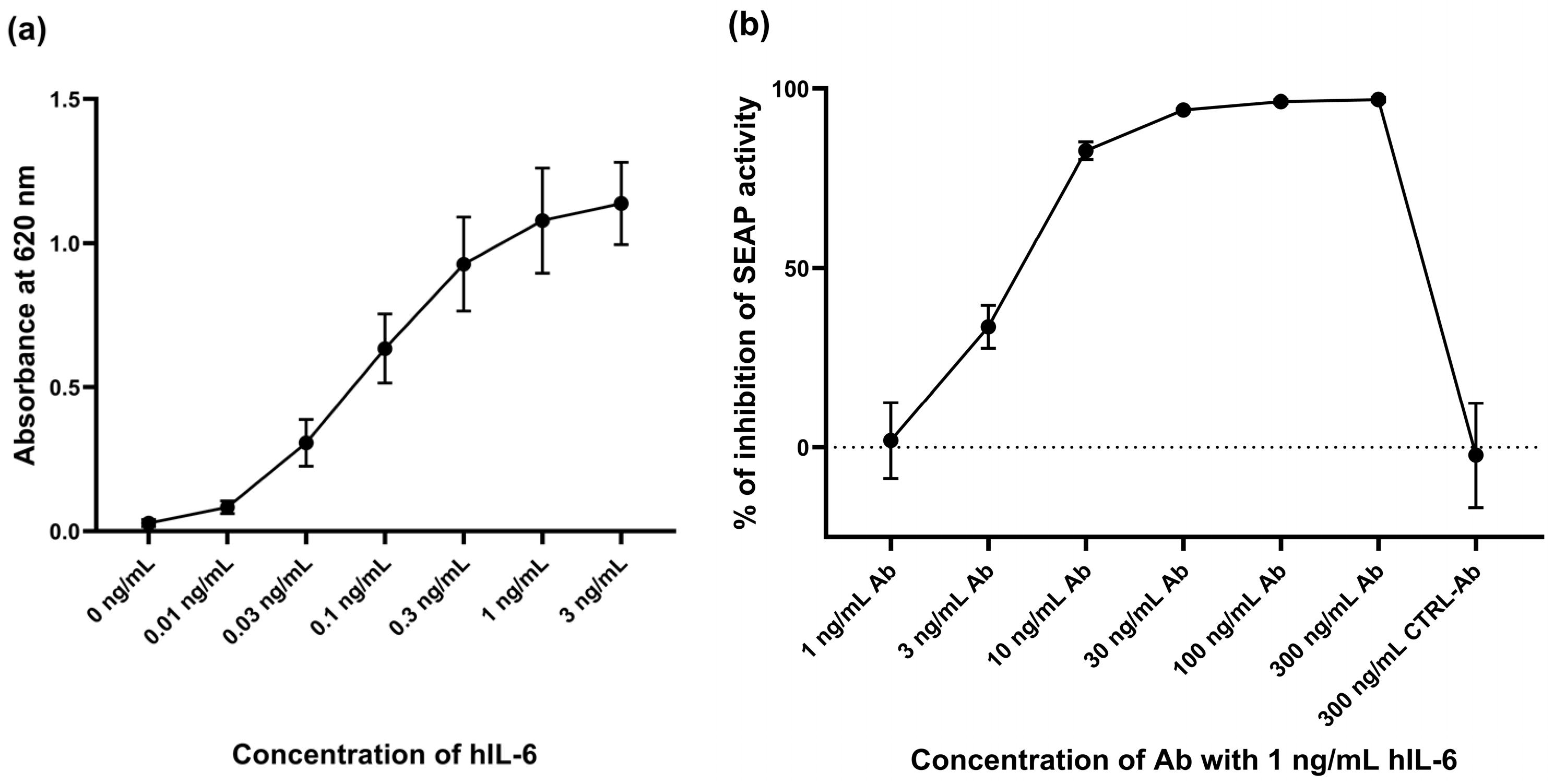
2.1. Response of HEK-Blue™ IL-6 Cell Line to hIL-6
2.2. Activation of the IL-6/JAK/STAT3 Signaling Pathway in Three Responsive Cell Lines
2.3. Putative Changes in the Expression of Magnesiotropic Genes Triggered by hIL-6
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. IL-6 Response Colorimetric Assay
4.3. Western Blot Analysis
4.4. RT-qPCR and Data Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahi, A.; Aslani, S.; Ataollahi, M.; Mahmoudi, M. The Role of Magnesium in Different Inflammatory Diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal Magnesium Status in the United States: Are the Health Consequences Underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Rosanoff, A.; West, C.; Elin, R.J.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.-C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an Updated Standardization of Serum Magnesium Reference Ranges. Eur. J. Nutr. 2022, 61, 3697–3706. [Google Scholar] [CrossRef]
- Nielsen, F.H. Effects of Magnesium Depletion on Inflammation in Chronic Disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 525–530. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Lainampetch, J.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Prangthip, P.; Soonthornworasiri, N.; Puduang, S.; Wechjakwen, N.; Kwanbunjan, K. Association of Tumor Necrosis Factor Alpha, Interleukin 6, and C-Reactive Protein with the Risk of Developing Type 2 Diabetes: A Retrospective Cohort Study of Rural Thais. J. Diabetes Res. 2019, 2019, 9051929. [Google Scholar] [CrossRef]
- López-Baltanás, R.; Encarnación Rodríguez-Ortiz, M.; Canalejo, A.; Díaz-Tocados, J.M.; Herencia, C.; Leiva-Cepas, F.; Torres-Peña, J.D.; Ortíz-Morales, A.; Muñoz-Castañeda, J.R.; Rodríguez, M.; et al. Magnesium Supplementation Reduces Inflammation in Rats with Induced Chronic Kidney Disease. Eur. J. Clin. Investig. 2021, 51, e13561. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Banach, M. Effect of Magnesium Supplements on Serum C-Reactive Protein: A Systematic Review and Meta-Analysis. Arch. Med. Sci. 2018, 14, 707–716. [Google Scholar] [CrossRef]
- Dmitrašinović, G.; Pešić, V.; Stanić, D.; Plećaš-Solarović, B.; Dajak, M.; Ignjatović, S. ACTH, Cortisol and IL-6 Levels in Athletes Following Magnesium Supplementation. J. Med. Biochem. 2016, 35, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Romani, A.M.; Valentin-Torres, A.M.; Luciano, A.A.; Ramirez Kitchen, C.M.; Funderburg, N.; Mesiano, S.; Bernstein, H.B. Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J. Immunol. 2012, 188, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ding, B.; Zhou, L.; Gao, X.; Guo, H.; Xu, H. Magnesium Sulfate Provides Neuroprotection in Lipopolysaccharide-Activated Primary Microglia by Inhibiting NF-κB Pathway. J. Surg. Res. 2013, 184, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, D.N.; Pathak, A.; Agrawal, A. IL-6 Regulates Induction of C-Reactive Protein Gene Expression by Activating STAT3 Isoforms. Mol. Immunol. 2022, 146, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-T.; Sun, Y.-H.; Zong, S.-H.; Xiang, Y.-B. Serum Levels of IL-6 and TNF-α May Correlate with Activity and Severity of Rheumatoid Arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Signalling in Health and Disease. F1000Research 2020, 9, 1013. [Google Scholar] [CrossRef]
- Tesoriere, A.; Dinarello, A.; Argenton, F. The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions. Biomedicines 2021, 9, 956. [Google Scholar] [CrossRef]
- Fahmi, A.; Smart, N.; Punn, A.; Jabr, R.; Marber, M.; Heads, R. P42/P44-MAPK and PI3K Are Sufficient for IL-6 Family Cytokines/Gp130 to Signal to Hypertrophy and Survival in Cardiomyocytes in the Absence of JAK/STAT Activation. Cell. Signal. 2013, 25, 898–909. [Google Scholar] [CrossRef]
- Jain, N.; Zhang, T.; Kee, W.H.; Li, W.; Cao, X. Protein Kinase C δ Associates with and Phosphorylates Stat3 in an Interleukin-6-Dependent Manner. J. Biol. Chem. 1999, 274, 24392–24400. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Lu, Y.; Guan, Y.; Chen, F. JNK-Dependent Stat3 Phosphorylation Contributes to Akt Activation in Response to Arsenic Exposure. Toxicol. Sci. 2012, 129, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.J.; Koetz, L.; Levy, D.E. The MEK-ERK Pathway Is Necessary for Serine Phosphorylation of Mitochondrial STAT3 and Ras-Mediated Transformation. PLoS ONE 2013, 8, e83395. [Google Scholar] [CrossRef]
- Wen, Z.; Zhong, Z.; Darnell, J.E. Maximal Activation of Transcription by Statl and Stat3 Requires Both Tyrosine and Serine Phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kunimoto, H.; Katayama, B.; Zhao, H.; Shiromizu, T.; Wang, L.; Ozawa, T.; Tomonaga, T.; Tsuruta, D.; Nakajima, K. Phospho-Ser727 Triggers a Multistep Inactivation of STAT3 by Rapid Dissociation of pY705–SH2 through C-Terminal Tail Modulation. Int. Immunol. 2019, 32, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, R.; Kunimoto, H.; Tanino, K.; Kojima, H.; Inoue, A.; Shintaku, H.; Nakajima, K. Phospho-Ser727 of STAT3 Regulates STAT3 Activity by Enhancing Dephosphorylation of Phospho-Tyr705 Largely through TC45. Genes Cells 2012, 17, 132–145. [Google Scholar] [CrossRef]
- Paulson, M.; Pisharody, S.; Pan, L.; Levy, D.E.; Guadagno, S.; Mui, A.L. Stat Protein Transactivation Domains Recruit P300/CBP through Widely Divergent Sequences. J. Biol. Chem. 1999, 274, 25343–25349. [Google Scholar] [CrossRef]
- Ohbayashi, N.; Ikeda, O.; Taira, N.; Yamamoto, Y.; Muromoto, R.; Sekine, Y.; Sugiyama, K.; Honjoh, T.; Matsuda, T. LIF- and IL-6-Induced Acetylation of STAT3 at Lys-685 through PI3K/Akt Activation. Biol. Pharm. Bull. 2007, 30, 1860–1864. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Huang, J.; Faouzi, M.; Schmitz, C.; Penner, R.; Fleig, A. The TRPM6 Kinase Domain Determines the Mg·ATP Sensitivity of TRPM7/M6 Heteromeric Ion Channels. J. Biol. Chem. 2014, 289, 5217–5227. [Google Scholar] [CrossRef]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 Is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with Secondary Hypocalcemia Is Caused by Mutations in TRPM6, a New Member of the TRPM Gene Family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Launay, P.; Beck, A.; Sponder, G.; Serafini, N.; Brenkus, M.; Froschauer, E.M.; Martens, H.; Fleig, A.; Schweigel, M. SLC41A1 Is a Novel Mammalian Mg2+ Carrier. J. Biol. Chem. 2008, 283, 16235–16247. [Google Scholar] [CrossRef] [PubMed]
- Arjona, F.J.; Latta, F.; Mohammed, S.G.; Thomassen, M.; van Wijk, E.; Bindels, R.J.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. SLC41A1 Is Essential for Magnesium Homeostasis in Vivo. Pflugers Arch.—Eur. J. Physiol. 2019, 471, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M.; Kawaguchi, T.; Tsunoda, T.; Watanabe, M.; Takeda, A.; et al. Genome-Wide Association Study Identifies Common Variants at Four Loci as Genetic Risk Factors for Parkinson’s Disease. Nat. Genet. 2009, 41, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.R.; Kolisek, M.; Sponder, G. Solute Carrier 41A3 Encodes for a Mitochondrial Mg2+ Efflux System. Sci. Rep. 2016, 6, 27999. [Google Scholar] [CrossRef]
- Nie, M.; Bal, M.S.; Liu, J.; Yang, Z.; Rivera, C.; Wu, X.-R.; Hoenderop, J.G.J.; Bindels, R.J.M.; Marciano, D.K.; Wolf, M.T.F. Uromodulin Regulates Renal Magnesium Homeostasis through the Ion Channel Transient Receptor Potential Melastatin 6 (TRPM6). J. Biol. Chem. 2018, 293, 16488–16502. [Google Scholar] [CrossRef]
- Matsuda-Lennikov, M.; Biancalana, M.; Zou, J.; Ravell, J.C.; Zheng, L.; Kanellopoulou, C.; Jiang, P.; Notarangelo, G.; Jing, H.; Masutani, E.; et al. Magnesium Transporter 1 (MAGT1) Deficiency Causes Selective Defects in N-Linked Glycosylation and Expression of Immune-Response Genes. J. Biol. Chem. 2019, 294, 13638–13656. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Fang, M.; Chen, J.; Liu, L.; Lu, Z.; Hou, J.; Luo, M. Epigenetic Activation of the TUSC3 Gene as a Potential Therapy for XMEN Disease. J. Allergy Clin. Immunol. 2023, 151, 1622–1633.e10. [Google Scholar] [CrossRef]
- Kolisek, M.; Sponder, G.; Pilchova, I.; Cibulka, M.; Tatarkova, Z.; Werner, T.; Racay, P. Magnesium Extravaganza: A Critical Compendium of Current Research into Cellular Mg2+ Transporters Other than TRPM6/7. In Reviews of Physiology, Biochemistry and Pharmacology 176; de Tombe, P., Gudermann, T., Jahn, R., Lill, R., Eds.; Reviews of Physiology, Biochemistry and Pharmacology; Springer International Publishing: Cham, Switzerland, 2018; Volume 176, pp. 65–105. ISBN 978-3-030-14026-7. [Google Scholar]
- Zhang, H.; Wu, Y.; Jiang, Y. CNNM2-Related Disorders: Phenotype and Its Severity Were Associated with the Mode of Inheritance. Front. Pediatr. 2021, 9, 699568. [Google Scholar] [CrossRef]
- Nemoto, T.; Tagashira, H.; Kita, T.; Kita, S.; Iwamoto, T. Functional Characteristics and Therapeutic Potential of SLC41 Transporters. J. Pharmacol. Sci. 2023, 151, 88–92. [Google Scholar] [CrossRef]
- Blache, D.; Devaux, S.; Joubert, O.; Loreau, N.; Schneider, M.; Durand, P.; Prost, M.; Gaume, V.; Adrian, M.; Laurant, P.; et al. Long-Term Moderate Magnesium-Deficient Diet Shows Relationships between Blood Pressure, Inflammation and Oxidant Stress Defense in Aging Rats. Free Radic. Biol. Med. 2006, 41, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, Y.; Li, A.; Yu, P.; Song, L.; Liang, J.; Cao, N.; Gao, J.; Xu, R.; Ma, Y.; et al. Curcumin Reverses Hepatic Epithelial Mesenchymal Transition Induced by Trichloroethylene by Inhibiting IL-6R/STAT3. Toxicol. Mech. Methods 2021, 31, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Link, S.; Toffol-Schmidt, U. Cytostatic and Cytocidal Effects of Mistletoe (Viscum Album L.) Quercus Extract Iscador. Arzneimittelforschung 2006, 56, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Block, K.M.; Hanke, N.T.; Maine, E.A.; Baker, A.F. IL-6 Stimulates STAT3 and Pim-1 Kinase in Pancreatic Cancer Cell Lines. Pancreas 2012, 41, 773–781. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, F.; Wang, J.; Zhao, Y.; Luo, Z.; Gao, Y.; Shi, J. Regulation of TRPM7 Function by IL-6 through the JAK2-STAT3 Signaling Pathway. PLoS ONE 2016, 11, e0152120. [Google Scholar] [CrossRef]
- Fetahu, I.S.; Hummel, D.M.; Manhardt, T.; Aggarwal, A.; Baumgartner-Parzer, S.; Kállay, E. Regulation of the Calcium-Sensing Receptor Expression by 1,25-Dihydroxyvitamin D3, Interleukin-6, and Tumor Necrosis Factor Alpha in Colon Cancer Cells. J. Steroid Biochem. Mol. Biol. 2014, 144, 228–231. [Google Scholar] [CrossRef]
- Brocke-Heidrich, K.; Kretzschmar, A.K.; Pfeifer, G.; Henze, C.; Löffler, D.; Koczan, D.; Thiesen, H.-J.; Burger, R.; Gramatzki, M.; Horn, F. Interleukin-6–Dependent Gene Expression Profiles in Multiple Myeloma INA-6 Cells Reveal a Bcl-2 Family–Independent Survival Pathway Closely Associated with Stat3 Activation. Blood 2004, 103, 242–251. [Google Scholar] [CrossRef]
- Schust, J.; Sperl, B.; Hollis, A.; Mayer, T.U.; Berg, T. Stattic: A Small-Molecule Inhibitor of STAT3 Activation and Dimerization. Chem. Biol. 2006, 13, 1235–1242. [Google Scholar] [CrossRef]
- de Oliveira, A.R.S.; Cruz, K.J.C.; Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Araújo, R.S.; Marreiro, D.D.N. Hypomagnesemia and Its Relation with Chronic Low-Grade Inflammation in Obesity. Rev. Assoc. Med. Bras. 2017, 63, 156–163. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium Deficiency and Increased Inflammation: Current Perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Arancibia-Hernández, Y.L.; Hernández-Cruzk, E.Y.; Pedraza-Chaverri, J. Magnesium (Mg2+) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cell. Physiol. Biochem. 2022, 57, 1–23. [Google Scholar] [CrossRef]
- Rios, F.J.; Zou, Z.-G.; Harvey, A.P.; Harvey, K.Y.; Nosalski, R.; Anyfanti, P.; Camargo, L.L.; Lacchini, S.; Ryazanov, A.G.; Ryazanova, L.; et al. Chanzyme TRPM7 Protects against Cardiovascular Inflammation and Fibrosis. Cardiovasc. Res. 2020, 116, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tsai, P.S.; Hung, Y.C.; Huang, C.J. L-Type Calcium Channels Are Involved in Mediating the Anti-Inflammatory Effects of Magnesium Sulphate. Br. J. Anaesth. 2010, 104, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, B.; Shu, H.-F.; Zhang, X.-Y.; Wang, X.; Kuang, F.; Liu, L.; Peng, Z.-W.; Wu, R.; Zhou, Z.; et al. Interleukin-6 Increases Intracellular Ca2+ Concentration and Induces Catecholamine Secretion in Rat Carotid Body Glomus Cells. J. Neurosci. Res. 2009, 87, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.K.; Kam, T.-I.; Guttha, S.; Park, H.; Baumann, B.; Mehrabani-Tabari, A.A.; Schultz, H.; Anderson, B.; Alnemri, A.; Chou, S.-C.; et al. Interleukin-6 Triggers Toxic Neuronal Iron Sequestration in Response to Pathological α-Synuclein. Cell Rep. 2022, 38, 110358. [Google Scholar] [CrossRef]
- Zuo, S.; Li, L.; Jiang, L.; Jiang, C.; Li, X.; Li, S.; Wen, S.; Bai, R.; Du, X.; Dong, J.; et al. Pravastatin Alleviates Intracellular Calcium Dysregulation Induced by Interleukin-6 via the Mitochondrial ROS Pathway in Adult Ventricular Myocytes. J. Pharmacol. Sci. 2020, 143, 141–147. [Google Scholar] [CrossRef]
- Wu, C.-K.; Lee, J.-K.; Chiang, F.-T.; Yang, C.-H.; Huang, S.-W.; Hwang, J.-J.; Lin, J.-L.; Tseng, C.-D.; Chen, J.-J.; Tsai, C.-T. Plasma Levels of Tumor Necrosis Factor-α and Interleukin-6 Are Associated with Diastolic Heart Failure through Downregulation of Sarcoplasmic Reticulum Ca2+ ATPase. Crit. Care Med. 2011, 39, 984–992. [Google Scholar] [CrossRef]
- Ma, S.-H.; Li, B.; Huang, H.-W.; Peng, Y.-P.; Qiu, Y.-H. Interleukin-6 Inhibits L-Type Calcium Channel Activity of Cultured Cerebellar Granule Neurons. J. Physiol. Sci. 2012, 62, 385–392. [Google Scholar] [CrossRef]
- Gruol, D.; Nelson, T. Purkinje Neuron Physiology Is Altered by the Inflammatory Factor Interleukin-6. Cerebellum 2005, 4, 198–205. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hypoferremia of Inflammation: Innate Host Defense against Infections. Blood Cells Mol. Dis. 2024, 104, 102777. [Google Scholar] [CrossRef]
- Fromonot, J.; Gette, M.; Ben Lassoued, A.; Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Guieu, R. Hypozincemia in the Early Stage of COVID-19 Is Associated with an Increased Risk of Severe COVID-19. Clin. Nutr. 2022, 41, 3115–3119. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 Regulates the Zinc Transporter Zip14 in Liver and Contributes to the Hypozincemia of the Acute-Phase Response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Ruz, M.; Andrews-Guzmán, M.; Arredondo-Olguín, M. Modulation of Zinc Transporter Expressions by Additional Zinc in C2C12 Cells Cultured in a High Glucose Environment and in the Presence of Insulin or Interleukin-6. Biol. Trace Elem. Res. 2023, 201, 3428–3437. [Google Scholar] [CrossRef] [PubMed]
- Miyai, T.; Hojyo, S.; Ikawa, T.; Kawamura, M.; Irié, T.; Ogura, H.; Hijikata, A.; Bin, B.-H.; Yasuda, T.; Kitamura, H.; et al. Zinc Transporter SLC39A10/ZIP10 Facilitates Antiapoptotic Signaling during Early B-Cell Development. Proc. Natl. Acad. Sci. USA 2014, 111, 11780–11785. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 Signaling in Immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Abid, H.; Ryan, Z.C.; Delmotte, P.; Sieck, G.C.; Lanza, I.R. Extramyocellular Interleukin-6 Influences Skeletal Muscle Mitochondrial Physiology through Canonical JAK/STAT Signaling Pathways. FASEB J. 2020, 34, 14458–14472. [Google Scholar] [CrossRef]
- Fujita, K.; Shindo, Y.; Katsuta, Y.; Goto, M.; Hotta, K.; Oka, K. Intracellular Mg2+ Protects Mitochondria from Oxidative Stress in Human Keratinocytes. Commun. Biol. 2023, 6, 868. [Google Scholar] [CrossRef]
- Kolisek, M.; Zsurka, G.; Samaj, J.; Weghuber, J.; Schweyen, R.J.; Schweigel, M. Mrs2p Is an Essential Component of the Major Electrophoretic Mg2+ Influx System in Mitochondria. EMBO J. 2003, 22, 1235–1244. [Google Scholar] [CrossRef]
- Schindl, R.; Weghuber, J.; Romanin, C.; Schweyen, R.J. Mrs2p Forms a High Conductance Mg2+ Selective Channel in Mitochondria. Biophys. J. 2007, 93, 3872–3883. [Google Scholar] [CrossRef]
- Rosa, T.L.S.A.; Leal-Calvo, T.; Tavares, I.F.; Mendes, M.A.; Dias, A.A.; Piauy, M.H.D.S.; Barboza, M.F.D.S.; Kapuscinski, M.; Costa, F.D.M.R.; Marques, M.A.M.; et al. Whole Blood Transcriptomics Reveals the Enrichment of Neutrophil Activation Pathways during Erythema Nodosum Leprosum Reaction. Front. Immunol. 2024, 15, 1366125. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; Van Den Hoff, M.J.B.; Moorman, A.F.M. Amplification Efficiency: Linking Baseline and Bias in the Analysis of Quantitative PCR Data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-Free Analysis of Quantitative Real-Time Polymerase Chain Reaction (PCR) Data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 7 October 2024).
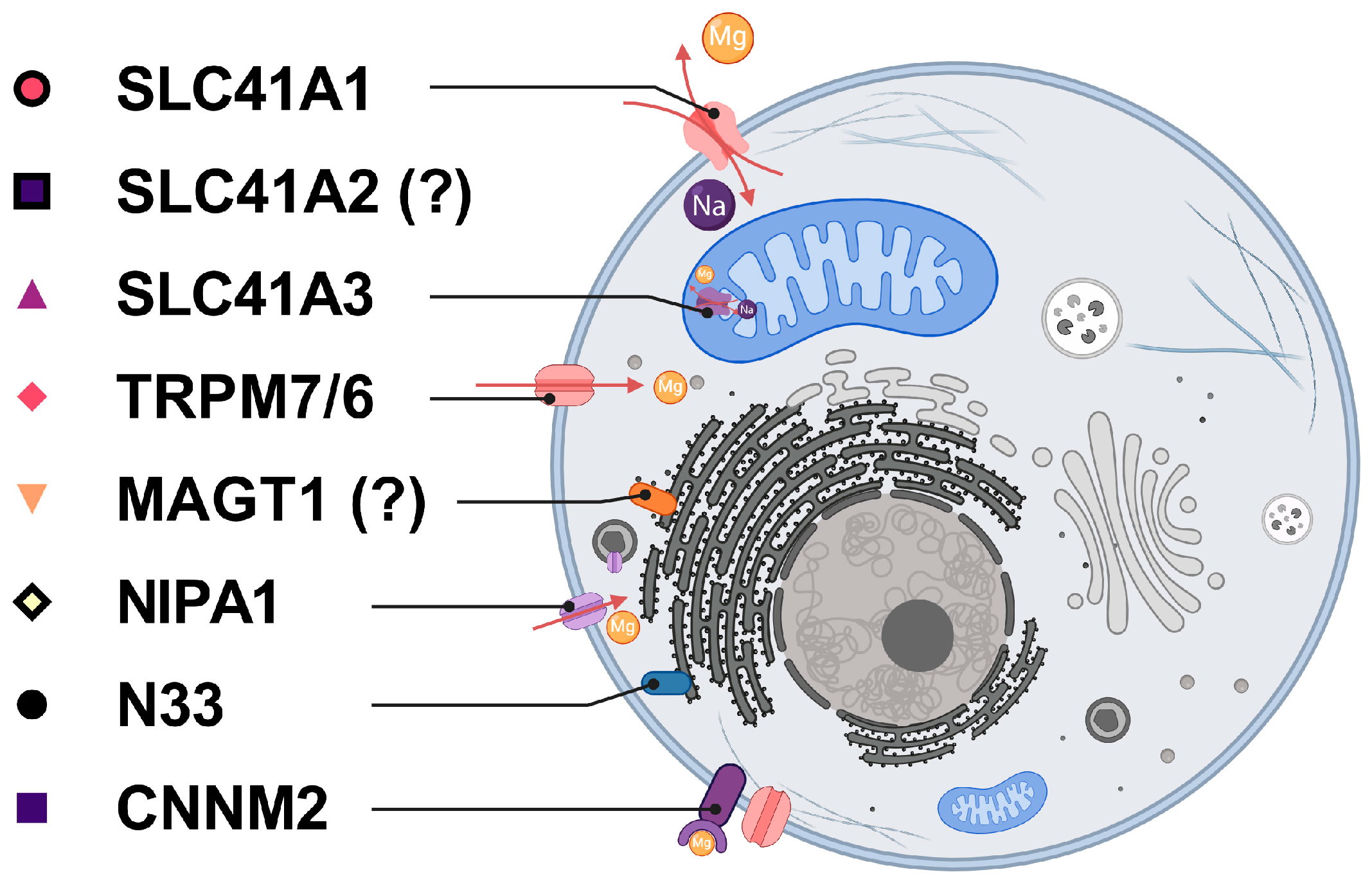



| Protein Name | Protein Role | References |
|---|---|---|
| SLC41A1 | Na+/Mg2+ exchanger, regulated extrusion through plasma membrane. | [32,33] |
| SLC41A2 | Mg2+ transporter, presumed localization in the plasma or organelle membrane. | [41] |
| SLC41A3 | Na+/Mg2+ exchanger, release of Mg2+ from mitochondria. | [35] |
| TRPM6 | Mg2+ uptake through the plasma membrane, epithelial cells of the kidney, and colon. | [30,36] |
| TRPM7 | Mg2+ uptake through the plasma membrane, ubiquitously expressed. | [28] |
| MAGT1 & N33 | Mg-homeostatic factors, integral parts of the protein N-glycosylation complex. | [37,38] |
| NIPA1 | The Mg2+ transporter, with its highest abundance in brain tissue, may be important for the maintenance of the nervous system. | [39] |
| CNNM2 | Mg-homeostatic factor, the pathogenic form of CNNM2 is associated with hypomagnesemia and epilepsy. | [40] |
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| SLC41A1 | GATTCTCCTGTACATCGCAGAC | CCCCTATGAGCCAGAGAACA |
| SLC41A2 | TGGTTATAAGTAGCATTGGGGGCCT | TCCTGCTAGCCTGAATGGCCA |
| SLC41A3 | CACAAAGATAGTCGGTATCTGACG | GACCATGGCCAGGATGATT |
| TRPM6 | GGATCTCTCTGCCCTGACTG | TTCTCTCCAGCGATCTCCAT |
| TRPM7 | TGGGAAGGCTGAATATGAGG | TCGCTGTCATCCATTGTCAT |
| MAGT1 | GGGATTGCTTTTGGCTGTTA | TATGGGCATATGGTGGTCCT |
| NIPA1 | AACAACCCGTCCAGTCAGAG | GTAGTAGATGGCCCCGAACA |
| N33 | ATGGAATGGAGTTCCAGACG | TCATTAGCTTGCCTGCACAC |
| CNNM2 | TGCAGGTGATCTTCATTTCG | GCAGTGAGCACAGCAGGTAG |
| ACTB | AACGGCTCCGGCATGTGCAAG | CACATAGGAATCCTTCTGACC |
| GAPDH | CTTTGGTATCGTGGAAGGAC | TAGAGGCAGGGATGATGTTC |
| YWHAZ | CTGTAACTGAGCAAGGAGCTG | ATACTTGAGACGACCCTCCA |
| Step | Temperature | Time | |
|---|---|---|---|
| Polymerase activation | 95 °C | 2 min | |
| 40× | Denaturation | 95 °C | 5 s |
| Annealing & polymerization | 58 °C | 30 s | |
| Melt curve | 58–95 °C | 1.99 °C/s; 15 s | |
| 95–60 °C | 1.77 °C/s; 1 min | ||
| 60–95 °C | 0.075 °C/s; 15 s | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodnanova, M.; Cibulka, M.; Grendar, M.; Gondas, E.; Kolisek, M. IL-6 Does Not Influence the Expression of SLC41A1 and Other Mg-Homeostatic Factors. Int. J. Mol. Sci. 2024, 25, 13274. https://doi.org/10.3390/ijms252413274
Brodnanova M, Cibulka M, Grendar M, Gondas E, Kolisek M. IL-6 Does Not Influence the Expression of SLC41A1 and Other Mg-Homeostatic Factors. International Journal of Molecular Sciences. 2024; 25(24):13274. https://doi.org/10.3390/ijms252413274
Chicago/Turabian StyleBrodnanova, Maria, Michal Cibulka, Marian Grendar, Eduard Gondas, and Martin Kolisek. 2024. "IL-6 Does Not Influence the Expression of SLC41A1 and Other Mg-Homeostatic Factors" International Journal of Molecular Sciences 25, no. 24: 13274. https://doi.org/10.3390/ijms252413274
APA StyleBrodnanova, M., Cibulka, M., Grendar, M., Gondas, E., & Kolisek, M. (2024). IL-6 Does Not Influence the Expression of SLC41A1 and Other Mg-Homeostatic Factors. International Journal of Molecular Sciences, 25(24), 13274. https://doi.org/10.3390/ijms252413274







