Fluorine-Terminated Liquid Polybutadiene: A Novel Approach to Enhancing Oil Resistance and Thermal Stability in Natural Rubber
Abstract
:1. Introduction
2. Results and Discussion
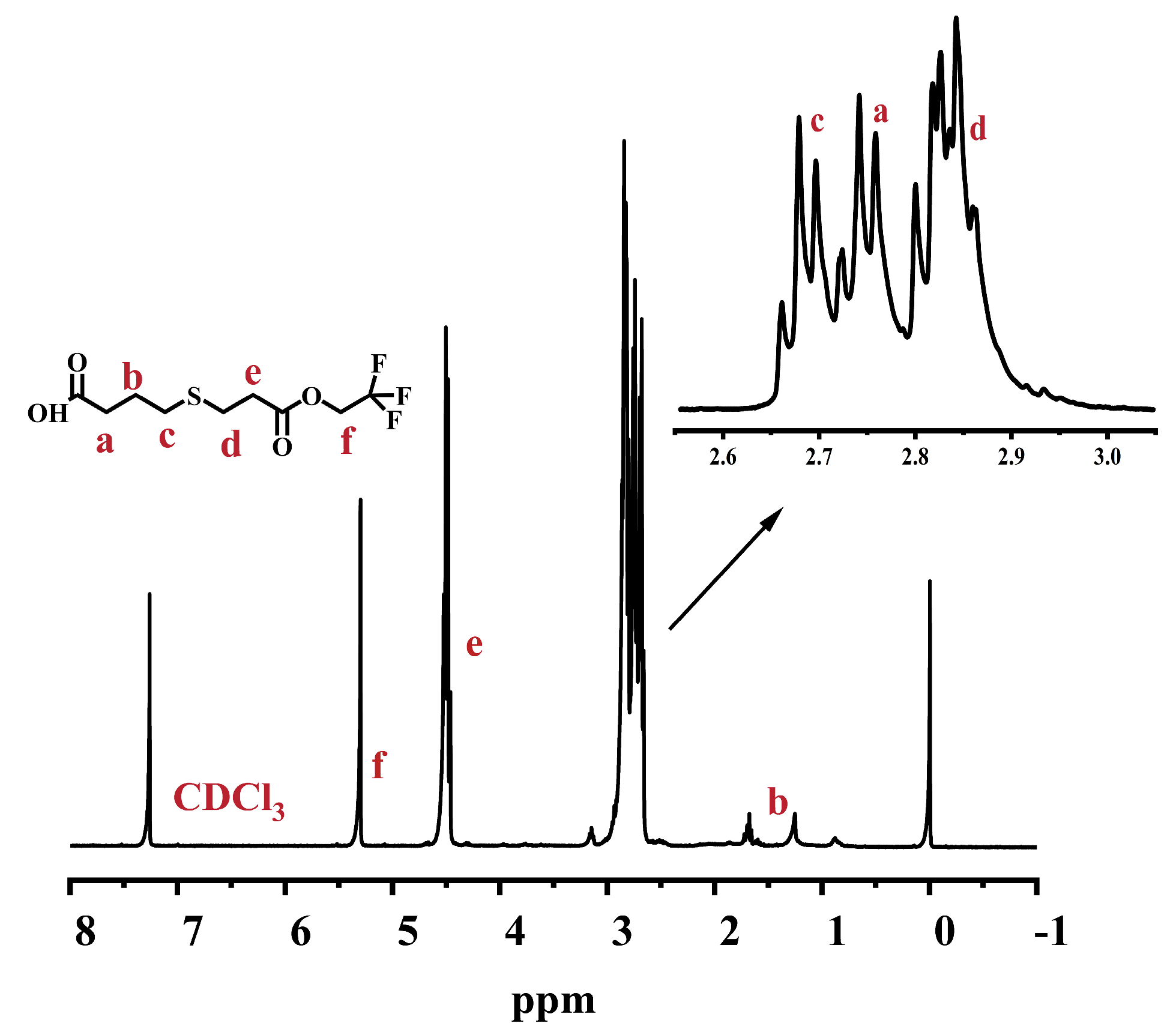
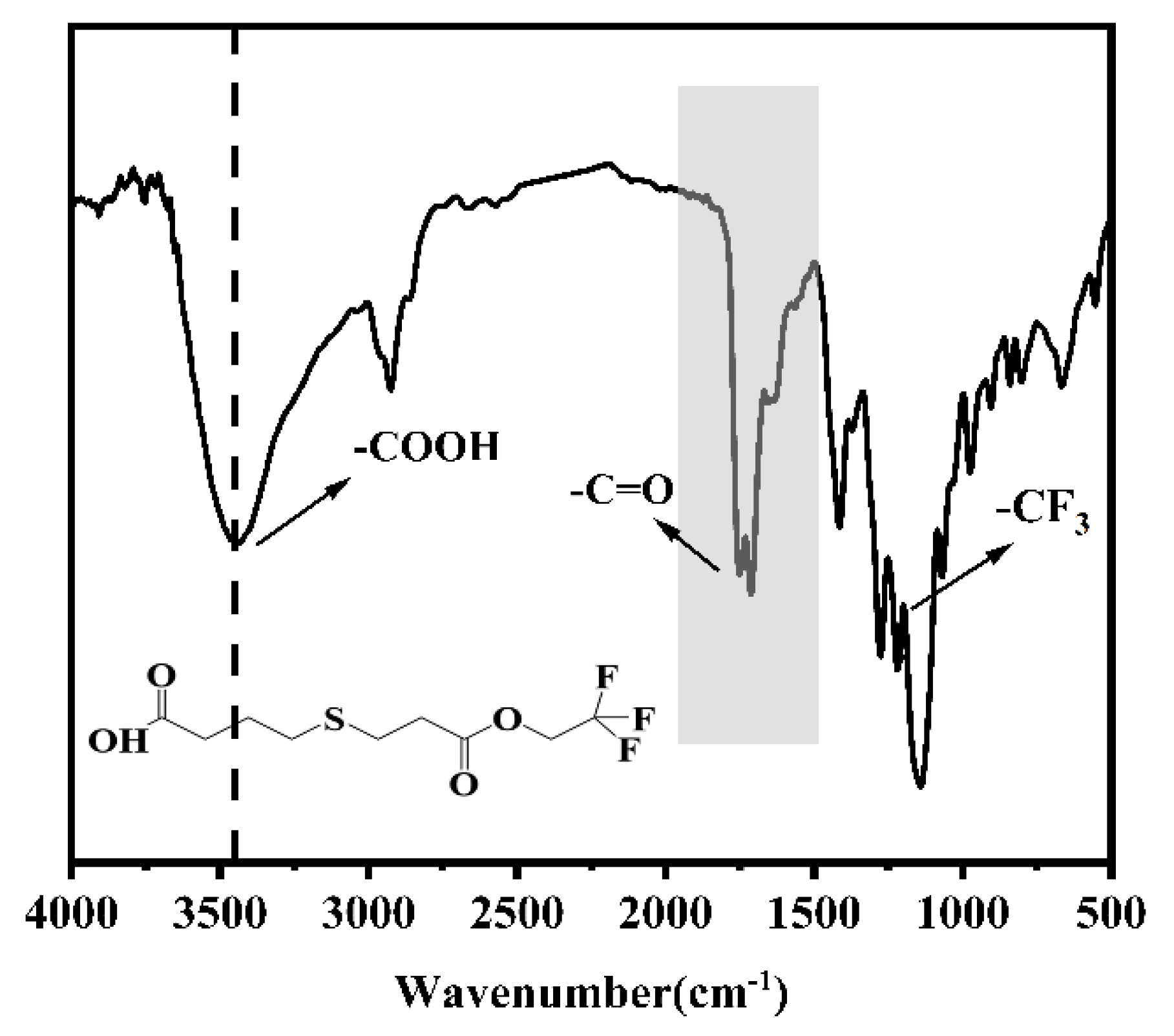
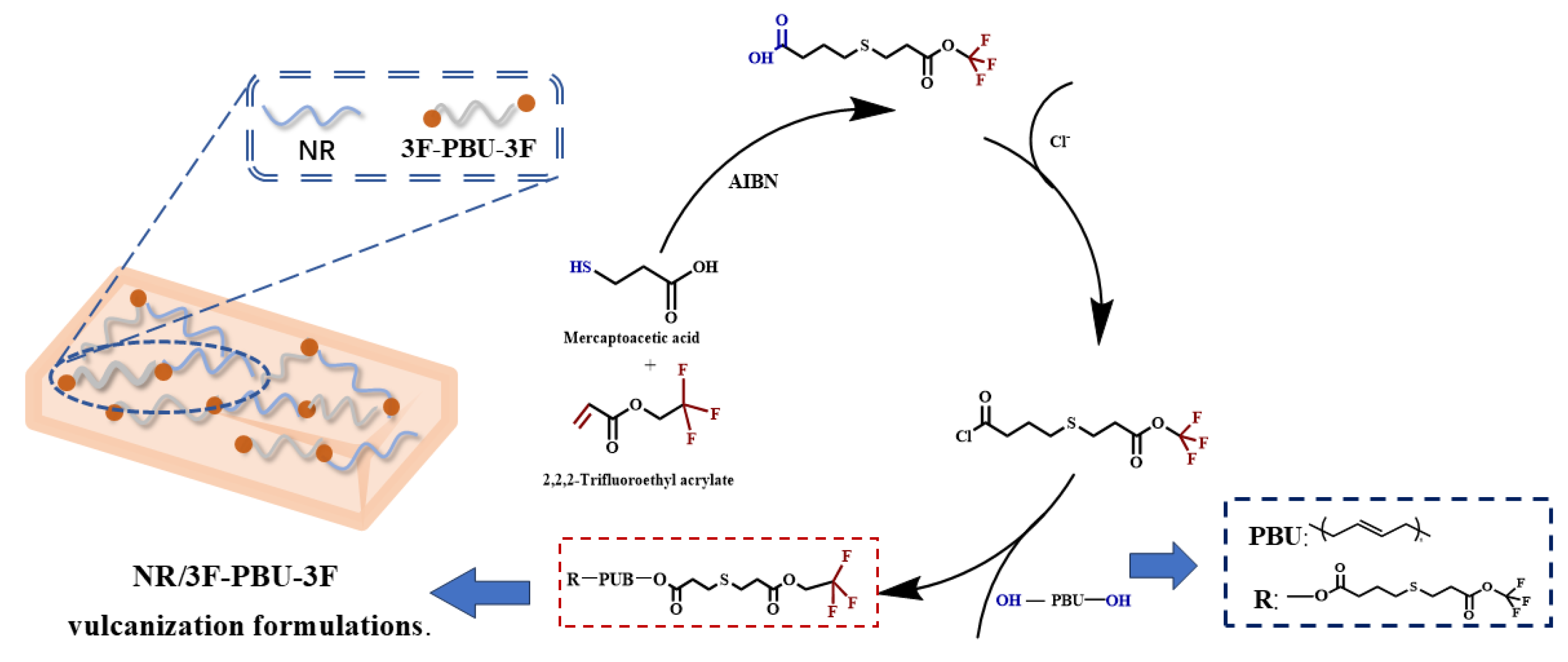
2.1. Synthesis of 4-((3-Oxo-3-(2,2,2-trifluoroethoxy)propyl)thio)butanoic Acid
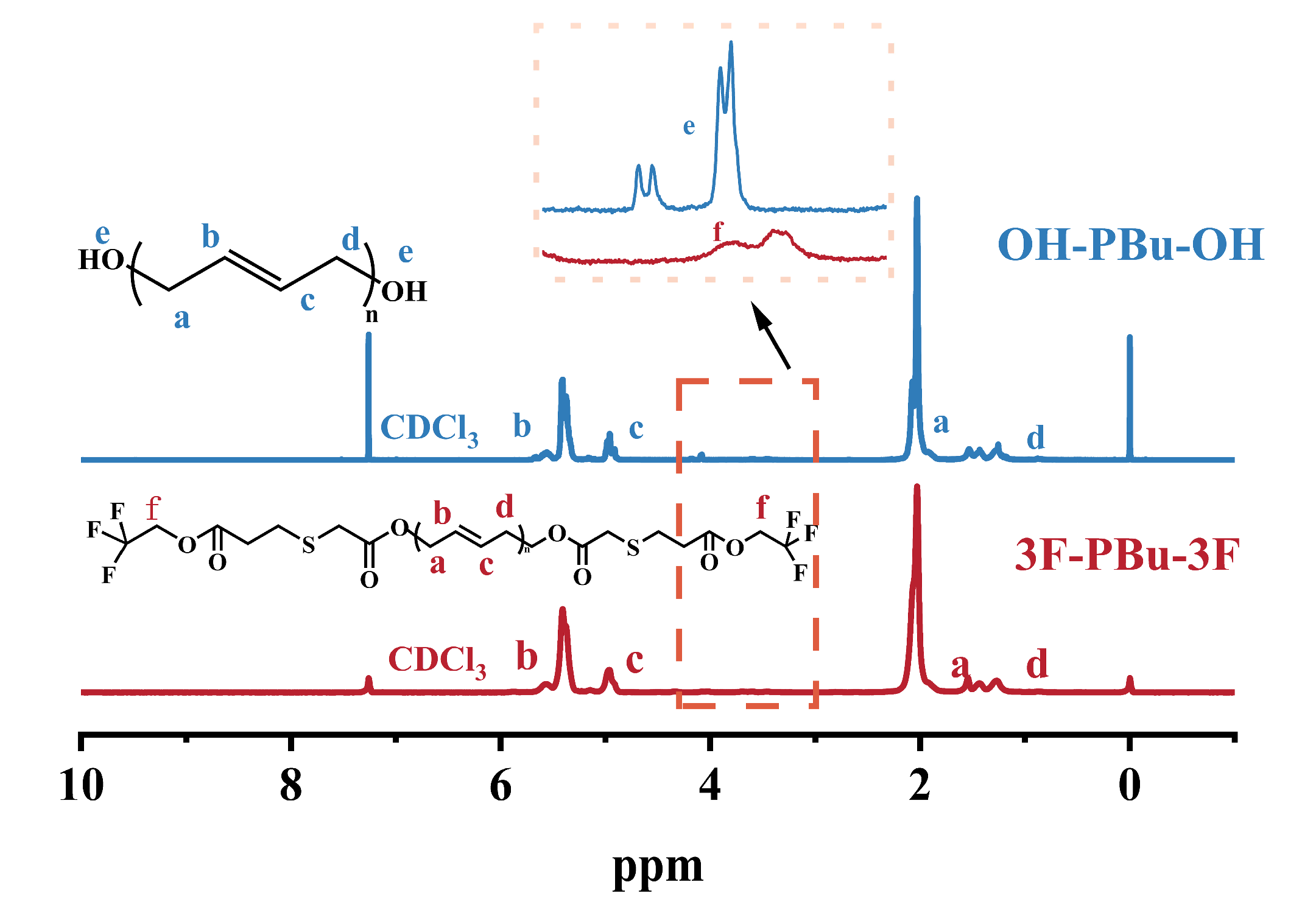
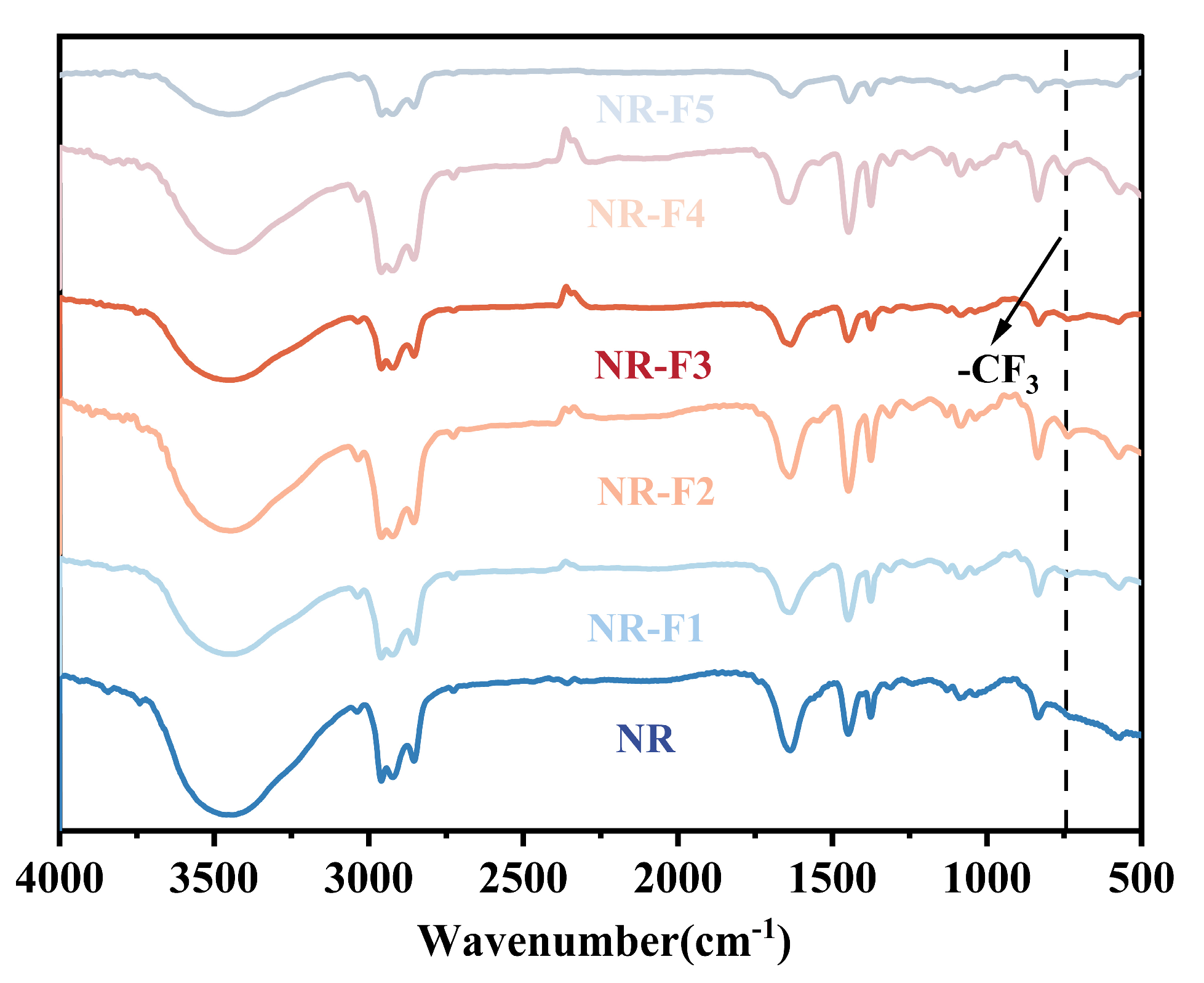
2.2. Structure Analysis of Fluoro-Terminated Polybutadiene (3F-PBu-3F)
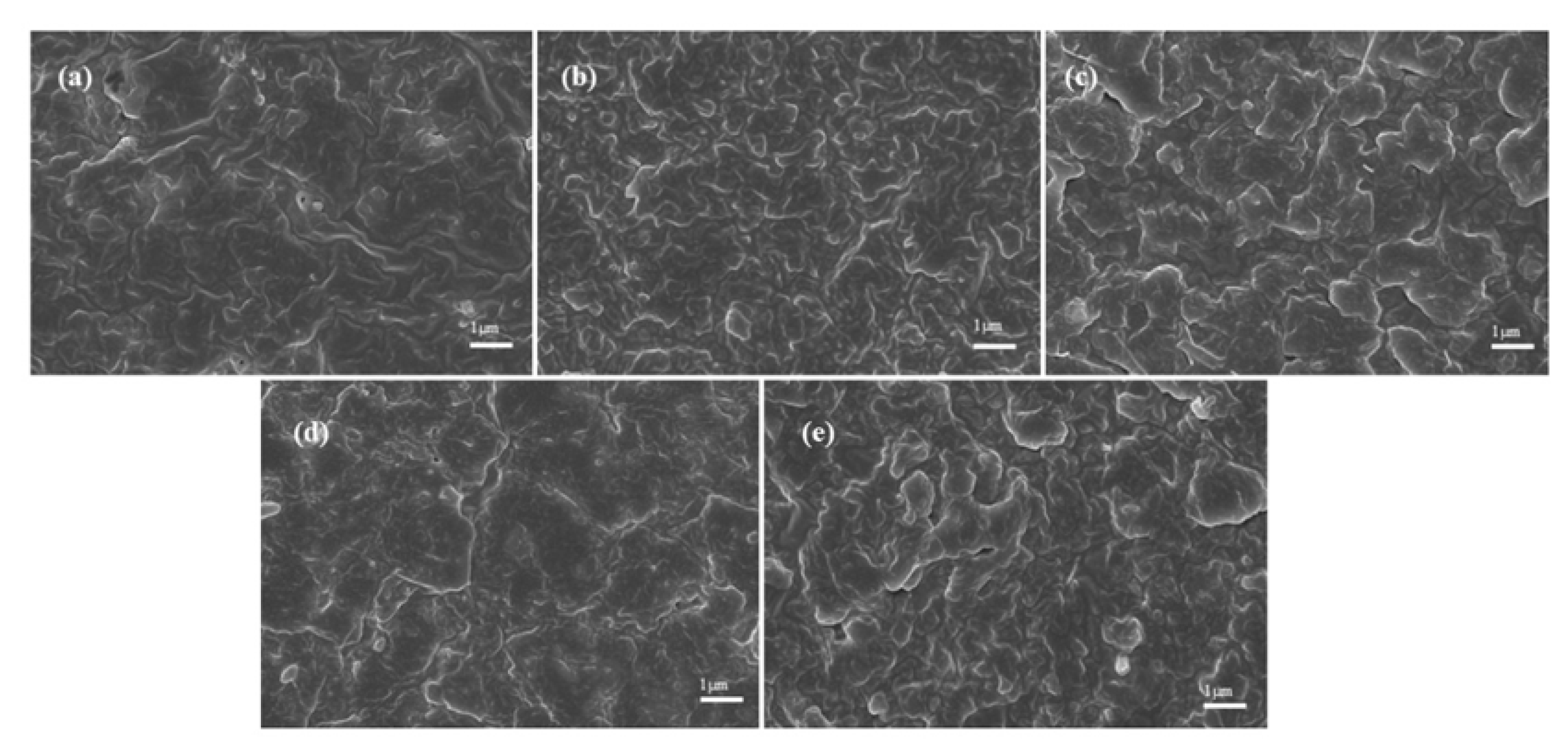
2.3. Morphologies of NR/3F-PBu-3F Vulcanizates
2.4. Crosslink Density and Oil Resistance of NR/3F-PBu-3F Vulcanizates
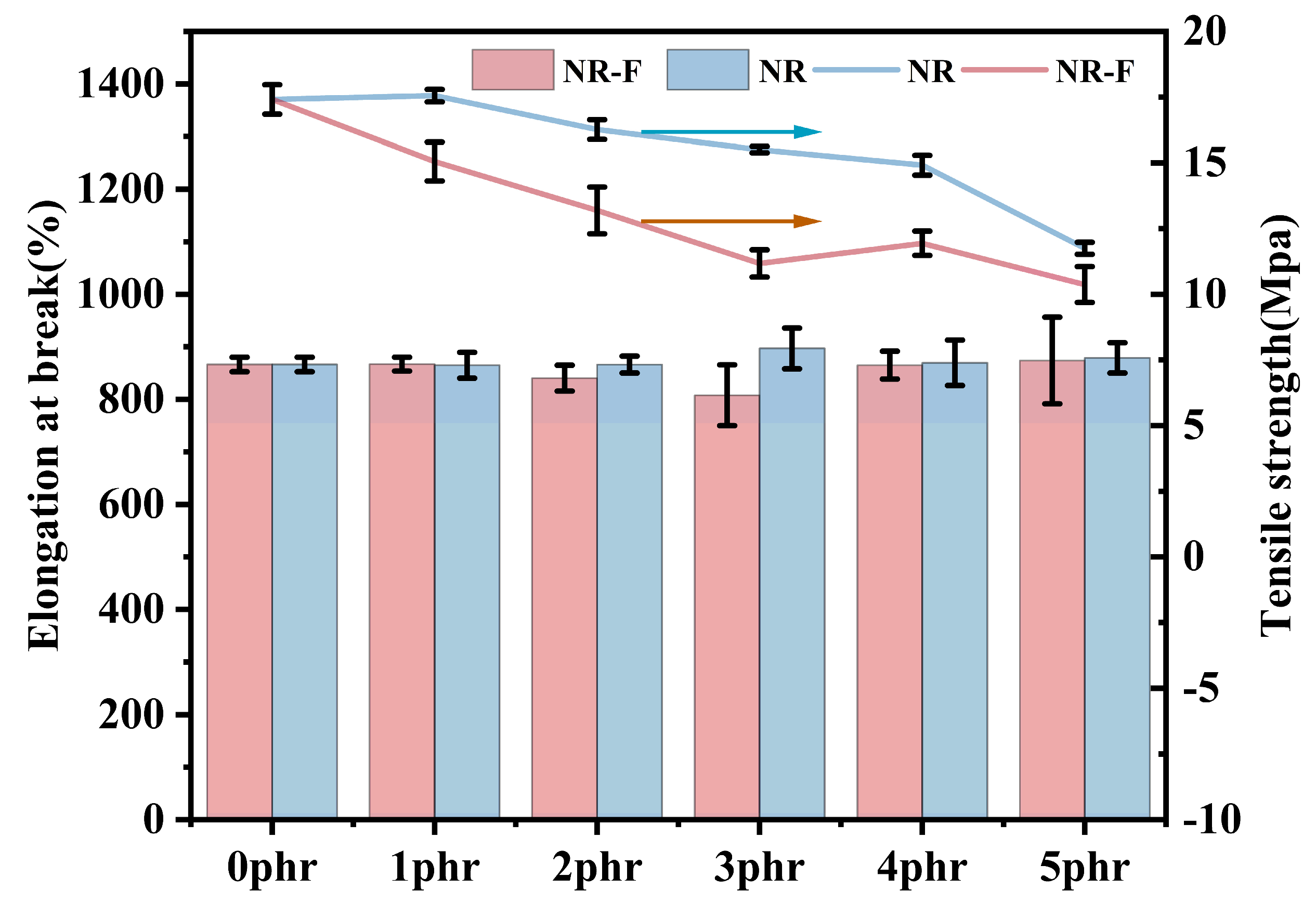
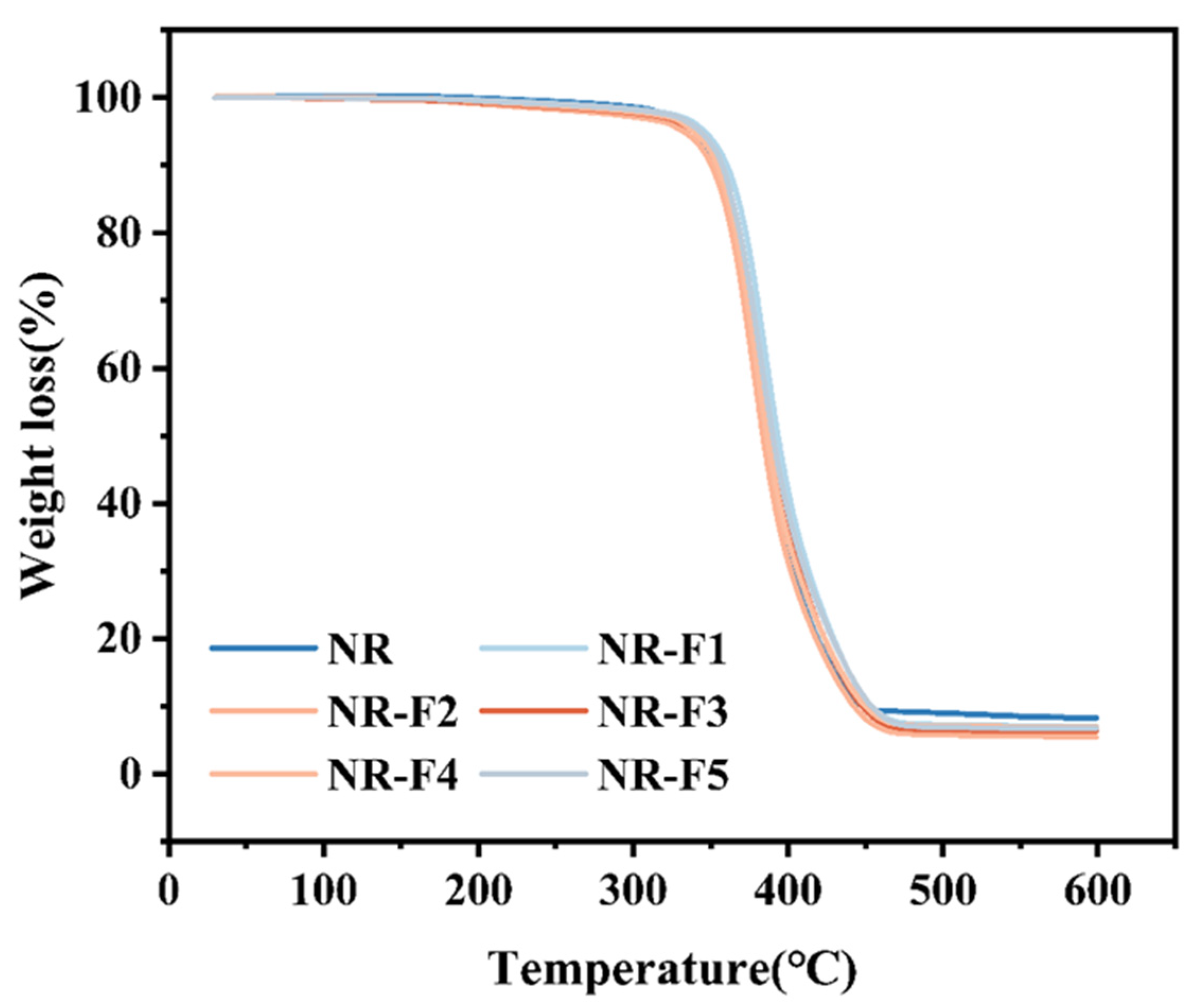
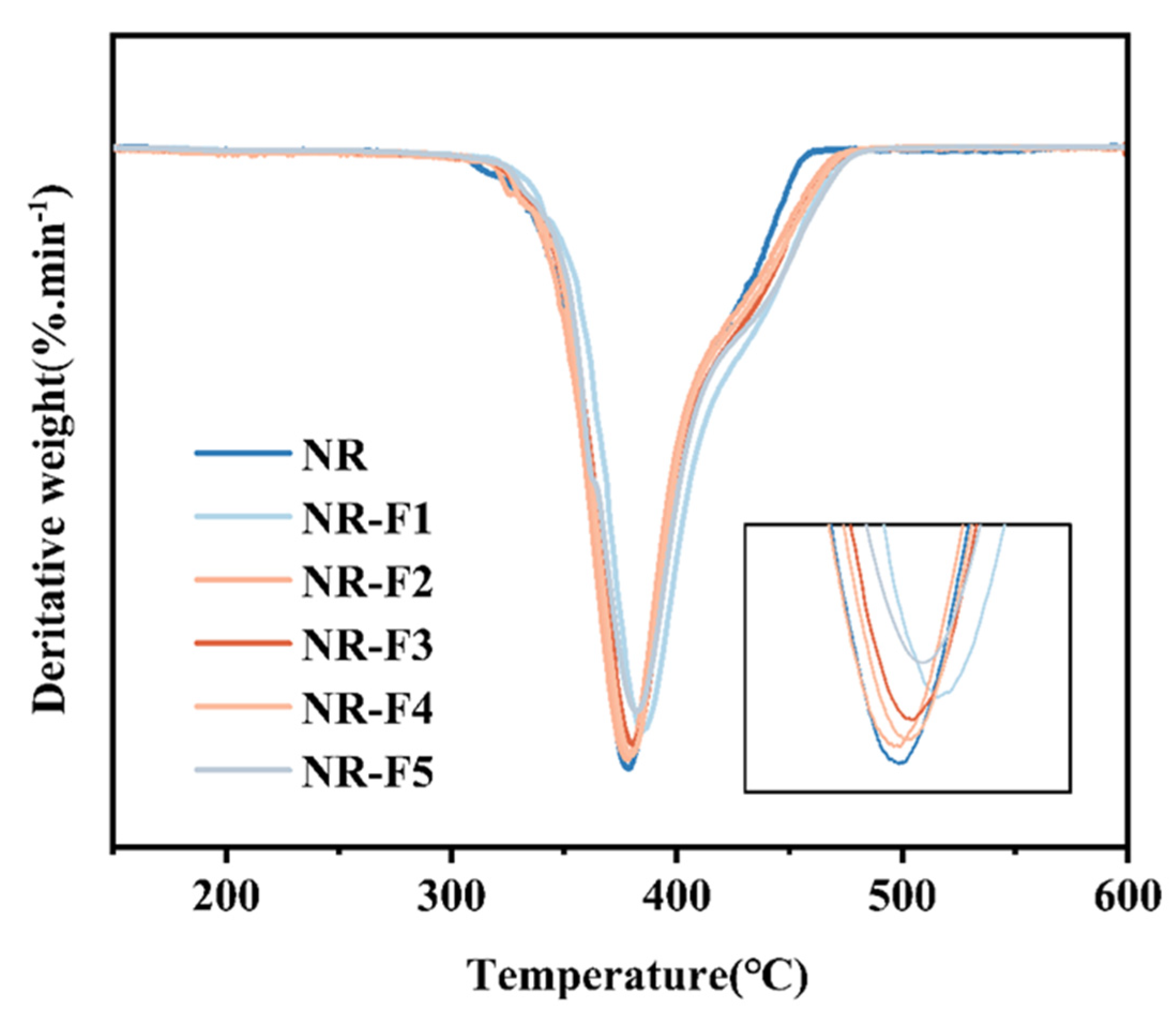
2.5. Mechanical and Thermal Stability Properties of NR/3F-PBu-3F Vulcanizates
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Fluorine-Terminated Polybutadiene (3F-PBu-3F)
3.2.2. Preparation of NR/3F-PBu-3F Vulcanizates
3.3. Characterization and Measurement
3.3.1. Nuclear Magnetic Resonance Spectroscopy (1H NMR)
3.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Oil Resistance and Crosslink Density
3.3.5. Mechanical Properties
3.3.6. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, P.; Bhowmick, A.K. Sustainable Rubbers and Rubber Additives. J. Appl. Polym. Sci. 2018, 135, 45701. [Google Scholar] [CrossRef]
- Whba, R.; Su’ait, M.S.; Whba, F.; Sahinbay, S.; Altin, S.; Ahmad, A. Intrinsic Challenges and Strategic Approaches for Enhancing the Potential of Natural Rubber and Its Derivatives: A Review. Rubber Chem. Technol. 2024, 97, 133796. [Google Scholar] [CrossRef]
- Ando, S.; Ito, K. Recent Progress and Future Perspective in Slide-Ring Based Polymeric Materials. Macromolecules 2025, 58, 2157–2177. [Google Scholar] [CrossRef]
- Marrero Nunes, F.; Emmel Silva, A.L.; May, J.; da Silva Szarblewski, M.; Flemming, L.; Assmann, E.E.; Ribas Moraes, J.A.; Machado, Ê.L. Environmental impacts associated with the life cycle of natural rubbers: A review and scientometric analysis. Ind. Crops Prod. 2025, 224, 120350. [Google Scholar] [CrossRef]
- Klei, B.; Koenig, J.L. NMR imaging of the competitive vulcanization of natural rubber and polybutadiene blends. Acta Polym. 2003, 48, 199–207. [Google Scholar] [CrossRef]
- Vieyres, A.; Pérez-Aparicio, R.; Albouy, P.-A.; Sanseau, O.; Saalwächter, K.; Long, D.R.; Sotta, P. Sulfur-Cured Natural Rubber Elastomer Networks: Correlating Cross-Link Density, Chain Orientation, and Mechanical Response by Combined Techniques. Macromolecules 2013, 46, 889–899. [Google Scholar] [CrossRef]
- Siriyong, T.; Keawwattana, W.J.A.; Resources, N. Utilization of different curing systems and natural zeolite as filler and absorbent for natural rubber/nitrile rubber blend. J. Reinf. Plast. Compos. 2012, 46, 918–930. [Google Scholar]
- Venkatanarasimhan, S.; Raghavachari, D. Epoxidized natural rubber–magnetite nanocomposites for oil spill recovery. J. Mater. Chem. A 2013, 1, 868–876. [Google Scholar] [CrossRef]
- Riyajan, S.A.; Keawittarit, P. A novel natural rubber-graft-cassava starch foam for oil/gasohol absorption. Polym. Int. 2016, 65, 491–502. [Google Scholar] [CrossRef]
- Ramesan, M.T. Thermogravimetric analysis, flammability and oil resistance properties in natural rubber and dichlorocarbene modified styrene butadiene rubber blends. React. Funct. Polym. 2004, 59, 267–274. [Google Scholar] [CrossRef]
- Sirisinha, C.; Saeoui, P.; Guaysomboon, J. Oil and thermal aging resistance in compatibilized and thermally stabilized chlorinated polyethylene/natural rubber blends. Polymer 2004, 45, 4909–4916. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A comprehensive review on the recent advancements in natural rubber nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef]
- Hinchiranan, N.; Lertweerasirikun, W.; Poonsawad, W.; Rempel, G.L.; Prasassarakich, P. Hydrogenated Natural Rubber Blends: Aspect on Thermal Stability and Oxidative Behavior. J. Appl. Polym. Sci. 2009, 113, 1566–1575. [Google Scholar] [CrossRef]
- Simma, K.; Rempel, G.L.; Prasassarakich, P. Improving thermal and ozone stability of skim natural rubber by diimide reduction. Polym. Degrad. Stab. 2009, 94, 1914–1923. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Synthesis and chemical structure of natural rubber adduct with SO2 and study of the thermal stability. Polym. Degrad. Stab. 2009, 94, 921–928. [Google Scholar] [CrossRef]
- Piya-areetham, P.; Rempel, G.L.; Prasassarakich, P. Hydrogenated nanosized polyisoprene as a thermal and ozone stabilizer for natural rubber blends. Polym. Degrad. Stab. 2014, 102, 112–121. [Google Scholar] [CrossRef]
- Zhong, B.; Lin, J.; Liu, M.; Jia, Z.; Luo, Y.; Jia, D.; Liu, F. Preparation of halloysite nanotubes loaded antioxidant and its antioxidative behaviour in natural rubber. Polym. Degrad. Stab. 2017, 141, 19–25. [Google Scholar] [CrossRef]
- Song, P.; Wu, X.; Wang, S. Effect of styrene butadiene rubber on the light pyrolysis of the natural rubber. Polym. Degrad. Stab. 2018, 147, 168–176. [Google Scholar] [CrossRef]
- Dohi, H.; Horiuchi, S. Heterogeneity of a vulcanized rubber by the formation of ZnS clusters. Polymer 2007, 48, 2526–2530. [Google Scholar] [CrossRef]
- Hou, F.; Song, Y.; Zheng, Q. Payne effect of thermo-oxidatively aged isoprene rubber vulcanizates. Polymer 2020, 195, 122432. [Google Scholar] [CrossRef]
- Amédur, B. The Promising Future of Fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar] [CrossRef]
- Indulekha, K.; Mathew, A.; Rajeev, R.S.; Ninan, K.N.; Gouri, C. A Facile Route to Fluoroalkylsiloxane Polymers Having Resistance to Corrosive Acidic Conditions: Synthesis and Characterization. Eur. Polym. J. 2017, 97, 120–132. [Google Scholar] [CrossRef]
- Gopala Krishnan, P.S.; Ayyaswamy, K.; Nayak, S.K. Hydroxy Terminated Polybutadiene: Chemical Modifications and Applications. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 128–138. [Google Scholar] [CrossRef]
- Jana, T.; Jana, S.; Dhara, M.; Ganivada, M.N. Synthetic Routes to Modify Hydroxyl Terminated Polybutadiene for Various Potential Applications. J. Macromol. Sci. Part A 2022, 59, 167–179. [Google Scholar] [CrossRef]
- Chen, F.; Qian, J. Studies on the thermal degradation of polybutadiene. Fuel Process. Technol. 2000, 67, 53–60. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Liu, Y.; Zhang, A.; Yuan, C.; Zhang, W. Cross-linking process of cis-polybutadiene rubber with peroxides studied by two-dimensional infrared correlation spectroscopy: A detailed tracking. RSC Adv. 2015, 5, 10231–10242. [Google Scholar] [CrossRef]
- Shao, H.; Guo, Q.; He, A. Strategy for the NR/BR blends with improved thermo-oxidative resistance. Polym. Degrad. Stab. 2021, 191, 109665. [Google Scholar] [CrossRef]
- Halladay, J.R.; Hedlund, C. A Solution to the Age-Stiffening Problem in Natural Rubber and Polybutadiene Elastomers. In Proceedings of the 40th European Rotorcraft Forum; ERF: Southampton, UK, 2014; pp. 1–12. Available online: http://hdl.handle.net/20.500.11881/3498 (accessed on 11 March 2025).
- Bhagawan, S.S.; Tripathy, D.K. Morphology and mechanical behavior of 1,2 polybutadiene—Natural rubber blends. Mater. Chem. Phys. 1987, 17, 415–432. [Google Scholar] [CrossRef]
- Portal, J.; Carrot, C.; Majesté, J.C.; Cocard, S.; Pélissier, V.; Baran, K.; Anselme-Bertrand, I. Coupling of Various Methods for the Investigation of the Morphology of Blends of Natural Rubber and Polybutadiene. Polym. Eng. Sci. 2008, 48, 1068–1076. [Google Scholar] [CrossRef]
- Milani, G.; Milani, F. Quasi-Analytical Kinetic Model for Natural Rubber and Polybutadiene Rubber Blends. React. Kinet. Mech. Catal. 2018, 123, 351–365. [Google Scholar] [CrossRef]
- Milani, G.; Milani, F. Numerical kinetic model with regularization for NR–PB natural and poly-butadiene rubber blends: Implementation and validation against experimental data. J. Math. Chem. 2019, 57, 1019–1034. [Google Scholar] [CrossRef]
- Klonos, P.; Kulyk, K.; Borysenko, M.V.; Gun’ko, V.M.; Kyritsis, A.; Pissis, P. Effects of Molecular Weight below the Entanglement Threshold on Interfacial Nanoparticles/Polymer Dynamics. Macromolecules 2016, 49, 9457–9473. [Google Scholar] [CrossRef]
- Wang, S.Q. Chain dynamics in entangled polymers: Diffusion versus rheology and their comparison. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1589–1604. [Google Scholar] [CrossRef]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Khalaf, A.; Yehia, A.; Ismail, M.; El-Sabbagh, H. High Performance Oil Resistant Rubber. Open J. Org. Polym. Mater. 2012, 2, 13–18. [Google Scholar] [CrossRef]
- Shi, R.; Han, X.; Zhu, L.; Hao, J.; Wang, H.; Zhou, C. Understanding the influence of interfacial interaction and fluorine content on the mechanical properties and oil resistance in composites of fluorosilicone rubber/silica. Polymer 2024, 308, 127428. [Google Scholar] [CrossRef]
- Jurkowska, B.; Jurkowski, B.; Nadolny, K.; Krasnov, A.P.; Studniev, Y.N.; Pesetskii, S.S.; Koval, V.N.; Pinchuk, L.S.; Olkhov, Y.A. Influence of fluorine-containing lubricant on properties of NR/BR rubber. Eur. Polym. J. 2006, 42, 1676–1687. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Yaowsang, C.; Na-Ranong, N.; Potiyaraj, P. Oil resistance and physical properties of in situ epoxidized natural rubber from high ammonia concentrated latex. J. Appl. Polym. Sci. 2006, 100, 3948–3955. [Google Scholar] [CrossRef]
- Salaeh, S.; Thitithammawong, A.; Banerjee, S.S. A new strategy applying ternary blends of modified natural rubber with fluoroplastic and fluorocarbon elastomer for high-performance thermoplastic vulcanizate. Polym. Test. 2024, 140, 108594. [Google Scholar] [CrossRef]
- Mousa, A.; Ishiaku, U.S.; Ishak, Z.A.M. Oil-resistance studies of dynamically vulcanized poly(vinyl chloride)/epoxidized natural rubber thermoplastic elastomer. J. Appl. Polym. Sci. 1998, 69, 1357–1366. [Google Scholar] [CrossRef]
- Raksaksri, L.; Chuayjuljit, S.; Chaiwutthinan, P.; Boonmahitthisud, A. Vinyl acetate ethylene copolymer and nanosilica reinforced epoxidized natural rubber: Effects of sulfur curing systems on cure characteristics, tensile properties, thermal stability, dynamic mechanical properties, and oil resistance. J. Vinyl Addit. Technol. 2019, 25, E28–E38. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Zhang, X.; Mi, Z. Thermal stability and kinetic of decomposition of nitrated HTPB. J. Hazard. Mater. 2009, 172, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yuan, M.; Lu, X.; Xin, Z. Fluorine and silicon-containing main-chain type polybenzoxazine with low dielectric constant and high thermal stability. Polymer 2024, 300, 126995. [Google Scholar] [CrossRef]
- Xu, H.; Wang, A.; Liu, X.; Feng, D.; Wang, S.; Chen, J.; An, Y.; Zhang, L. A new fluorine-containing star-branched polymer as electrolyte for all-solid-state lithium-ion batteries. Polymer 2018, 146, 249–255. [Google Scholar] [CrossRef]



| Modification Method | Material System | Mass Change Rate (%) | Reference |
|---|---|---|---|
| Fluorinated end-group | NR/3F-PBu-3F | 40–45 | This work |
| Epoxidation | ENR | 10–30 | [39] |
| Fluorinated | PVDF/MGNR | 15–25 | [40] |
| Chlorinated | PVC/NRF | 30–40 | [41] |
| Nanocomposite | ENR40/VAE/nSiO2 | 30–80 | [42] |
| Sample | NR (phr) | 3F-PBu-3F (phr) | ZnO (phr) | SA (phr) | MBT (phr) | S (phr) |
|---|---|---|---|---|---|---|
| S0 | 100 | 0 | 6 | 0.5 | 0.5 | 3.5 |
| S1 | 100 | 1 | 6 | 0.5 | 0.5 | 3.5 |
| S2 | 100 | 2 | 6 | 0.5 | 0.5 | 3.5 |
| S3 | 100 | 3 | 6 | 0.5 | 0.5 | 3.5 |
| S4 | 100 | 4 | 6 | 0.5 | 0.5 | 3.5 |
| S5 | 100 | 5 | 6 | 0.5 | 0.5 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Li, M.; Fu, G.; Yu, R.; Liao, J. Fluorine-Terminated Liquid Polybutadiene: A Novel Approach to Enhancing Oil Resistance and Thermal Stability in Natural Rubber. Int. J. Mol. Sci. 2025, 26, 3410. https://doi.org/10.3390/ijms26073410
Luo X, Li M, Fu G, Yu R, Liao J. Fluorine-Terminated Liquid Polybutadiene: A Novel Approach to Enhancing Oil Resistance and Thermal Stability in Natural Rubber. International Journal of Molecular Sciences. 2025; 26(7):3410. https://doi.org/10.3390/ijms26073410
Chicago/Turabian StyleLuo, Xue, Mengyan Li, Guliang Fu, Rentong Yu, and Jianhe Liao. 2025. "Fluorine-Terminated Liquid Polybutadiene: A Novel Approach to Enhancing Oil Resistance and Thermal Stability in Natural Rubber" International Journal of Molecular Sciences 26, no. 7: 3410. https://doi.org/10.3390/ijms26073410
APA StyleLuo, X., Li, M., Fu, G., Yu, R., & Liao, J. (2025). Fluorine-Terminated Liquid Polybutadiene: A Novel Approach to Enhancing Oil Resistance and Thermal Stability in Natural Rubber. International Journal of Molecular Sciences, 26(7), 3410. https://doi.org/10.3390/ijms26073410








