MDMA for the Treatment of Negative Symptoms in Schizophrenia
Abstract
:1. Introduction
2. Negative Symptoms
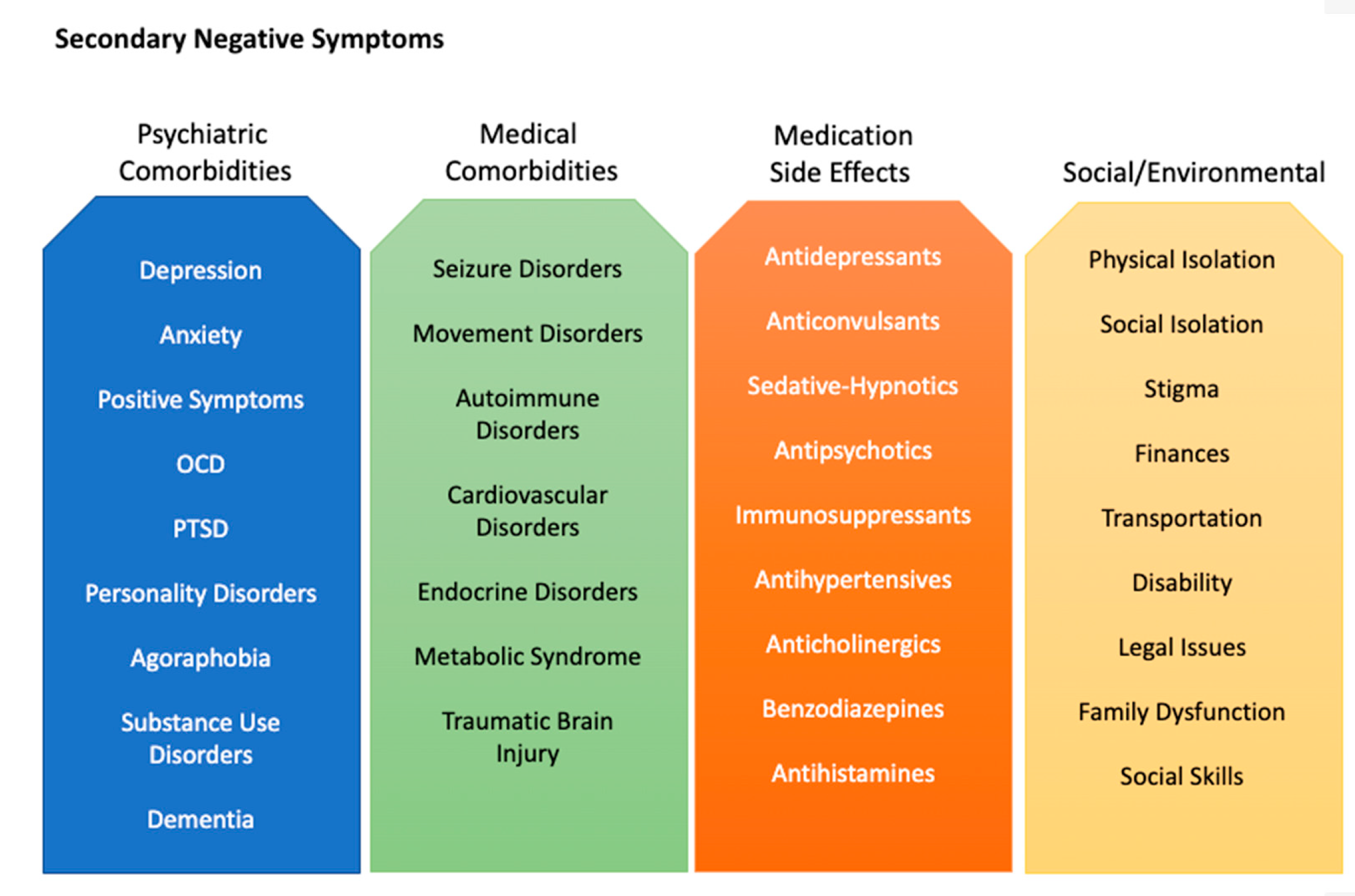
2.1. Secondary Negative Symptoms
2.2. Cognition and Negative Symptoms
2.3. Treatments for Negative Symptoms
3. MDMA
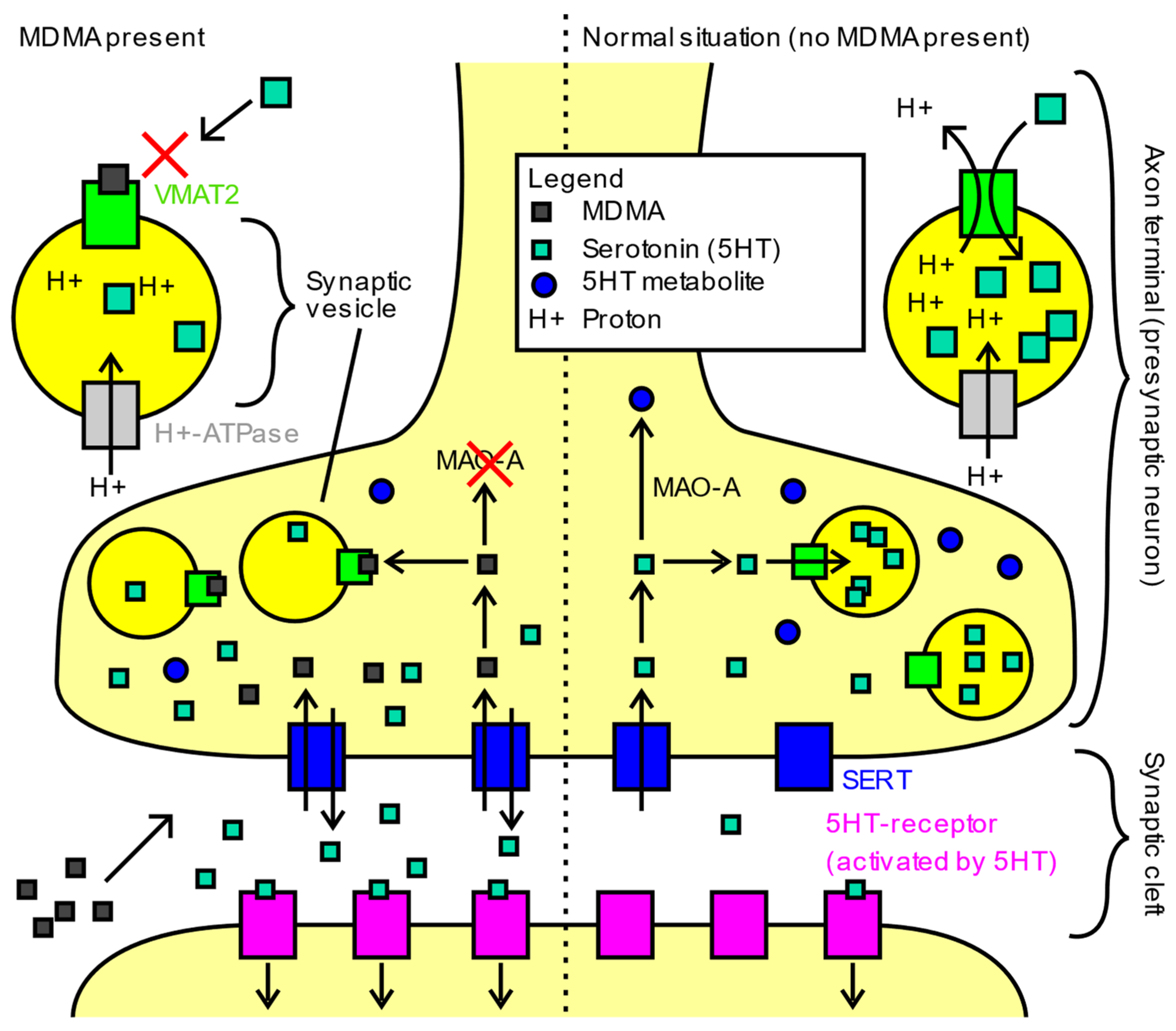
3.1. Pharmacology
3.2. Drug Interactions
3.3. Safety Concerns
3.4. Current Applications of MDMA
4. MDMA in Schizophrenia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultz, S.H.; North, S.W.; Shields, C.G. Schizophrenia: A review. Am. Fam. Physician 2007, 75, 1821–1829. [Google Scholar]
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings from the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature Mortality Among Adults with Schizophrenia in the United States. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.A.; Pankratz, V.S.; Bostwick, J.M. The lifetime risk of suicide in schizophrenia: A reexamination. Arch. Gen. Psychiatry 2005, 62, 247–253. [Google Scholar] [CrossRef]
- Schoenbaum, M.; Sutherland, J.M.; Chappel, A.; Azrin, S.; Goldstein, A.B.; Rupp, A.; Heinssen, R.K. Twelve-Month Health Care Use and Mortality in Commercially Insured Young People with Incident Psychosis in the United States. Schizophr. Bull. 2017, 43, 1262–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Mental Health: A New Understanding, New Hope. The World Health Report; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.C.; Kotirum, S.; Chiou, C.F.; Chaiyakunapruk, N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373. [Google Scholar] [CrossRef] [Green Version]
- Bobes, J.; Arango, C.; Garcia-Garcia, M.; Rejas, J. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: Findings from the CLAMORS study. J. Clin. Psychiatry 2010, 71, 280–286. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Werbeloff, N.; Caers, I.; Mandel, F.S.; Stauffer, V.; Menard, F.; Kinon, B.J.; Kapur, S. Negative symptoms in schizophrenia—The remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr. Res. 2013, 150, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Carbon, M.; Correll, C.U. Thinking and acting beyond the positive: The role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014, 19 (Suppl. 1), 38–52. [Google Scholar] [CrossRef]
- Sicras-Mainar, A.; Maurino, J.; Ruiz-Beato, E.; Navarro-Artieda, R. Impact of negative symptoms on healthcare resource utilization and associated costs in adult outpatients with schizophrenia: A population-based study. BMC Psychiatry 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.; Scott, J.G.; Chatterton, M.L. Healthcare costs and resource use associated with negative symptoms of schizophrenia: A systematic literature review. Schizophr. Res. 2022, 241, 251–259. [Google Scholar] [CrossRef]
- Lepow, L.; Morishita, H.; Yehuda, R. Critical Period Plasticity as a Framework for Psychedelic-Assisted Psychotherapy. Front. Neurosci. 2021, 15, 710004. [Google Scholar] [CrossRef]
- Nardou, R.; Lewis, E.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 2019, 569, 116–120. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’Alora, M.G.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Pearce, J.M. Positive and negative cerebral symptoms: The roles of Russell Reynolds and Hughlings Jackson. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1148. [Google Scholar] [CrossRef] [Green Version]
- Mosolov, S.N.; Yaltonskaya, P.A. Primary and Secondary Negative Symptoms in Schizophrenia. Front. Psychiatry 2022, 12, 766692. [Google Scholar] [CrossRef]
- Blom, J.D.; van Praag, H.M. Schizophrenia: It’s broken and it can’t be fixed. A conceptual analysis at the centenary of Bleuler’s Dementia praecox oder Gruppe der Schizophrenien. Isr. J. Psychiatry Relat. Sci. 2011, 48, 240–248. [Google Scholar]
- Andreasen, N.C. Negative symptoms in schizophrenia. Definition and reliability. Arch. Gen. Psychiatry 1982, 39, 784–788. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fenton, W.S.; Carpenter, W.T.; Marder, S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006, 32, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Marder, S.R.; Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017, 16, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Kring, A.M.; Moran, E.K. Emotional response deficits in schizophrenia: Insights from affective science. Schizophr. Bull. 2008, 34, 819–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, K.; Mass, R.; Kiefer, F.; Wiedemann, K.; Naber, D. Characterization of the facial expression of emotions in schizophrenia patients: Preliminary findings with a new electromyography method. Can. J. Psychiatry 2006, 51, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Brüne, M.; Sonntag, C.; Abdel-Hamid, M.; Lehmkämper, C.; Juckel, G.; Troisi, A. Nonverbal behavior during standardized interviews in patients with schizophrenia spectrum disorders. J. Nerv. Ment. Dis. 2008, 196, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Trémeau, F. A review of emotion deficits in schizophrenia. Dialogues Clin. Neurosci. 2006, 8, 59–70. [Google Scholar] [CrossRef]
- Fervaha, G.; Takeuchi, H.; Foussias, G.; Agid, O.; Remington, G. Using poverty of speech as a case study to explore the overlap between negative symptoms and cognitive dysfunction. Schizophr. Res. 2016, 176, 411–416. [Google Scholar] [CrossRef]
- Cohen, A.S.; Mitchell, K.R.; Elvevåg, B. What do we really know about blunted vocal affect and alogia? A meta-analysis of objective assessments. Schizophr. Res. 2014, 159, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Barch, D.M.; Pagliaccio, D.; Luking, K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr. Top. Behav. Neurosci. 2016, 27, 411–449. [Google Scholar]
- Kollias, C.T.; Kontaxakis, V.P.; Havaki-Kontaxaki, B.J.; Stamouli, S.; Margariti, M.; Petridou, E. Association of physical and social anhedonia with depression in the acute phase of schizophrenia. Psychopathology 2008, 41, 365–370. [Google Scholar] [CrossRef]
- Strauss, G.P.; Waltz, J.A.; Gold, J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 2014, 40 (Suppl. 2), S107–S116. [Google Scholar] [CrossRef] [Green Version]
- Strauss, G.P.; Gold, J.M. A Psychometric Comparison of the Clinical Assessment Interview for Negative Symptoms and the Brief Negative Symptom Scale. Schizophr. Bull. 2016, 42, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.S.; Minor, K.S. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophr. Bull. 2010, 36, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Foussias, G.; Remington, G. Negative symptoms in schizophrenia: Avolition and Occam’s razor. Schizophr. Bull. 2010, 36, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foussias, G.; Mann, S.; Zakzanis, K.; van Reekum, R.; Agid, O.; Remington, G. Prediction of longitudinal functional outcomes in schizophrenia: The impact of baseline motivational deficits. Schizophr. Res. 2011, 132, 24–27. [Google Scholar] [CrossRef]
- Myin-Germeys, I.; Delespaul, P.A.; Devries, M.W. Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr. Bull. 2000, 26, 847–854. [Google Scholar] [CrossRef]
- Oorschot, M.; Lataster, T.; Thewissen, V.; Lardinois, M.; Wichers, M.; van Os, J.; Delespaul, P.; Myin-Germeys, I. Emotional experience in negative symptoms of schizophrenia--no evidence for a generalized hedonic deficit. Schizophr. Bull. 2013, 39, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.; Dubois, B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex 2006, 16, 916–928. [Google Scholar] [CrossRef] [Green Version]
- Waltz, J.A.; Frank, M.J.; Robinson, B.M.; Gold, J.M. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol. Psychiatry 2007, 62, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Couture, S.M.; Penn, D.L.; Losh, M.; Adolphs, R.; Hurley, R.; Piven, J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: More convergence than divergence. Psychol. Med. 2010, 40, 569–579. [Google Scholar] [CrossRef]
- Felice Reddy, L.; Green, M.F.; Rizzo, S.; Sugar, C.A.; Blanchard, J.J.; Gur, R.E.; Kring, A.M.; Horan, W.P. Behavioral approach and avoidance in schizophrenia: An evaluation of motivational profiles. Schizophr. Res. 2014, 159, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Grant, P.M.; Huh, G.A.; Perivoliotis, D.; Chang, N.A. Dysfunctional attitudes and expectancies in deficit syndrome schizophrenia. Schizophr. Bull. 2013, 39, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Huang, C.L.; Chang, Y.C.; Chen, P.W.; Lin, C.Y.; Tsai, G.E.; Lane, H.Y. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr. Res. 2013, 146, 231–237. [Google Scholar] [CrossRef]
- Strauss, G.P.; Keller, W.R.; Koenig, J.; Gold, J.M.; Ossenfort, K.L.; Buchanan, R.W. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophr. Res. 2015, 162, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschner, M.; Aleman, A.; Kaiser, S. Secondary negative symptoms—A review of mechanisms, assessment and treatment. Schizophr. Res. 2017, 186, 29–38. [Google Scholar] [CrossRef]
- Kumari, S.; Malik, M.; Florival, C.; Manalai, P.; Sonje, S. An Assessment of Five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used Symptoms Rating Scales in Schizophrenia and Comparison to Newer Scales (CAINS, BNSS). J. Addict. Res. Ther. 2017, 8, 324. [Google Scholar] [CrossRef]
- Parola, A.; Simonsen, A.; Bliksted, V.; Fusaroli, R. Voice patterns in schizophrenia: A systematic review and Bayesian meta-analysis. Schizophr. Res. 2020, 216, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Bishay, M.; Palasek, P.; Priebe, S.; Patras, I. SchiNet: Automatic Estimation of Symptoms of Schizophrenia from Facial Behaviour Analysis. IEEE Trans. Affect. Comput. 2021, 12, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Tron, T.; Grinsphoon, A.; Weinshall, D. Automated Facial Expressions Analysis in Schizophrenia: A Continuous Dynamic Approach. In Pervasive Computing Paradigms for Mental Health; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Kupper, Z.; Ramseyer, F.; Hoffmann, H.; Kalbermatten, S.; Tschacher, W. Video-based quantification of body movement during social interaction indicates the severity of negative symptoms in patients with schizophrenia. Schizophr. Res. 2010, 121, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.K.; Culbreth, A.J.; Barch, D.M. Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. J. Abnorm. Psychol. 2017, 126, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Rocca, P.; the Italian Network for Research on Psychoses; Brasso, C.; Montemagni, C.; Bellino, S.; Rossi, A.; Bertolino, A.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. Accuracy of self-assessment of real-life functioning in schizophrenia. NPJ Schizophr. 2021, 7, 11. [Google Scholar] [CrossRef]
- Sabbag, S.; Twamley, E.W.; Vella, L.; Heaton, R.K.; Patterson, T.L.; Harvey, P.D. Predictors of the accuracy of self assessment of everyday functioning in people with schizophrenia. Schizophr. Res. 2012, 137, 190–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, J.M.; Waltz, J.A.; Prentice, K.J.; Morris, S.E.; Heerey, E.A. Reward processing in schizophrenia: A deficit in the representation of value. Schizophr. Bull. 2008, 34, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Addington, D.; Maticka-Tyndale, E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr. Res. 1991, 5, 123–134. [Google Scholar] [CrossRef]
- Berman, I.; Viegner, B.; Merson, A.; Allan, E.; Pappas, D.; Green, A.I. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr. Res. 1997, 25, 1–10. [Google Scholar] [CrossRef]
- Jagannath, V.; Theodoridou, A.; Gerstenberg, M.; Franscini, M.; Heekeren, K.; Correll, C.U.; Rössler, W.; Grünblatt, E.; Walitza, S. Prediction Analysis for Transition to Schizophrenia in Individuals at Clinical High Risk for Psychosis: The Relationship of DAO, DAOA, and NRG1 Variants with Negative Symptoms and Cognitive Deficits. Front. Psychiatry 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Mwansisya, T.E.; Wang, Z.; Tao, H.; Zhang, H.; Hu, A.; Guo, S.; Liu, Z. The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia. Schizophr. Res. 2013, 150, 144–150. [Google Scholar] [CrossRef]
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Bueno, B.; Bioque, M.; Mac-Dowell, K.S.; Barcones, M.F.; Martínez-Cengotitabengoa, M.; Pina-Camacho, L.; Rodríguez-Jiménez, R.; Sáiz, P.A.; Castro, C.; Lafuente, A.; et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: Toward an integrative inflammatory hypothesis of schizophrenia. Schizophr. Bull. 2014, 40, 376–387. [Google Scholar] [CrossRef] [Green Version]
- Green, M.F.; Horan, W.P.; Barch, D.M.; Gold, J.M. Effort-Based Decision Making: A Novel Approach for Assessing Motivation in Schizophrenia. Schizophr. Bull. 2015, 41, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.F.; Horan, W.P.; Barch, D.M.; Buchanan, R.W.; Dunayevich, E.; Gold, J.M.; Lyons, N.; Marder, S.R.; Treadway, M.T.; Wynn, J.; et al. Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 1—Psychometric Characteristics of 5 Paradigms. Schizophr. Bull. 2015, 41, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive Deficits and Functional Outcome in Schizophrenia: Are We Measuring the “Right Stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar] [PubMed]
- Markou, A.; Salamone, J.D.; Bussey, T.J.; Mar, A.C.; Brunner, D.; Gilmour, G.; Balsam, P. Measuring reinforcement learning and motivation constructs in experimental animals: Relevance to the negative symptoms of schizophrenia. Neurosci. Biobehav. Rev. 2013, 37 Pt 9, 2149–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, J.M.; Kool, W.; Botvinick, M.M.; Hubzin, L.; August, S.; Waltz, J.A. Cognitive effort avoidance and detection in people with schizophrenia. Cogn. Affect. Behav. Neurosci. 2015, 15, 145–154. [Google Scholar] [CrossRef]
- Gold, J.M.; Strauss, G.P.; Waltz, J.A.; Robinson, B.M.; Brown, J.K.; Frank, M.J. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiatry 2013, 74, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, L.F.; Horan, W.P.; Barch, D.M.; Buchanan, R.W.; Gold, J.M.; Marder, S.R.; Wynn, J.K.; Young, J.; Green, M.F. Understanding the Association Between Negative Symptoms and Performance on Effort-Based Decision-Making Tasks: The Importance of Defeatist Performance Beliefs. Schizophr. Bull. 2018, 44, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Green, M.F.; Horan, W.P.; Lee, J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015, 16, 620–631. [Google Scholar] [CrossRef]
- Sergi, M.J.; Rassovsky, Y.; Widmark, C.; Reist, C.; Erhart, S.; Braff, D.L.; Marder, S.R.; Green, M.F. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophr. Res. 2007, 90, 316–324. [Google Scholar] [CrossRef]
- Harvey, P.O.; Zaki, J.; Lee, J.; Ochsner, K.; Green, M.F. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr. Bull. 2013, 39, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Mackes, N.K.; Golm, D.; O’Daly, O.G.; Sarkar, S.; Sonuga-Barke, E.; Fairchild, G.; Mehta, M.A. Tracking emotions in the brain—Revisiting the Empathic Accuracy Task. Neuroimage 2018, 178, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.M.; Correll, C.U. The acute efficacy of antipsychotics in schizophrenia: A review of recent meta-analyses. Ther. Adv. Psychopharmacol. 2018, 8, 303–318. [Google Scholar] [CrossRef] [Green Version]
- Leucht, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Davis, J.M. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2012, 5, Cd008016. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899. [Google Scholar] [CrossRef]
- Krause, M.; Zhu, Y.; Huhn, M.; Schneider-Thoma, J.; Bighelli, I.; Nikolakopoulou, A.; Leucht, S. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2018, 268, 625–639. [Google Scholar] [CrossRef]
- Németh, G.; Laszlovszky, I.; Czobor, P.; Szalai, E.; Szatmári, B.; Harsányi, J.; Barabássy, Á.; Debelle, M.; Durgam, S.; Bitter, I.; et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: A randomised, double-blind, controlled trial. Lancet 2017, 389, 1103–1113. [Google Scholar] [CrossRef]
- Galling, B.; Wadhwa, A.; Grudnikoff, E.; Tsoy-Podosenin, M.; Poyurovsky, M.; A Vernon, J.; Pagsberg, A.K.; Seidman, A.J.; Kane, J.M.; Correll, C.U. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr. Scand. 2018, 137, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, J.-P.; Nasrallah, H.; Pucci, M.; James, S.; Citrome, L. A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: Challenges and therapeutic opportunities. Schizophr. Res. 2013, 147, 241–252. [Google Scholar] [CrossRef]
- Aleman, A.; Lincoln, T.; Bruggeman, R.; Melle, I.; Arends, J.; Arango, C.; Knegtering, H. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr. Res. 2017, 186, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Wahlbeck, K.; Kiviniemi, V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2009, 109, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.R.; Woolley, J.D. Oxytocin effects in schizophrenia: Reconciling mixed findings and moving forward. Neurosci. Biobehav. Rev. 2017, 80, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Carter, C.S.; Drogos, L.; Pournajafi-Nazarloo, H.; Sweeney, J.A.; Maki, P. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr. Res. 2010, 124, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Kéri, S.; Kiss, I.; Kelemen, O. Sharing secrets: Oxytocin and trust in schizophrenia. Soc. Neurosci. 2009, 4, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Oya, K.; Matsuda, Y.; Matsunaga, S.; Kishi, T.; Iwata, N. Efficacy and safety of oxytocin augmentation therapy for schizophrenia: An updated systematic review and meta-analysis of randomized, placebo-controlled trials. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 439–450. [Google Scholar] [CrossRef]
- Williams, D.R.; Bürkner, P.C. Effects of intranasal oxytocin on symptoms of schizophrenia: A multivariate Bayesian meta-analysis. Psychoneuroendocrinology 2017, 75, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.W.; Javitt, D.C.; Marder, S.R.; Schooler, N.R.; Gold, J.M.; McMahon, R.P.; Heresco-Levy, U.; Carpenter, W.T. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. Am. J. Psychiatry 2007, 164, 1593–1602. [Google Scholar] [CrossRef] [Green Version]
- Bugarski-Kirola, D.; Wang, A.; Abi-Saab, D.; Blättler, T. A phase II/III trial of bitopertin monotherapy compared with placebo in patients with an acute exacerbation of schizophrenia—Results from the CandleLyte study. Eur. Neuropsychopharmacol. 2014, 24, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, V. Meta-Analysis of the Efficacy of Adjunctive NMDA Receptor Modulators in Chronic Schizophrenia. CNS Drugs 2011, 25, 859–885. [Google Scholar] [CrossRef]
- De Lucena, D.; Fernandes, B.S.; Berk, M.; Dodd, S.; Medeiros, D.W.; Pedrini, M.; Kunz, M.; Gomes, F.A.; Giglio, L.F.; Lobato, M.I.; et al. Improvement of negative and positive symptoms in treatment-refractory schizophrenia: A double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J. Clin. Psychiatry 2009, 70, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerman, S.R.T.; Schulte, P.F.J.; Smith, J.D.; De Haan, L. Memantine augmentation in clozapine-refractory schizophrenia: A randomized, double-blind, placebo-controlled crossover study. Psychol. Med. 2016, 46, 1909–1921. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- Turner, D.T.; van der Gaag, M.; Karyotaki, E.; Cuijpers, P. Psychological Interventions for Psychosis: A Meta-Analysis of Comparative Outcome Studies. Am. J. Psychiatry 2014, 171, 523–538. [Google Scholar] [CrossRef]
- Elis, O.; Caponigro, J.M.; Kring, A.M. Psychosocial treatments for negative symptoms in schizophrenia: Current practices and future directions. Clin. Psychol. Rev. 2013, 33, 914–928. [Google Scholar] [CrossRef] [Green Version]
- Velthorst, E.; Koeter, M.; van der Gaag, M.; Nieman, D.; Fett, A.-K.; Smit, F.; Staring, A.B.P.; Meijer, C.; De Haan, L. Adapted cognitive–behavioural therapy required for targeting negative symptoms in schizophrenia: Meta-analysis and meta-regression. Psychol. Med. 2015, 45, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykes, T.; Steel, C.; Everitt, B.; Tarrier, N. Cognitive Behavior Therapy for Schizophrenia: Effect Sizes, Clinical Models, and Methodological Rigor. Schizophr. Bull. 2008, 34, 523–537. [Google Scholar] [CrossRef]
- Calvo, A.; Moreno, M.; Ruiz-Sancho, A.; Rapado-Castro, M.; Moreno, C.; Sánchez-Gutiérrez, T.; Arango, C.; Mayoral, M. Intervention for Adolescents with Early-Onset Psychosis and Their Families: A Randomized Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 688–696. [Google Scholar] [CrossRef]
- Dyck, D.G.; Short, R.A.; Hendryx, M.S.; Norell, D.; Myers, M.; Patterson, T.; McDonell, M.G.; Voss, W.D.; McFarlane, W.R. Management of Negative Symptoms Among Patients with Schizophrenia Attending Multiple-Family Groups. Psychiatr. Serv. 2000, 51, 513–519. [Google Scholar] [CrossRef]
- Shulgin, A.T. The background and chemistry of MDMA. J. Psychoact. Drugs 1986, 18, 291–304. [Google Scholar] [CrossRef]
- Greer, G.; Tolbert, R. Subjective Reports of the Effects of MDMA in a Clinical Setting. J. Psychoact. Drugs 1986, 18, 319–327. [Google Scholar] [CrossRef]
- Hysek, C.M.; Schmid, Y.; Simmler, L.D.; Domes, G.; Heinrichs, M.; Eisenegger, C.; Preller, K.H.; Quednow, B.B.; Liechti, M.E. MDMA enhances emotional empathy and prosocial behavior. Soc. Cogn. Affect. Neurosci. 2014, 9, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Passie, T. The early use of MDMA (‘Ecstasy’) in psychotherapy (1977–1985). Drug Sci. Policy Law 2018, 4, 2050324518767442. [Google Scholar] [CrossRef] [Green Version]
- Bernschneider-Reif, S.; Oxler, F.; Freudenmann, R.W. The origin of MDMA (“ecstasy”)—Separating the facts from the myth. Pharmazie 2006, 61, 966–972. [Google Scholar]
- Danforth, A.L.; Struble, C.M.; Yazar-Klosinski, B.; Grob, C.S. MDMA-assisted therapy: A new treatment model for social anxiety in autistic adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Grinspoon, L.; Bakalar, J.B. Can Drugs Be Used to Enhance the Psychotherapeutic Process? Am. J. Psychother. 1986, 40, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Camí, J. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef]
- Betzler, F.; Viohl, L.; Romanczuk-Seiferth, N. Decision-making in chronic ecstasy users: A systematic review. Eur. J. Neurosci. 2017, 45, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.; Greb, A.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Lin, S.H.; Lee, L.T.; Yang, Y.K. Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clin. Psychopharmacol. Neurosci. 2014, 12, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, H.Y. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999, 21 (Suppl. 2), 106s–115s. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Hysek, C.M.; Domes, G.; Liechti, M.E. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology 2012, 222, 293–302. [Google Scholar] [CrossRef]
- Liechti, M.E.; Baumann, C.; Gamma, A.; Vollenweider, F.X. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 2000, 22, 513–521. [Google Scholar] [CrossRef]
- Liechti, M.E.; Saur, M.R.; Gamma, A.; Hell, D.; Vollenweider, F.X. Psychological and physiological effects of MDMA (“Ecstasy”) after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology 2000, 23, 396–404. [Google Scholar] [CrossRef]
- Kuypers, K.P.; de la Torre, R.; Farre, M.; Yubero-Lahoz, S.; Dziobek, I.; Bos, W.V.D.; Ramaekers, J.G. No evidence that MDMA-induced enhancement of emotional empathy is related to peripheral oxytocin levels or 5-HT1a receptor activation. PLoS ONE 2014, 9, e100719. [Google Scholar] [CrossRef]
- Van Wel, J.H.; Kuypers, K.P.; Theunissen, E.L.; Bosker, W.M.; Bakker, K.; Ramaekers, J.G. Effects of acute MDMA intoxication on mood and impulsivity: Role of the 5-HT2 and 5-HT1 receptors. PLoS ONE 2012, 7, e40187. [Google Scholar] [CrossRef] [PubMed]
- Dölen, G. The Pharmacology and Neuroscience of Psychedelics in Horizons: Perspectives on Psychadelics; Horizons: New York, NY, USA, 2021. [Google Scholar]
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan Res. 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kroeze, W.K.; Hufeisen, S.J.; A Popadak, B.; Renock, S.M.; Steinberg, S.; Ernsberger, P.; Jayathilake, K.; Meltzer, H.Y.; Roth, B.L. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E.; Vollenweider, F.X. Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. Eur. Neuropsychopharmacol. 2000, 10, 289–295. [Google Scholar] [CrossRef]
- Baumann, M.H.; Wang, X.; Rothman, R.B. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: A reappraisal of past and present findings. Psychopharmacology 2007, 189, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.H.; Rothman, R.B. Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA). Int. Rev. Neurobiol. 2009, 88, 257–296. [Google Scholar] [PubMed] [Green Version]
- Hudson, A.L.; Lalies, M.D.; Baker, G.B.; Wells, K.; Aitchison, K.J. Ecstasy, legal highs and designer drug use: A Canadian perspective. Drug Sci. Policy Law 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Szigeti, B.; Winstock, A.R.; Erritzoe, D.; Maier, L.J. Are ecstasy induced serotonergic alterations overestimated for the majority of users? J. Psychopharmacol. 2018, 32, 741–748. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Pennings, E.; van den Brink, W. Fatal and non-fatal health incidents related to recreational ecstasy use. J. Psychopharmacol. 2020, 34, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, M.C.; Feduccia, A.A.; Jerome, L.; Mithoefer, A.; Wagner, M.; Walsh, Z.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Doblin, R. MDMA-assisted psychotherapy for treatment of PTSD: Study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology 2019, 236, 2735–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grob, C.S.; Poland, R.E.; Chang, L.; Ernst, T. Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: Methodological considerations and preliminary observations. Behav. Brain Res. 1995, 73, 103–107. [Google Scholar] [CrossRef]
- Grob, C. MDMA research: Preliminary investigations with human subjects. Int. J. Drug Policy 1998, 9, 119–124. [Google Scholar] [CrossRef]
- Check, E. The ups and downs of ecstasy. Nature 2004, 429, 126–128. [Google Scholar] [CrossRef]
- Doblin, R. A Clinical Plan for MDMA (Ecstasy) in the Treatment of Posttraumatic Stress Disorder (PTSD): Partnering with the FDA. J. Psychoact. Drugs 2002, 34, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Lieb, R.; Schuetz, C.G.; Pfister, H.; von Sydow, K.; Wittchen, H.-U. Mental disorders in ecstasy users: A prospective-longitudinal investigation. Drug Alcohol Depend. 2002, 68, 195–207. [Google Scholar] [CrossRef] [Green Version]
- MDMA. 2022. Available online: https://maps.org/mdma/ (accessed on 1 May 2022).
- Hua, Y.S.; Liang, R.; Liang, L.; Huang, G.Z. Contraction band necrosis in two ecstasy abusers: A latent lethal lesion associated with ecstasy. Am. J. Forensic Med. Pathol. 2009, 30, 295–297. [Google Scholar] [CrossRef]
- Shenouda, S.K.; Varner, K.J.; Carvalho, F.; Lucchesi, P.A. Metabolites of MDMA induce oxidative stress and contractile dysfunction in adult rat left ventricular myocytes. Cardiovasc. Toxicol. 2009, 9, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Droogmans, S.; Cosyns, B.; D’Haenen, H.; Creeten, E.; Weytjens, C.; Franken, P.R.; Scott, B.; Schoors, D.; Kemdem, A.; Close, L.; et al. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am. J. Cardiol. 2007, 100, 1442–1445. [Google Scholar] [CrossRef]
- Kaye, S.; Darke, S.; Duflou, J. Methylenedioxymethamphetamine (MDMA)-related fatalities in Australia: Demographics, circumstances, toxicology and major organ pathology. Drug Alcohol Depend. 2009, 104, 254–261. [Google Scholar] [CrossRef]
- Montgomery, C.; Roberts, C.A. Neurological and cognitive alterations induced by MDMA in humans. Exp. Neurol. 2022, 347, 113888. [Google Scholar] [CrossRef]
- Roberts, C.A.; Jones, A.; Montgomery, C. Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users. Neurosci. Biobehav. Rev. 2016, 63, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Benmansour, S.; Cecchi, M.; Morilak, D.A.; Gerhardt, G.A.; Javors, M.A.; Gould, G.; Frazer, A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J. Neurosci. 1999, 19, 10494–10501. [Google Scholar] [CrossRef] [Green Version]
- Frazer, A.; Benmansour, S. Delayed pharmacological effects of antidepressants. Mol. Psychiatry 2002, 7 (Suppl. 1), S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; O’Callaghan, J.P.; Miller, D.B.; Kramer, M. Comparative study of fluoxetine, sibutramine, sertraline and dexfenfluramine on the morphology of serotonergic nerve terminals using serotonin immunohistochemistry. Brain Res. 2000, 858, 92–105. [Google Scholar] [CrossRef]
- Buchert, R.; Thomasius, R.; Nebeling, B.; Petersen, K.; Obrocki, J.; Jenicke, L.; Wilke, F.; Wartberg, L.; Zapletalova, P.; Clausen, M. Long-Term Effects of “Ecstasy” Use on Serotonin Transporters of the Brain Investigated by PET. J. Nucl. Med. 2003, 44, 375. [Google Scholar] [PubMed]
- Cohen, R.S.; Cocores, J. Neuropsychiatric manifestations following the use of 3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1997, 21, 727–734. [Google Scholar] [CrossRef]
- McGuire, P.; Fahy, T. Chronic paranoid psychosis after misuse of MDMA (“ecstasy”). BMJ 1991, 302, 697. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Moreland, T.; Haq, F.; Siddiqui, F.; Mikul, M.; Qadir, H.; Raza, S. Persistent Psychosis after a Single Ingestion of “Ecstasy” (MDMA). Prim. Care Companion CNS Disord. 2011, 13, 27095. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.W.; Sicignano, D.J.; Hernandez, A.V.; White, C.M. MDMA-Assisted Psychotherapy for Treatment of Posttraumatic Stress Disorder: A Systematic Review with Meta-Analysis. J. Clin. Pharmacol. 2022, 62, 463–471. [Google Scholar] [CrossRef]
- Sanson, A.; Riva, M.A. Anti-Stress Properties of Atypical Antipsychotics. Pharmaceuticals 2020, 13, 322. [Google Scholar] [CrossRef]
- Avram, M.; Müller, F.; Rogg, H.; Korda, A.; Andreou, C.; Holze, F.; Vizeli, P.; Ley, L.; Liechti, M.E.; Borgwardt, S. Characterizing thalamocortical (dys)connectivity following d-amphetamine, LSD, and MDMA administration. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022. [Google Scholar] [CrossRef]
- Woodward, N.D.; Karbasforoushan, H.; Heckers, S. Thalamocortical Dysconnectivity in Schizophrenia. Am. J. Psychiatry 2012, 169, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Barch, D.M.; Carter, C.S. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr. Res. 2005, 77, 43–58. [Google Scholar] [CrossRef]
- Koreen, A.R.; Lieberman, J.A.; Alvir, J.; Chakos, M. The Behavioral Effect of m-Chlorophenylpiperazine (mCPP) and methylphenidate in first-episode schizophrenia and normal controls. Neuropsychopharmacology 1997, 16, 61–68. [Google Scholar] [CrossRef]
- Pandurangi, A.K.; Goldberg, S.C.; Brink, D.D.; Hill, M.H.; Gulati, A.N.; Hamer, R.M. Amphetamine Challenge Test, response to treatment, and lateral ventricle size in schizophrenia. Biol. Psychiatry 1989, 25, 207–214. [Google Scholar] [CrossRef]
- Sharma, R.P.; Javaid, J.I.; Pandey, G.N.; Janicak, P.G.; Davis, J.M. Behavioral and biochemical effects of methylphenidate in schizophrenic and nonschizophrenic patients. Biol. Psychiatry 1991, 30, 459–466. [Google Scholar] [CrossRef]
- Danforth, A.L.; Grob, C.S.; Struble, C.; Feduccia, A.A.; Walker, N.; Jerome, L.; Yazar-Klosinski, B.; Emerson, A. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: A randomized, double-blind, placebo-controlled pilot study. Psychopharmacology 2018, 235, 3137–3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, A.F.; Gunderson, J.G. The Role of the Therapeutic Alliance in the Treatment of Schizophrenia: Relationship to Course and Outcome. Arch. Gen. Psychiatry 1990, 47, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Eack, S.M.; Wojtalik, J.; Keshavan, M.S.; Minshew, N.J. Social-cognitive brain function and connectivity during visual perspective-taking in autism and schizophrenia. Schizophr. Res. 2017, 183, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Gordon, I.; Jack, A.; Pretzsch, C.; Wyk, B.V.; Leckman, J.F.; Feldman, R.; Pelphrey, K.A. Intranasal Oxytocin Enhances Connectivity in the Neural Circuitry Supporting Social Motivation and Social Perception in Children with Autism. Sci. Rep. 2016, 6, 35054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bois, C.; Levita, L.; Ripp, I.; Owens, D.C.; Johnstone, E.C.; Whalley, H.; Lawrie, S. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: A cross-sectional comparison. Schizophr. Res. 2015, 165, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.J.; Loughry, B.; O’Reilly, R.C. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cogn. Affect. Behav. Neurosci. 2001, 1, 137–160. [Google Scholar] [CrossRef] [Green Version]
- Colado, M.I.; O’Shea, E.; Green, A.R. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology 2004, 173, 249–263. [Google Scholar] [CrossRef]
- Muench, J.; Hamer, A.M. Adverse effects of antipsychotic medications. Am. Fam. Physician 2010, 81, 617–622. [Google Scholar]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Siafis, S.; Fernando, P.; Falkai, P.; Honer, W.G.; Röh, A.; Siskind, D.; Leucht, S.; Hasan, A. Efficacy and safety of clozapine in psychotic disorders—A systematic quantitative meta-review. Transl. Psychiatry 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Park, S.H. Seizure Associated with Clozapine: Incidence, Etiology, and Management. CNS Drugs 2015, 29, 101–111. [Google Scholar] [CrossRef]
- Agin-Liebes, G.I.; Malone, T.; Yalch, M.M.; Mennenga, S.E.; Ponté, K.L.; Guss, J.; Bossis, A.P.; Grigsby, J.; Fischer, S.; Ross, S. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J. Psychopharmacol. 2020, 34, 155–166. [Google Scholar] [CrossRef]
- Jerome, L.; Feduccia, A.A.; Wang, J.B.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; Mithoefer, M.C.; Doblin, R. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: A longitudinal pooled analysis of six phase 2 trials. Psychopharmacology 2020, 237, 2485–2497. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnovitz, M.D.; Spitzberg, A.J.; Davani, A.J.; Vadhan, N.P.; Holland, J.; Kane, J.M.; Michaels, T.I. MDMA for the Treatment of Negative Symptoms in Schizophrenia. J. Clin. Med. 2022, 11, 3255. https://doi.org/10.3390/jcm11123255
Arnovitz MD, Spitzberg AJ, Davani AJ, Vadhan NP, Holland J, Kane JM, Michaels TI. MDMA for the Treatment of Negative Symptoms in Schizophrenia. Journal of Clinical Medicine. 2022; 11(12):3255. https://doi.org/10.3390/jcm11123255
Chicago/Turabian StyleArnovitz, Mitchell D., Andrew J. Spitzberg, Ashkhan J. Davani, Nehal P. Vadhan, Julie Holland, John M. Kane, and Timothy I. Michaels. 2022. "MDMA for the Treatment of Negative Symptoms in Schizophrenia" Journal of Clinical Medicine 11, no. 12: 3255. https://doi.org/10.3390/jcm11123255
APA StyleArnovitz, M. D., Spitzberg, A. J., Davani, A. J., Vadhan, N. P., Holland, J., Kane, J. M., & Michaels, T. I. (2022). MDMA for the Treatment of Negative Symptoms in Schizophrenia. Journal of Clinical Medicine, 11(12), 3255. https://doi.org/10.3390/jcm11123255





