Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value
Abstract
:1. Introduction
2. Nutritional Value of Mushrooms
3. Nutraceuticals
4. Bioactive Compounds from Macrofungi and Their Medicinal Properties
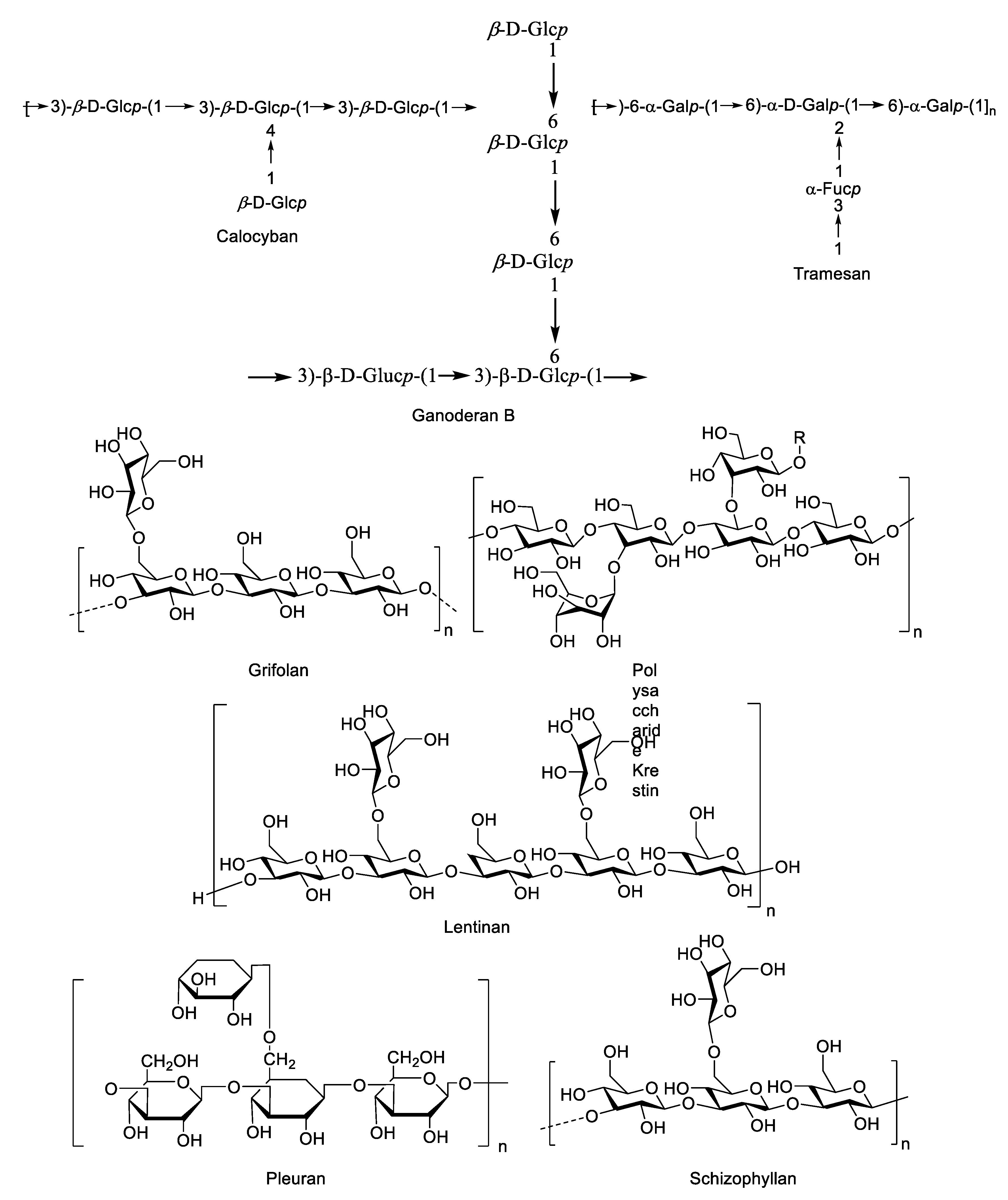
4.1. Mushroom Species Containing Bioactive Polysaccharides
4.2. Macrofungal β-Glucans
4.3. Proteins
4.4. Fats
4.5. Phenolic Compounds
4.6. Vitamins
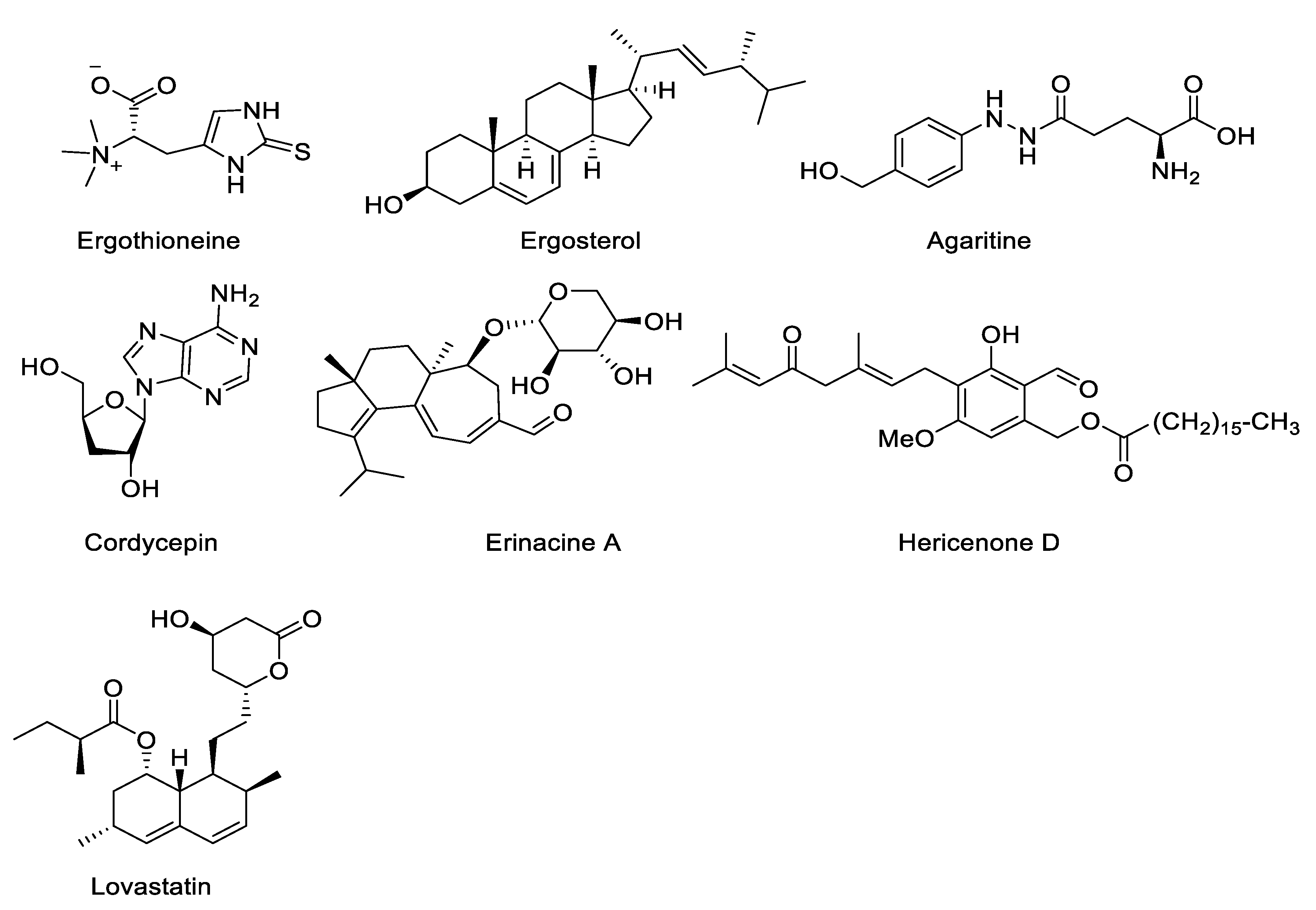
4.7. Other Bioactive Compounds
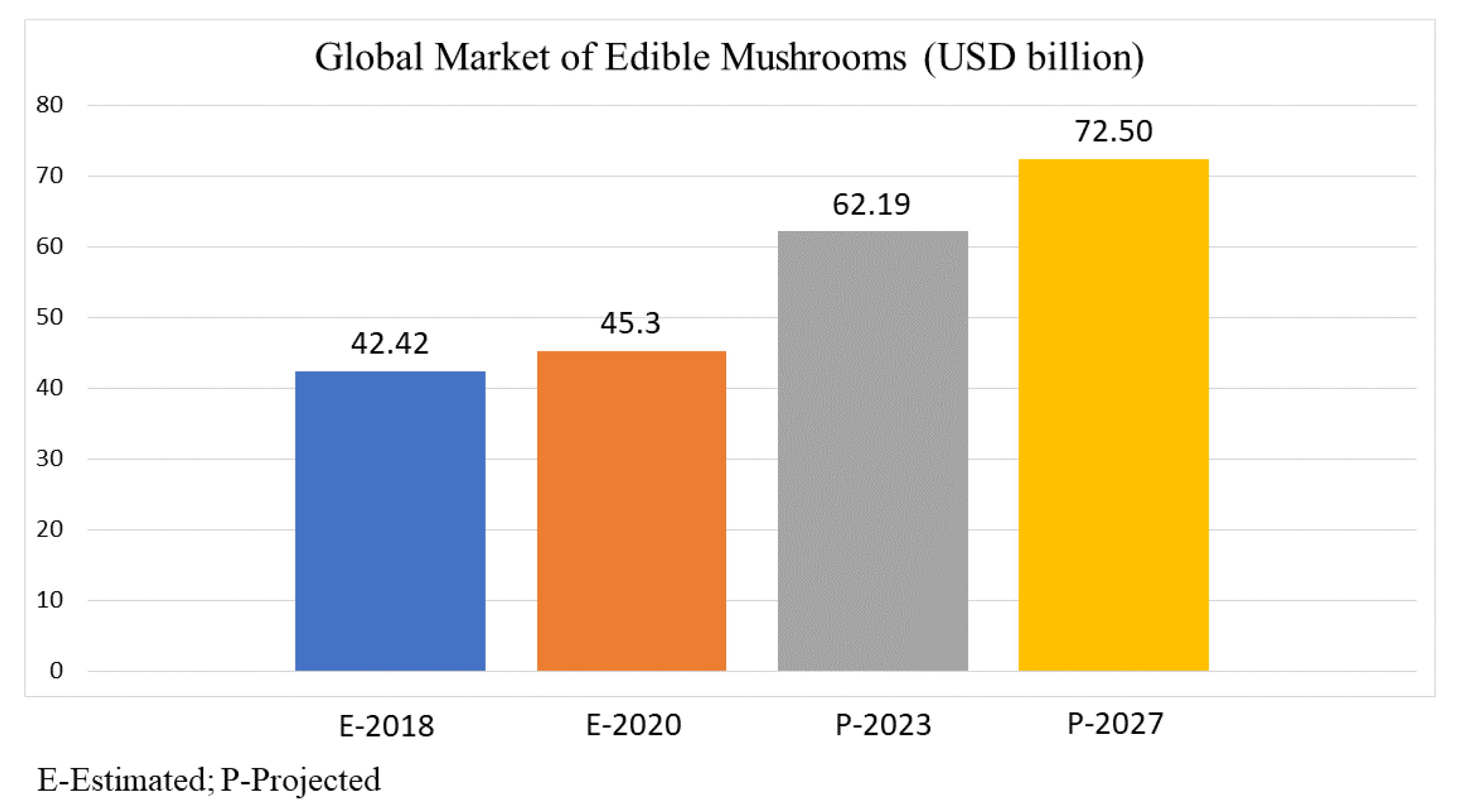
5. Macrofungi Nutraceutical Market Overview
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, S.T.; Wasser, S.P. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products (review). Int. J. Med. Mushrooms 2018, 20, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Willis, K.J. State of the World’s Fungi 2018. Available online: https://stateoftheworldsfungi.org/ (accessed on 18 March 2021).
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T.; et al. Mushrooms and health summit proceedings. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Samsudin, N.I.P.; Abdullah, N. Edible mushrooms from Malaysia; a literature review on their nutritional and medicinal properties. Int. Food Res. J. 2019, 26, 11–31. [Google Scholar]
- Huang, J.; Ou, Y.; Yew, T.W.D.; Liu, J.; Leng, B.; Lin, Z.; Su, Y.; Zhuang, Y.; Lin, J.; Li, X.; et al. Hepatoprotective effects of polysaccharide isolated from Agaricus bisporus industrial wastewater against CCl4-induced hepatic injury in mice. Int. J. Biol. Macromol. 2016, 82, 678–686. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Hu, Q. Development situation on processing technology and product innovation of edible mushroom in China. J. Food Sci. Technol. 2019, 37, 13–18. [Google Scholar] [CrossRef]
- Agrawal, S.; Jha, K.N. Medicinal mushrooms. J. Crit. Rev. 2020, 7, 1401–1407. [Google Scholar] [CrossRef]
- Technavio Medicinal Mushrooms Market 2018–2022. Available online: https://www.technavio.com/report/global-medicinal-mushrooms-market-analysis-share-2018#:~:text=The%20medicinal%20mushrooms%20market%20size,%2C%20APAC%2C%20and%20EMEA (accessed on 10 March 2021).
- Shamtsyan, M. Bioactive compounds in mushrooms. In Encyclopedia of Biotechnology in Agriculture and Food; Heldman, D., Wheeler, M., Hoover, D., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 76–81. [Google Scholar]
- Raman, J.; Lee, S.-K.; Im, J.-H.; Oh, M.-J.; Oh, Y.-L.; Jang, K.-Y. Current prospects of mushroom production and industrial growth in India. J. Mushrooms 2018, 4, 239–249. [Google Scholar] [CrossRef]
- Research and Markets Global edible Mushrooms Market—Industry Trends, Opportunities and Forecasts to 2023. Available online: https://www.researchandmarkets.com/reports/4451952/global-edible-mushrooms-market-industry-trends (accessed on 20 March 2021).
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Mollet, B.; Rowland, I. Functional foods: At the frontier between food and pharma. Curr. Opin. Biotechnol. 2002, 13, 483–485. [Google Scholar] [CrossRef]
- Sobieralski, K.; Siwulski, M.; Lisiecka, J.; Jedryczka, M.; Sas-Golak, I.; Fruzyńska-Jóźwiak, D. Fungi-derived β-glucans as a component of functional food. Acta Sci. Pol. Hortorum Cultus 2012, 11, 111–128. [Google Scholar]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- De Silva, D.D.; Rapior, S.; Fons, F.; Bahkali, A.H.; Hyde, K.D. Medicinal mushrooms in supportive cancer therapies: An approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012, 55, 1–35. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Rapior, S. The neurotrophic and neuroprotective potential of macrofungi. In Medicinal Herbs and Fungi; Springer Nature Singapore: Singapore, 2021; pp. 37–77. [Google Scholar]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Varghese, R.; Dalvi, Y.B.; Lamrood, P.Y.; Shinde, B.P.; Nair, C.K.K. Historical and current perspectives on therapeutic potential of higher basidiomycetes: An overview. 3 Biotech 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, K. A review on edible straw mushrooms: A source of high nutritional supplement, biologically active diverse structural polysaccharides. J. Sci. Res. 2020, 64, 295–304. [Google Scholar] [CrossRef]
- Semwal, K.; Lemma, H.; Dhyani, A.; Equar, G.; Amhare, S. Mushroom: Nature’s treasure in Ethiopia. Momona Ethiop. J. Sci. 2014, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef]
- Govorushko, S.; Rezaee, R.; Dumanov, J.; Tsatsakis, A. Poisoning associated with the use of mushrooms: A review of the global pattern and main characteristics. Food Chem. Toxicol. 2019, 128, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Öztürk, M.; Tel-Çayan, G.; Muhammad, A.; Terzioğlu, P.; Duru, M.E. Mushrooms. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 363–456. [Google Scholar]
- Cheung, P.C.K. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Temesgen, T. Application of mushroom as food and medicine. Adv. Biotechnol. Microbiol. 2018, 11, 555817. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M. Nutritional and medicinal importance of Pleurotus mushrooms: An overview. Food Rev. Int. 2012, 28, 313–329. [Google Scholar] [CrossRef]
- Wannet, W.J.B.; Hermans, J.H.M.; van der Drift, C.; Op den Camp, H.J.M. HPLC detection of soluble carbohydrates involved in mannitol and trehalose metabolism in the edible mushroom Agaricus bisporus. J. Agric. Food Chem. 2000, 48, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Food Data Central Mushroom Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169251/nutrients (accessed on 18 March 2021).
- Malinowska, E.; Szefer, P.; Falandysz, J. Metals bioaccumulation by bay bolete, Xerocomus badius, from selected sites in Poland. Food Chem. 2004, 84, 405–416. [Google Scholar] [CrossRef]
- Rudawska, M.; Leski, T. Macro- and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 2005, 92, 499–506. [Google Scholar] [CrossRef]
- Goyal, R.; Grewal, R.B.; Goyal, R.K. Nutritional attributes of Agaricus bisporus and Pleurotus sajor caju mushrooms. Nutr. Health 2006, 18, 179–184. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130. [Google Scholar] [CrossRef] [Green Version]
- Ouzouni, P.K.; Petridis, D.; Koller, W.-D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Bandara, A.R.; Karunarathna, S.C.; Mortimer, P.E.; Hyde, K.D.; Khan, S.; Kakumyan, P.; Xu, J. First successful domestication and determination of nutritional and antioxidant properties of the red ear mushroom Auricularia thailandica (Auriculariales, Basidiomycota). Mycol. Prog. 2017, 16, 1029–1039. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Targeted metabolites analysis in wild Boletus species. LWT Food Sci. Technol. 2011, 44, 1343–1348. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Vieira, V.; Fernandes, Â.; Barros, L.; Glamočlija, J.; Ćirić, A.; Stojković, D.; Martins, A.; Soković, M.; Ferreira, I.C.F.R. Wild Morchella conica Pers. from different origins: A comparative study of nutritional and bioactive properties. J. Sci. Food Agric. 2016, 96, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Télessy, I.G. Nutraceuticals. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–421. ISBN 978-0-12-813148-0. [Google Scholar]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS PharmSci 2003, 5, 27–28. [Google Scholar] [CrossRef] [Green Version]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, P.; Gupta, R.K. Nutraceuticals. In Food Safety in the 21st Century; Dudeja, P., Kumar, R., Amarjeet, G., Minhas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 491–496. ISBN 9780128017739. [Google Scholar]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 5–13. [Google Scholar]
- Chang, S.T.; Buswell, J.A. Mushroom nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Olawuyi, I.F.; Lee, W.Y. Quality and antioxidant properties of functional rice muffins enriched with shiitake mushroom and carrot pomace. Int. J. Food Sci. Technol. 2019, 54, 2321–2328. [Google Scholar] [CrossRef]
- Kumar, K. Role of edible mushroom as functional foods: A review. South Asian J. Food Technol. Environ. 2015, 1, 211–218. [Google Scholar] [CrossRef]
- Raghavendra, V.B.; Venkitasamy, C.; Pan, Z.; Nayak, C. Functional foods from mushroom. In Microbial Functional Foods and Nutraceuticals; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 65–91. [Google Scholar]
- Wu, F.; Zhou, L.-W.; Yang, Z.-L.; Bau, T.; Li, T.-H.; Dai, Y.-C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Tangen, J.-M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Badalyan, S.; Badalyan, S.; Barkhudaryan, A. The cardioprotective properties of Agaricomycetes mushrooms growing in the territory of Armenia (review). Int. J. Med. Mushrooms 2021, 23, 21–31. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of mannogalactoglucan-type polysaccharides from Lentinus edodes (Berkeley) Singer. Cent. Eur. J. Immunol. 2016, 1, 47–53. [Google Scholar] [CrossRef]
- Rubel, R.; Santa, H.S.D.; dos Santos, L.F.; Fernandes, L.C.; Figueiredo, B.C.; Soccol, C.R. Immunomodulatory and antitumoral properties of Ganoderma lucidum and Agaricus brasiliensis (Agaricomycetes) medicinal mushrooms. Int. J. Med. Mushrooms 2018, 20, 393–403. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhang, J.; Wang, W.; Wang, X.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; Gong, Z.; et al. The antioxidative, antiaging, and hepatoprotective effects of alkali-extractable polysaccharides by Agaricus bisporus. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- do Rocio Andrade Pires, A.; Ruthes, A.C.; Cadena, S.M.S.C.; Iacomini, M. Cytotoxic effect of a mannogalactoglucan extracted from Agaricus bisporus on HepG2 cells. Carbohydr. Polym. 2017, 170, 33–42. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Wang, W.; Wang, X.; Zhang, C.; Zhang, J.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; et al. Antioxidant and anti-aging effects of acidic-extractable polysaccharides by Agaricus bisporus. Int. J. Biol. Macromol. 2018, 106, 1297–1306. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Su, L.; Wang, Q.; Li, Y. Protective effect of polysaccharide from Agaricus bisporus in Tibet area of China against tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2018, 118, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hou, P.; Xin, H.; Zhang, Y.; Zhou, A.; Lai, C.; Xie, J. A glucogalactomanan polysaccharide isolated from Agaricus bisporus causes an inflammatory response via the ERK/MAPK and IκB/NFκB pathways in macrophages. Int. J. Biol. Macromol. 2020, 151, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xing, J.; Zheng, S.; Bo, R.; Luo, L.; Huang, Y.; Niu, Y.; Li, Z.; Wang, D.; Hu, Y.; et al. Ganoderma lucidum polysaccharides encapsulated in liposome as an adjuvant to promote Th1-bias immune response. Carbohydr. Polym. 2016, 142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, L.; Zhao, T.; Zhang, Z.; Zhang, R.; Jin, J.; Cai, Y.; Wang, F. Restoration of the tumor-suppressor function to mutant p53 by Ganoderma lucidum polysaccharides in colorectal cancer cells. Oncol. Rep. 2017, 37, 594–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Liao, Y.; Li, W.; Guo, L. Neuroprotective effects of Ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis. Neural Regen. Res. 2017, 12, 953. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, D.; Liu, Y.; Li, C.; Zhao, X.; Li, Y.; Li, W. Ganoderma lucidum polysaccharide inhibits prostate cancer cell migration via the protein arginine methyltransferase 6 signaling pathway. Mol. Med. Rep. 2017, 17, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and immunomodulatory activities of Ganoderma lucidum polysaccharides in glioma-bearing rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Y.; Zhang, W.; Sun, M.; Zhang, Z. Hypoglycemic effect of inulin combined with Ganoderma lucidum polysaccharides in T2DM rats. J. Funct. Foods 2019, 55, 381–390. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Meng, Q.; Guo, W.; Chen, Q.; Zhang, Y. Grifola frondosa polysaccharides induce breast cancer cell apoptosis via the mitochondrial-dependent apoptotic pathway. Int. J. Mol. Med. 2017, 40, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Tang, Y.; Liu, A.; Jin, X.; Zhu, J.; Lu, X. Oral administration of Grifola frondosa polysaccharides improves memory impairment in aged rats via antioxidant action. Mol. Nutr. Food Res. 2017, 61, 1700313. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 105, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ren, Z.; Huang, Y.; Song, Y.; Lin, D.; Li, J.; Ma, Y.; Wu, X.; Qiu, F.; Xiao, Q. Selenizing Hericium erinaceus polysaccharides induces dendritic cells maturation through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2017, 97, 287–298. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yin, J.-Y.; Zhao, M.-M.; Liu, S.-Y.; Nie, S.-P.; Xie, M.-Y. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. Carbohydr. Polym. 2018, 186, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhou, C.; Liu, T.; Dai, Y.; Huang, H. A novel Hericium erinaceus polysaccharide: Structural characterization and prevention of H2O2-induced oxidative damage in GES-1 cells. Int. J. Biol. Macromol. 2020, 154, 1460–1470. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hou, X.X.; Li, Z.Y.; Shan, S.H.; Chang, M.C.; Feng, C.P.; Wei, Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int. J. Biol. Macromol. 2020, 157, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, Z.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Luo, Y.; Zhang, W.; Huang, Y.; et al. Structural characterization of enzymatic modification of Hericium erinaceus polysaccharide and its immune-enhancement activity. Int. J. Biol. Macromol. 2021, 166, 1396–1408. [Google Scholar] [CrossRef]
- Du, J.; Wang, R.; Zhang, W.; Zhang, C.; Li, X.; Shi, X.; Hu, M.; Ma, F.; Ma, C.; Wang, X.; et al. A polysaccharide derived from Lentinus edodes impairs the immunosuppressive function of myeloid-derived suppressor cells via the p38 pathways. RSC Adv. 2017, 7, 36533–36540. [Google Scholar] [CrossRef] [Green Version]
- Ya, G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2017, 103, 676–682. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, W.; Song, X.; Qi, Y.; Zhang, C.; Gao, Z.; Zhang, J.; Jia, L. Antioxidant and anti-inflammation of enzymatic-hydrolysis residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 120, 811–822. [Google Scholar] [CrossRef]
- Song, X.; Ren, Z.; Wang, X.; Jia, L.; Zhang, C. Antioxidant, anti-inflammatory and renoprotective effects of acidic-hydrolytic polysaccharides by spent mushroom compost (Lentinula edodes) on LPS-induced kidney injury. Int. J. Biol. Macromol. 2020, 151, 1267–1276. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Huang, X.; Hu, L.; Huang, Y.; Chen, H.; Fang, Q.; Dong, N.; Li, M.; Tang, W.; et al. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts. J. Funct. Foods 2020, 66, 103791. [Google Scholar] [CrossRef]
- Xiang, F.; Lin, L.; Hu, M.; Qi, X. Therapeutic efficacy of a polysaccharide isolated from Cordyceps sinensis on hypertensive rats. Int. J. Biol. Macromol. 2016, 82, 308–314. [Google Scholar] [CrossRef]
- Zhengqi, Z.; Qiuli, C.; Yufeng, W.; Hui, Z.; Ying, W.; Hongping, Y.; Long, Y. Protective effects of Cordyceps sinensis polysaccharide CPS-A on angiotensin ll-induced injury of liver L02 cells. J. China Pharm. Univ. 2017, 48, 490–495. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Gu, Y.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides attenuate cyclophosphamide-induced intestinal barrier injury in mice. J. Funct. Foods 2019, 62, 103523. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Wang, W.; Qiu, W.; Liu, L.; Ning, A.; Cao, J.; Huang, M.; Zhong, M. Polysaccharides isolated from Cordyceps sinensis contribute to the progression of NASH by modifying the gut microbiota in mice fed a high-fat diet. PLoS ONE 2020, 15, e0232972. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Han, L.; Shi, N.; Zhu, H.; Wang, J. Development, in vitro biocompatibility, and antitumor efficacy of acetic acid-modified Cordyceps sinensis polysaccharide nanoparticle drug delivery system. Brazilian J. Pharm. Sci. 2020, 56, e18470. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, N.; Guo, J.; Yuan, L.; Yang, X. Chemical characterization of Pleurotus eryngii polysaccharide and its tumor-inhibitory effects against human hepatoblastoma HepG-2 cells. Carbohydr. Polym. 2016, 138, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Sha, O.; Xu, W.; Wang, S. Hypolipidaemic and hypoglycaemic activities of polysaccharide from Pleurotus eryngii in Kunming mice. Int. J. Biol. Macromol. 2016, 93, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Zhao, Y.; Jia, W.; Chang, X.; Liu, H.; Liu, N. Optimization of extraction parameters of Pleurotus eryngii polysaccharides and evaluation of the hypolipidemic effect. RSC Adv. 2020, 10, 11918–11928. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Guo, J.Y.; Cheng, H.; Lin, L.; Liu, Y.; Shi, Y.; Xu, J.; Yu, H.T. Protective effects of the king oyster culinary-medicinal mushroom, Pleurotus eryngii (Agaricomycetes), Polysaccharides on β-Amyloid-induced neurotoxicity in PC12 cells and aging rats, in vitro and in vivo studies. Int. J. Med. Mushrooms 2020, 22, 325–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Wisbeck, E.; Facchini, J.M.; Alves, E.P.; Silveira, M.L.L.; Gern, R.M.M.; Ninow, J.L.; Furlan, S.A. A polysaccharide fraction extracted from Pleurotus ostreatus mycelial biomass inhibit Sarcoma 180 tumor. An. Acad. Bras. Cienc. 2017, 89, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Z.; Jin, G.; Yang, X.; Zhou, H. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017, 101, 107–116. [Google Scholar] [CrossRef]
- Uddin Pk, M.M.; Islam, M.S.; Pervin, R.; Dutta, S.; Talukder, R.I.; Rahman, M. Optimization of extraction of antioxidant polysaccharide from Pleurotus ostreatus (Jacq.) P. Kumm and its cytotoxic activity against murine lymphoid cancer cell line. PLoS ONE 2019, 14, e0209371. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Deng, Z.; Li, T.; He, Y.; Yang, Y.; Zhong, S. Physicochemical characterization and antitumor activity in vitro of a selenium polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 2020, 165, 2934–2946. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhu, C.; Wu, Y.; Du, B.; Ji, H. Structural characterization of phosphorylated Pleurotus ostreatus polysaccharide and its hepatoprotective effect on carbon tetrachloride-induced liver injury in mice. Int. J. Biol. Macromol. 2020, 162, 533–547. [Google Scholar] [CrossRef]
- Jhan, M.-H.; Yeh, C.-H.; Tsai, C.-C.; Kao, C.-T.; Chang, C.-K.; Hsieh, C.-W. Enhancing the antioxidant ability of Trametes versicolor polysaccharopeptides by an enzymatic hydrolysis process. Molecules 2016, 21, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca-Lema, D.; Martinez-Iglesias, O.; de Ana Portela, C.F.; Rodríguez-Blanco, A.; Valladares-Ayerbes, M.; Díaz-Díaz, A.; Casas-Pais, A.; Prego, C.; Figueroa, A. In vitro anti-proliferative and anti-invasive effect of polysaccharide-rich extracts from Trametes versicolor and Grifola frondosa in colon cancer cells. Int. J. Med. Sci. 2019, 16, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zhang, M.; Wang, Y.; Zhang, S.; Jiang, X. Extracellular and intracellular polysaccharide extracts of Trametes versicolor improve lipid profiles via serum regulation of lipid-regulating enzymes in hyperlipidemic mice. Curr. Microbiol. 2020, 77, 3526–3537. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, J.; Wen, C.; Sedem Dzah, C.; Chidimma Juliet, I.; Duan, Y.; Zhang, H. Recent advances in Agaricus bisporus polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Process Biochem. 2020, 94, 39–50. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Zhang, C.; Tian, C.; Wang, X.; Song, X.; Jing, H.; Gao, Z.; Ren, Z.; Liu, W.; et al. Purification, in vitro antioxidant and in vivo anti-aging activities of soluble polysaccharides by enzyme-assisted extraction from Agaricus bisporus. Int. J. Biol. Macromol. 2018, 109, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Koyyalamudi, S.R.; Jeong, Y.T.; Song, C.H.; Pang, G. Macrophage immunomodulating and antitumor activities of polysaccharides isolated from Agaricus bisporus white button mushrooms. J. Med. Food 2012, 15, 58–65. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Yang, L.; Zhuang, Y.; Qian, X.; Shen, Y. Ganoderma lucidum polysaccharide exerts anti-tumor activity via MAPK pathways in HL-60 acute leukemia cells. J. Recept. Signal Transduct. 2016, 36, 6–13. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wu, X. Ganoderma lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-κB-IDO pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Huang, S.; Mao, J.; Ding, K.; Zhou, Y.; Zeng, X.; Yang, W.; Wang, P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of Alzheimer’s disease. Stem Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhang, L.; Wang, H. The biological activities of the antitumor drug Grifola frondosa polysaccharide. Prog. Mol. Biol. Transl. Sci. 2019, 163, 221–261. [Google Scholar] [CrossRef]
- Mizuno, T.; Wasa, T.; Ito, H.; Suzuki, C.; Ukai, N. Antitumor-active polysaccharides isolated from the fruiting body of Hericium erinaceum, an edible and medicinal mushroom called yamabushitake or houtou. Biosci. Biotechnol. Biochem. 1992, 56, 347–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic potential of Hericium erinaceus for depressive disorder. Int. J. Mol. Sci. 2019, 21, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, X.; Jia, W.; Gong, G.; Zhao, Y.; Li, G.; Zhou, J.; Li, X.; Zhao, Y.; Ma, W. Extraction, isolation, characterisation, antioxidant and anti-fatigue activities of Pleurotus eryngii polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 2492–2508. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Ellinger, S. Effect of the intake of oyster mushrooms (Pleurotus ostreatus) on cardiometabolic parameters—A systematic review of clinical trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef] [Green Version]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Bulam, S.; Şule Üstün, N.; Pekşen, A. β-Glucans: An important bioactive molecule of edible and medicinal mushrooms. In Proceedings of the International Technological Sciences and Design Symposium (ITESDES), Giresun, Turkey, 27–29 June 2018; pp. 1242–1258. [Google Scholar]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshige, K. Activation of macrophages by linear (1→3)-β-d-glucans. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetvicka, V.; Yvin, J.-C. Effects of marine β−1,3 glucan on immune reactions. Int. Immunopharmacol. 2004, 4, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Maity, K.K.; Bhunia, S.K.; Dey, B.; Patra, S.; Sikdar, S.R.; Islam, S.S. Chemical analysis of new water-soluble (1→6)-, (1→4)-α, β-glucan and water-insoluble (1→3)-, (1→4)-β-glucan (Calocyban) from alkaline extract of an edible mushroom, Calocybe indica (Dudh Chattu). Carbohydr. Res. 2010, 345, 2657–2663. [Google Scholar] [CrossRef]
- Datta, S.; Dubey, J.; Gupta, S.; Paul, A.; Gupta, P.; Mitra, A.K. Tropical milky white mushroom, Calocybe indica (Agaricomycetes): An effective antimicrobial agent working in synergism with standard antibiotics. Int. J. Med. Mushrooms 2020, 22, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Bera, T.; Pal, S. Antiproliferative, apoptotic, and antimigration property of ethyl acetate extract of Calocybe indica against HeLa and CaSki cell lines of cervical cancer, and its antioxidant and mycochemistry analysis. Middle East J. Cancer 2020, 11, 454–468. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, D.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gou, X.; Xue, H.; Liu, K. Ganoderan (GDN) regulates the growth, motility and apoptosis of non-small cell lung cancer cells through ERK signaling pathway in vitro and in vivo. Onco. Targets. Ther. 2019, 12, 8821–8832. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.-D.; He, H.-C.; Ou, R.-B.; Bi, X.-C.; Dai, Q.-S.; Han, Z.-D.; Liang, Y.-X.; Ye, Y.-K.; Qin, W.-J.; Li, Z.; et al. Protective effect of ganoderan on renal damage in rats with chronic glomerulonephritis. Clin. Investig. Med. 2008, 31, 212. [Google Scholar] [CrossRef] [Green Version]
- Hikino, H.; Ishiyama, M.; Suzuki, Y.; Konno, C. Mechanisms of hypoglycemic activity of Ganoderan B: A Glycan of Ganoderma lucidum fruit bodies. Planta Med. 1989, 55, 423–428. [Google Scholar] [CrossRef]
- Han, M.D.; Hoon, J.; Lee, J.W.; Back, S.J.; Kim, S.U.; Yoon, K.H. The composition and bioactivities of ganoderan by mycelial fractionation of Ganoderma lucidum IY009. Korean J. Mycol. 1995, 23, 285–297. [Google Scholar]
- Mao, C.F.; Hsu, M.C.; Hwang, W.H. Physicochemical characterization of grifolan: Thixotropic properties and complex formation with Congo Red. Carbohydr. Polym. 2007, 68, 502–510. [Google Scholar] [CrossRef]
- Takeyama, T.; Suzuki, I.; Ohno, N.; Oikawa, S.; Sato, K.; Ohsawa, M.; Yadomae, T. Host-mediated antitumor effect of grifolan NMF-5N, a polysaccharide obtained from Grifola frondosa. J. Pharmacobiodyn. 1987, 10, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, K.; Miura, N.N.; Adachi, Y.; Ohno, N.; Yadomae, T. Relationship between solubility of Grifolan, a Fungal 1,3-β-D-glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 2001, 65, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.R.; Patel, D.K.; Shin, W.C.; Sim, W.S.; Lee, O.H.; Lim, K.T. Structural elucidation and immune-enhancing effects of novel polysaccharide from Grifola frondosa. Biomed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Ulbricht, C.; Weissner, W.; Basch, E.; Giese, N.; Hammerness, P.; Rusie-Seamon, E.; Varghese, M.; Woods, J. Maitake mushroom (Grifola frondosa): Systematic review by the natural standard research collaboration. J. Soc. Integr. Oncol. 2009, 7, 66–72. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide Krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Ichikawa, S.; Uchida, S.; Komada, T. Effects of a protein-bound polysaccharide from a basidiomycetes against hepatocarcinogenesis induced by 3′-methyl-4-dimethylaminoazobenzene in rats. Clin. Ther. 1990, 12, 385–392. [Google Scholar]
- Hirose, K.; Hakozaki, M.; Matsunaga, K.; Yoshikumi, C.; Hotta, T.; Yanagisawa, M.; Yamamoto, M.; Endo, H. Cloning of sequences induced and suppressed by administration of PSK, antitumor protein-bound polysaccharide. Biochem. Biophys. Res. Commun. 1985, 126, 884–892. [Google Scholar] [CrossRef]
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal mushrooms and cancer therapy: Translating a traditional practice into western medicine. Perspect. Biol. Med. 2006, 49, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Hidaka, H.; Sugiura, M. Effects of coriolan, an antitumor polysaccharide, produced by Coriolus versicolor Iwade. Jpn. J. Pharmacol. 1979, 29, 953–957. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, B.; Zhu, G.; Li, W.; Tian, Y.; Wang, L.; Zong, S.; Sheng, P.; Li, M.; Chen, S.; et al. Selenium–lentinan inhibits tumor progression by regulating epithelial–mesenchymal transition. Toxicol. Appl. Pharmacol. 2018, 360, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Jiang, B.; He, C.; Liu, L. Lentinan inhibits oxidative stress and inflammatory cytokine production induced by benzo(a)pyrene in human keratinocytes. J. Cosmet. Dermatol. 2020, 19, 502–507. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Jiang, Y.; Li, X.; He, Y.; Zeng, P.; Guo, Z.; Chang, Y.; Luo, H.; Liu, Y.; et al. Lentinan as an immunotherapeutic for treating lung cancer: A review of 12 years clinical studies in China. J. Cancer Res. Clin. Oncol. 2018, 144, 2177–2186. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Parola, S.; Chiodaroli, L.; Orlandi, V.; Vannin, C.; Panno, L. Lentinula edodes and Pleurotus ostreatus: Functional food with antioxidant—Antimicrobial activity and an important source of Vitamin D and medicinal compounds. Funct. Foods Health Dis. 2017, 7, 773. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Zhou, Z.; Zhang, L. An overview of fungal glycan-based therapeutics. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 135–163. [Google Scholar]
- Fujimoto, K.; Tomonaga, M.; Goto, S. A case of recurrent ovarian cancer successfully treated with adoptive immunotherapy and lentinan. Anticancer Res. 2006, 26, 4015–4018. [Google Scholar] [PubMed]
- Wang, Y.; Jin, H.; Yu, J.; Qu, C.; Wang, Q.; Yang, S.; Ma, S.; Ni, J. Quality control and immunological activity of lentinan samples produced in China. Int. J. Biol. Macromol. 2020, 159, 129–136. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives (review). Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Peng, H.; Dong, L.; Chen, L.; Ma, X.; Peng, Y.; Dai, S.; Liu, Q. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int. Immunopharmacol. 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Markova, N.; Michailova, L.; Kussovski, V.; Jourdanova, M.; Radoucheva, T. Intranasal application of lentinan enhances bactericidal activity of rat alveolar macrophages against Mycobacterium tuberculosis. Pharmazie 2005, 60, 42–48. [Google Scholar]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int. Immunopharmacol. 2006, 6, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Drandarska, I.; Kussovski, V.; Nikolaeva, S.; Markova, N. Combined immunomodulating effects of BCG and Lentinan after intranasal application in guinea pigs. Int. Immunopharmacol. 2005, 5, 795–803. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Y.; Wang, D.; Ma, X.; Zhao, X.; Zhao, B.; Wang, J.; Liu, P. Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine 2009, 27, 660–665. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Q.; Zhang, Y.; Liu, J.; Cao, Y. The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses. Int. Immunopharmacol. 2009, 9, 455–462. [Google Scholar] [CrossRef]
- Gordon, M.; Guralnik, M.; Kaneko, Y.; Mimura, T.; Goodgame, J.; DeMarzo, C.; Pierce, D.; Baker, M.; Lang, W. A phase II controlled study of a combination of the immune modulator, lentinan, with didanosine (DDI) in HIV patients with CD4 cells of 200–500/MM3. J. Med. 1995, 26, 193–207. [Google Scholar]
- Gordon, M.; Bihari, B.; Goosby, E.; Gorter, R.; Greco, M.; Guralnik, M.; Mimura, T.; Rudinicki, V.; Wong, R.; Kaneko, Y. A placebo-controlled trial of the immune modulator, lentinan, in HIV- positive patients: A phase I/II trial. J. Med. 1998, 29, 305–330. [Google Scholar]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef]
- Pasnik, J.; Ślemp, A.; Cywinska-Bernas, A.; Zeman, K.; Jesenak, M. Preventive effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections—Open-label prospective study. Curr. Pediatr. Res. 2017, 21, 99–104. [Google Scholar]
- Urbancikova, I.; Hudackova, D.; Majtan, J.; Rennerova, Z.; Banovcin, P.; Jesenak, M. Efficacy of pleuran (β -glucan from Pleurotus ostreatus) in the management of Herpes simplex virus type 1 infection. Evid. Based Complement. Altern. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydrates Diet. Fibre 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Sung, K.H.; Josewski, J.; Dübel, S.; Blankenfeldt, W.; Rau, U. Structural insights into antigen recognition of an anti-β-(1,6)-β-(1,3)-D-glucan antibody. Sci. Rep. 2018, 8, 13652. [Google Scholar] [CrossRef]
- Leathers, T.D.; Nunnally, M.S.; Price, N.P. Co-production of schizophyllan and arabinoxylan from corn fiber. Biotechnol. Lett. 2006, 28, 623–626. [Google Scholar] [CrossRef]
- Scarpari, M.; Reverberi, M.; Parroni, A.; Scala, V.; Fanelli, C.; Pietricola, C.; Zjalic, S.; Maresca, V.; Tafuri, A.; Ricciardi, M.R.; et al. Tramesan, a novel polysaccharide from Trametes versicolor. Structural characterization and biological effects. PLoS ONE 2017, 12, e0171412. [Google Scholar] [CrossRef] [Green Version]
- Lakhanpal, T.N.; Rana, M. Medicinal and nutraceutical genetic resources of mushrooms. Plant Genet. Resour. 2005, 3, 288–303. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical antileukemia activity of Tramesan: A newly identified bioactive fungal metabolite. Oxid. Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Loncar, J.; Bellich, B.; Parroni, A.; Reverberi, M.; Rizzo, R.; Zjalić, S.; Cescutti, P. Oligosaccharides derived from Tramesan: Their structure and activity on mycotoxin inhibition in Aspergillus flavus and Aspergillus carbonarius. Biomolecules 2021, 11, 243. [Google Scholar] [CrossRef]
- Scala, V.; Pietricola, C.; Farina, V.; Beccaccioli, M.; Zjalic, S.; Quaranta, F.; Fornara, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; et al. Tramesan elicits durum wheat defense against the Septoria disease complex. Biomolecules 2020, 10, 608. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Reddy, G.; Reddy, B.; Shekar, P.; Sumanthi, J.; Chandra, K.P. Biological role of lectins: A review. J. Orofac. Sci. 2012, 4, 20. [Google Scholar] [CrossRef]
- Liu, Q.; Ng, T.; Wang, H. Isolation and characterization of a novel lectin from the wild mushroom Oudemansiella radicata (Relhan.: Fr.) sing. Biotechnol. Bioprocess Eng. 2013, 18, 465–471. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumaran, S.; Pandurangan, A.K.; Shenbhagaraman, R.; Esa, N.M. Isolation and characterization of lectin from the artist’s conk medicinal mushroom, Ganoderma applanatum (Agaricomycetes), and evaluation of its antiproliferative activity in HT-29 colon cancer cells. Int. J. Med. Mushrooms 2017, 19, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ditamo, Y.; Rupil, L.L.; Sendra, V.G.; Nores, G.A.; Roth, G.A.; Irazoqui, F.J. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 2016, 7, 262–269. [Google Scholar] [CrossRef]
- He, M.; Su, D.; Liu, Q.; Gao, W.; Kang, Y. Mushroom lectin overcomes hepatitis B virus tolerance via TLR6 signaling. Sci. Rep. 2017, 7, 5814. [Google Scholar] [CrossRef] [Green Version]
- Kawagishi, H.; Suzuki, H.; Watanabe, H.; Nakamura, H.; Sekiguchi, T.; Murata, T.; Usui, T.; Sugiyama, K.; Suganuma, H.; Inakuma, T.; et al. A lectin from an edible mushroom Pleurotus ostreatus as a food intake-suppressing substance. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 299–308. [Google Scholar] [CrossRef]
- Ismaya, W.T. In silico study to develop a lectin-like protein from mushroom Agaricus bisporus for pharmaceutical application. Sci. Pharm. 2016, 84, 203–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Wang, J.; Zhou, S.; Wang, Y.; Cai, M.; Yu, L.; Tang, Q. A study on the antioxidant properties and stability of ergothioneine from culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2020, 22, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, I.K.; Halliwell, B. Could ergothioneine aid in the treatment of coronavirus patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B. Flammulin: A novel ribosome-inactivating protein from fruiting bodies of the winter mushroom Flammulina velutipes. Biochem. Cell Biol. 2000, 78, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Chen, Y.; Gong, M.; Zhang, C. Crystallization and some characterization of flammulin purified from the fruit bodies of Flammulina velutipes. Bioresour. Technol. 1998, 64, 153–156. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakanishi, K.; Komatsu, N.; Sakabe, T.; Terakawa, H. Flammulin, an antitumor substance. Bull. Chem. Soc. Jpn. 1964, 37, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, N.; Terakawa, H.; Nakanishi, K.; Watanabe, Y. Flammulin, a basic protein of Flammulina velutipes with antitumor activities. J. Antibiot. 1963, 16. [Google Scholar] [CrossRef]
- Ng, T.B.; Ngai, P.H.K.; Xia, L. An agglutinin with mitogenic and antiproliferative activities from the mushroom Flammulina velutipes. Mycologia 2006, 98, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [Green Version]
- Nallathamby, N.; Malek, S.N.A.; Vidyadaran, S.; Phan, C.-W.; Sabaratnam, V. Lipids in an ethyl acetate fraction of caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), reduce nitric oxide production in BV2 cells via NRF2 and NF-κB pathways. Int. J. Med. Mushrooms 2020, 22, 1215–1223. [Google Scholar] [CrossRef]
- Phan, C.W.; Wong, W.L.; David, P.; Naidu, M.; Sabaratnam, V. Pleurotus giganteus (Berk.) Karunarathna & K.D. Hyde: Nutritional value and in vitro neurite outgrowth activity in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.W.; Lee, G.S.; Macreadie, I.G.; Malek, S.N.A.; Pamela, D.; Sabaratnam, V. Lipid constituents of the edible mushroom, Pleurotus giganteus demonstrate anti-Candida activity. Nat. Prod. Commun. 2013, 8, 1763–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a novel methodology for the analysis of ergosterol in mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamzivar, F.; Shams-Ghahfarokhi, M.; Khoramizadeh, M.; Yousefi, N.; Gholami-Shabani, M. Unraveling the importance of molecules of natural origin in antifungal drug development through targeting ergosterol biosynthesis pathway. Iran. J. Microbiol. 2020, 11, 448–459. [Google Scholar] [CrossRef]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O.; Sablani, S.S. Kinetics of the conversion of ergosterol in edible mushrooms. J. Food Eng. 2007, 79, 864–869. [Google Scholar] [CrossRef]
- Diallo, I.; Morel, S.; Vitou, M.; Michel, A.; Rapior, S.; Traoré, L.; Poucheret, P.; Fons, F. Ergosterol and amino acids contents of culinary-medicinal Shiitake from various culture conditions. Proceedings 2020, 70, 78. [Google Scholar] [CrossRef]
- Shimizu, T.; Kawai, J.; Ouchi, K.; Kikuchi, H.; Osima, Y.; Hidemi, R. Agarol, an ergosterol derivative from Agaricus blazei, induces caspase-independent apoptosis in human cancer cells. Int. J. Oncol. 2016, 48, 1670–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Bernas, E.; Jaworska, G.; Lisiewska, Z. Edible mushrooms as a source of valuable nutritive constituents. Acta Sci. Pol. Technol. Aliment. 2006, 5, 5–20. [Google Scholar]
- Pogoń, K.; Gabor, A.; Jaworska, G.; Bernaś, E. Effect of traditional canning in acetic brine on the antioxidants and vitamins in Boletus edulis and Suillus luteus mushrooms. J. Food Process. Preserv. 2017, 41, e12826. [Google Scholar] [CrossRef]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.; James, A.; Black, L. A review of mushrooms as a potential source of dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg, S. Molecular basis for differential action of vitamin D analogs. In Vitamin D; Academic Press: Burlington, VT, USA, 2005; Volume 2, pp. 1471–1488. [Google Scholar]
- El-Sharkawy, A.; Malki, A. Vitamin D signaling in inflammation and cancer: Molecular mechanisms and therapeutic implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. Mushrooms and agaritine: A mini-review. J. Funct. Foods 2010, 2, 91–98. [Google Scholar] [CrossRef]
- Hashida, C.; Hayashi, K.; Jie, L.; Haga, S.; Sakurai, M.; Shimizu, H. Quantities of agaritine in mushrooms (Agaricus bisporus) and the carcinogenicity of mushroom methanol extracts on the mouse bladder epithelium. [Nippon kōshū eisei zasshi] Jpn. J. Public Health 1990, 37, 400–405. [Google Scholar]
- Gao, W.N.; Wei, D.Q.; Li, Y.; Gao, H.; Xu, W.R.; Li, A.X.; Chou, K.C. Agaritine and its derivatives are potential inhibitors against HIV proteases. Med. Chem. 2007, 3, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Baig, M.T.; Jabeen, A.; Aslam, M.; Shahid, U. Therapeutic value of medicinal mushroom Agaricus blazei Murill. Pak. J. Med. Dent. 2021, 10, 83–89. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phyther. Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef]
- Qin, P.; Li, X.; Yang, H.; Wang, Z.-Y.; Lu, D. Therapeutic potential and biological applications of cordycepin and metabolic mechanisms in cordycepin-producing fungi. Molecules 2019, 24, 2231. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Meng, X.; Qiu, Z.; Su, Y.; Yu, P.; Qu, P. Anti-tumor and anti-metastatic roles of cordycepin, one bioactive compound of Cordyceps militaris. Saudi J. Biol. Sci. 2018, 25, 991–995. [Google Scholar] [CrossRef]
- Yoon, S.; Park, S.; Park, Y. The anticancer properties of cordycepin and their underlying mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Li, Y.; Wang, X.; Chen, Z.; Xu, H.; Wu, L.; Li, S.; Wang, C.; Luan, W.; Wang, X.; et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Health Dis. 2018, 17, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Lee, W.; Jung, K.; Kwon, Y.; Kim, D.; Hwang, G.; Kim, C.; Lee, S.; Kang, K.S. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism: An investigation using network pharmacology-based analysis. Biomolecules 2019, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of metabolites in Italian Hericium erinaceus mycelium, primordium, and sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mong, M.C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P.C. Prevention of early Alzheimer’s disease by erinacine A-enriched Hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Ren, L. Protective effect of ganoderic acid against the streptozotocin induced diabetes, inflammation, hyperlipidemia and microbiota imbalance in diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1961–1972. [Google Scholar] [CrossRef]
- Gill, B.S.; Navgeet; Kumar, S. Antioxidant potential of ganoderic acid in Notch-1 protein in neuroblastoma. Mol. Cell. Biochem. 2019, 456. [Google Scholar] [CrossRef]
- Hiraki, E.; Furuta, S.; Kuwahara, R.; Takemoto, N.; Nagata, T.; Akasaka, T.; Shirouchi, B.; Sato, M.; Ohnuki, K.; Shimizu, K. Anti-obesity activity of Yamabushitake (Hericium erinaceus) powder in ovariectomized mice, and its potentially active compounds. J. Nat. Med. 2017, 71, 482–491. [Google Scholar] [CrossRef]
- Mori, K.; Kikuchi, H.; Obara, Y.; Iwashita, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Oshima, Y.; Nakahata, N. Inhibitory effect of hericenone B from Hericium erinaceus on collagen-induced platelet aggregation. Phytomedicine 2010, 17, 1082–1085. [Google Scholar] [CrossRef]
- Boruta, T.; Bizukojc, M. Production of lovastatin and itaconic acid by Aspergillus terreus: A comparative perspective. World J. Microbiol. Biotechnol. 2017, 33, 34. [Google Scholar] [CrossRef] [Green Version]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Chyuan, I.; Shiue, C.; Yu, M.; Hsu, Y.; Hsu, M. Lovastatin-mediated MCF-7 cancer cell death involves LKB1-AMPK-p38MAPK-p53-survivin signalling cascade. J. Cell. Mol. Med. 2020, 24, 1822–1836. [Google Scholar] [CrossRef]
- Bardeleben, R.; Dunkern, T.; Kaina, B.; Fritz, G. The HMG-CoA reductase inhibitor lovastatin protects cells from the antineoplastic drugs doxorubicin and etoposide. Int. J. Mol. Med. 2002, 10, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Zambón, D.; Ros, E.; Rodriguez-Villar, C.; Laguna, J.C.; Vázquez, M.; Sanllehy, C.; Casals, E.; Sol, J.M.; Hernández, G. Randomized crossover study of gemfibrozil versus lovastatin in familial combined hyperlipidemia: Additive effects of combination treatment on lipid regulation. Metabolism 1999, 48, 47–54. [Google Scholar] [CrossRef]
- Ali, M.E.; Nizar, N.N.A. Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081018927. [Google Scholar]
- PMMI Business Intelligence 2019 Nutraceuticals Market Assessment. Available online: https://www.pmmi.org/report/2019-nutraceuticals-market-assessment (accessed on 10 March 2021).
- Chrzan, J. The Global Market for Nutraceuticals Set for Robust Growth. Available online: https://www.healthcarepackaging.com/markets/neutraceuticals-functional/article/13296428/the-global-market-for-nutraceuticals-set-for-robust-growth (accessed on 16 March 2021).
- Datam Intelligence Edible Mushrooms Market, Size, Share, Opportunities and Forecast, 2020–2027. Available online: https://www.datamintelligence.com/research-report/edible-mushrooms-market (accessed on 18 March 2021).
- Market Data Forecast Asia Pacific Edible Mushroom Market. Available online: https://www.marketdataforecast.com/market-reports/apac-edible-mushroom-market (accessed on 18 March 2021).
- Markets and Markets Dietary Supplements Market. Available online: https://www.marketsandmarkets.com/Market-Reports/dietary-supplements-market-973.html (accessed on 18 March 2021).
- Badalyan, S.M. Potential of mushroom bioactive molecules to develop healthcare biotech products. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products, New Delhi, India, 19–22 November 2014; pp. 373–378. [Google Scholar]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Industrial production and applications of α/β linear and branched glucans. Indian Chem. Eng. 2020, 1–15. [Google Scholar] [CrossRef]
- Coherent Market Insights Cordyceps sinensis and militaris Extract Market Analysis. Available online: https://www.coherentmarketinsights.com/market-insight/cordyceps-sinensis-and-militaris-extract-market-2578 (accessed on 18 March 2021).
- Market Watch Global Lentinan Market 2021 Analysis with Key Players, Applications, Trends and Forecasts by 2027. Available online: https://www.marketwatch.com/press-release/global-lentinan-market-2021-analysis-with-key-players-applications-trends-and-forecasts-by-2027-2021-03-09 (accessed on 18 March 2021).
- Research and Markets Statin Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020–2025. Available online: https://www.researchandmarkets.com/reports/5009067/statin-market-global-industry-trends-share?utm_source=dynamic&utm_medium=BW&utm_code=bmkdw4&utm_campaign=1375328+-+Statin+Market+Insights+and+Trends%2C+2020-2025%3A+Atorvastatin%2C+Fluvastatin%2C+Lovastat (accessed on 20 March 2021).
- Globe Newswire the Global Functional Food Market is Forecasted to Reach USD 34.3 Billion by 2024, Growing at a CAGR of 8.04% during the Forecast Period (2019 2024). Available online: http://www.globenewswire.com/news-release/2020/01/13/1969374/0/en/The-global-functional-food-market-is-forecasted-to-reach-USD-34-3-billion-by-2024-growing-at-a-CAGR-of-8-04-during-the-forecast-period-2019.html (accessed on 16 March 2021).
- Persistence Market Research Medicinal Mushrooms Market: Global Industry Trend Analysis 2013 to 2017 and Forecast 2018–2028. Available online: https://www.persistencemarketresearch.com/market-research/medicinal-mushrooms-market.asp (accessed on 10 March 2021).

| Species | Moisture (g/100 g FW or DW) | Ash (g/100 g DW) | Proteins (g/100 g DW) | Fat (g/100 g DW) | Carbohydrates (g/100 g DW) | References |
|---|---|---|---|---|---|---|
| Agaricus bisporus | 90.09 ± 0.07 (FW) | 9.17 ± 0.52 | 24.43 ± 0.10 | 3.06 ± 0.03 | 53.10 ± 0.56 | [38] |
| Agaricus brasiliensis | 6.5 ± 0.11 (DW) | 8.9 ± 0.09 | 37.3 ±0.22 | 9 2.4 ± 0.03 | 44.9 ± 2.5 | [39] |
| Agaricus campestris | 88.17 ± 0.44 (FW) | 23.16 ± 0.00 | 18.57 ± 0.00 | 0.11 ± 0.00 | 58.16 ± 0.00 | [40] |
| Agaricus comtulus | 87.94 ± 0.77 (FW) | 28.14 ± 0.18 | 21.29 ± 0.83 | 0.46 ± 0.00 | 50.11 ± 0.89 | [40] |
| Amanita battarrae (as A. umbrinolutea) | 73.60 ± 0.17 (FW) | 28.86 ± 0.00 | 16.78 ± 0.00 | 6.77 ± 0.00 | 47.59 ± 0.00 | [40] |
| Amanita caesarea | - | 6.05 ± 0.01 | 34.77 ± 0.06 | 3.50 ± 0.00 | 55.63 ± 0.06 | [41] |
| Armillaria mellea | 88.27 ± 0.60 (FW) | 6.78 ± 1.28 | 16.38 ± 1.34 | 5.56 ± 0.53 | 71.28 ± 1.06 | [42] |
| Armillaria mellea | - | 7.95 ± 0.02 | 24.47 ± 0.12 | 2.10 ± 0.02 | 65.47 ± 0.15 | [41] |
| Armillaria tabescens | - | 7.63 ± 0.15 | 22.90 ± 0.20 | 2.54 ± 0.03 | 66.87 ± 0.06 | [41] |
| Auricularia auricula-judae | 88.9 ± 0.02 (FW) | 3.15 ± 0.3 | 56.92 ± 0.01 | - | 18.67 ± 0.01 | [43] |
| Auricularia nigricans (as A. polytricha) | 82.01 ± 0.04 (FW) | 8.44 ± 0.8 | 42 ± 0.02 | - | 16.03 ± 0.02 | [43] |
| Auricularia thailandica | 80.75 ± 0.20 (FW) | 4.30 ± 0.02 | 12.99 ± 0.05 | 2.93 ± 0.66 | - | [44] |
| Boletus aereus | 91.65 ± 1.04 (FW) | 8.87 ± 0.10 | 17.86 ± 0.96 | 0.44 ± 0.08 | 72.83 ± 0.90 | [45] |
| Boletus aureus | - | 6.25 ± 0.02 | 27.17 ± 0.15 | 4.47 ± 0.02 | 62.10 ± 0.10 | [41] |
| Boletus edulis | 89.15 ± 0.90 (FW) | 5.53 ± 0.23 | 21.07 ± 0.66 | 2.45 ± 0.09 | 70.96 ± 0.66 | [45] |
| Boletus fragrans | 77.99 ± 0.07 (FW) | 4.74 ± 0.19 | 17.15 ± 0.04 | 1.83 ± 0.17 | 76.29 ± 0.27 | [46] |
| Boletus reticulatus | 91.10 ± 2.21 (FW) | 19.72 ± 0.25 | 22.57 ± 2.08 | 2.55 ± 0.01 | 55.16 ± 2.03 | [45] |
| Bovista aestivalis | 73.23 ± 0.93 (FW) | 31.86 ± 0.20 | 15.59 ± 1.23 | 0.18 ± 0.02 | 52.37 ± 1.31 | [40] |
| Bovista nigrescens | 76.41 ± 0.18 (FW) | 3.24 ± 0.17 | 20.94 ± 0.31 | 3.64 ± 0.96 | 72.18 ± 0.76 | [40] |
| Bovistella utriformis (as Calvatia utriformis) | 78.00 ± 1.36 (FW) | 17.81 ± 0.22 | 20.37 ± 0.49 | 1.90 ± 0.01 | 59.91 ± 0.40 | [46] |
| Calocybe gambosa | 90.92 ± 1.08 (FW) | 13.89 ± 1.41 | 15.46 ± 0.24 | 0.83 ± 0.11 | 69.83 ± 1.22 | [42] |
| Cantharellus cibarius | - | 9.44 ± 0.01 | 21.57 ± 0.21 | 2.88 ± 0.02 | 66.07 ± 0.23 | [41] |
| Chlorophyllum rhacodes | 88.28 ± 0.33 (FW) | 12.10 ± 0.31 | 19.32 ± 0.04 | 3.29 ± 0.33 | 65.29 ± 0.48 | [40] |
| Clavariadelphus pistillaris | 84.22 ± 1.78 (FW) | 20.77 ± 0.86 | 16.27 ± 0.24 | 0.59 ± 0.07 | 62.37 ± 0.48 | [40] |
| Clavariadelphus truncatus | 90.97 ± 1.29 (FW) | 12.86 ± 0.33 | 15.98 ± 0.15 | 1.54 ± 0.25 | 69.62 ± 0.37 | [40] |
| Clitocybe costata | 76.92 ± 2.11 (FW) | 10.87 ± 1.36 | 17.27 ± 0.25 | 1.50 ± 0.00 | 70.36 ± 1.10 | [40] |
| Clitocybe odora | 88.49 ± 3.03 (FW) | 9.55 ± 0.68 | 17.33 ± 1.37 | 2.46 ± 0.04 | 70.66 ± 1.09 | [42] |
| Clitopilus prunulus | 89.78 ± 1.46 (FW) | 30.19 ± 2.50 | 18.13 ± 0.37 | 1.01 ± 0.06 | 50.66 ± 2.21 | [46] |
| Coprinus comatus | 85.19 ± 0.50 (FW) | 12.85 ± 0.42 | 15.67 ± 0.23 | 1.13 ± 0.05 | 70.36 ± 0.26 | [42] |
| Coprinus comatus | 4.2 ± 0.06 (DW) | 13.2 ± 0.42 | 22.7 ± 0.37 | 1.3 ± 0.02 | 58.6 ± 5.1 | [39] |
| Cordyceps militaris | 7.7 ± 0.61 (DW) | 5.4 ± 0.16 | 29.7 ± 0.42 | 2.9 ± 0.18 | 54.3 ± 5.5 | [39] |
| Fistulina hepatica | - | 8.2 ± 0.10 | 22.6 ± 0.20 | 3.17 ± 0.02 | 66.0 ± 0.10 | [41] |
| Flammulina velutipes | 5.0 ± 0.13 (DW) | 8.3 ± 0.08 | 23.4 ± 0.19 | 2.1 ± 0.10 | 61.2 ± 4.3 | [39] |
| Ganoderma lingzhi/G. sichuanense (as Ganoderma lucidum) | 5.1 ± 0.16 (DW) | 1.0 ± 0.00 | 9.2 ± 0.32 | 1.1 ± 0.01 | 83.6 ± 4.4 | [39] |
| Grifola frondosa | 4.8 ± 0.08 (DW) | 4.7 ± 0.07 | 18.3 ± 0.34 | 5.3 ± 0.09 | 66.9 ± 8.4 | [39] |
| Hemileccinum impolitum (as Boletus impolitus) | 88.90 ± 1.45 (FW) | 24.43 ± 0.84 | 16.01 ± 0.02 | 2.94 ± 0.33 | 56.63 ± 0.84 | [40] |
| Hericium erinaceus | 6.2 ± 0.14 (DW) | 6.8 ± 0.22 | 20.8 ± 0.43 | 5.1 ± 0.11 | 61.1 ± 3.6 | [39] |
| Hortiboletus engelii (as Boletus armeniacus) | 71.50 ± 0.43 (FW) | 12.09 ± 0.35 | 18.25 ± 0.06 | 1.56 ± 0.42 | 68.10 ± 0.51 | [40] |
| Hygrophorus chrysodon | 92.09 ± 1.01 (FW) | 26.91 ± 1.99 | 15.11 ± 0.18 | 3.48 ± 0.09 | 54.51 ± 1.28 | [40] |
| Hygrophorus pustulatus | 93.03 ± 0.79 (FW) | 14.04 ± 0.14 | 18.64 ± 0.40 | 3.06 ± 0.51 | 64.26 ± 0.72 | [46] |
| Hygrophorus russula | - | 8.18 ± 0.02 | 32.47 ± 0.06 | 6.00 ± 0.10 | 53.33 ± 0.06 | [41] |
| Infundibulicybe gibba (as Clitocybe gibba) | 72.66 ± 0.99 (FW) | 20.68 ± 0.15 | 14.59 ± 0.27 | 4.29 ± 0.00 | 60.45 ± 0.23 | [40] |
| Lactifluus piperatus | 80.03 ± 0.02 (FW) | 5.38 ± 0.6 | 19.33 ± 0.02 | - | 9.2 ± 0.07 | [43] |
| Laetiporus sulphureus | 49.8 ± 0.02 (FW) | 4.81 ± 0.5 | 22.73 ± 0.01 | - | 7.65 ± 0.01 | [43] |
| Lentinula edodes | 7.3 ± 0.10 (DW) | 5.1 ± 0.05 | 18.5 ± 0.16 | 0.8 ± 0.01 | 68.3 ± 4.7 | [39] |
| Lentinula edodes | 82.8 ± 0.01 (FW) | 5.59 ± 0.3 | 43.81 ± 0.02 | - | 38.44 ± 0.01 | [43] |
| Lentinus sajor-caju | 85.1 ± 0.02 (FW) | 8.41 ± 0.2 | 62.27 ± 0.02 | - | 6.81 ± 0.01 | [43] |
| Lentinus sajor-caju (as Pleurotus sajor-caju) | 89.58 ± 0.19 (FW) | 7.46 ± 0.30 | 25.65 ± 0.05 | 1.96 ± 0.12 | 52.46 ± 0.43 | [38] |
| Lentinus squarrosulus | 87.3 ± 0.02 (FW) | 10.66 ± 0.4 | 27.86 ± 0.01 | - | 9.32 ± 0.01 | [43] |
| Lentinus squarrosulus var. squarrosulus | 86.2 ± 0.01 (FW) | 3.12 ± 0.2 | 18.77 ± 0.02 | - | 19.14 ± 0.01 | [43] |
| Lentinus tigrinus | 73.7 ± 0.04 (FW) | 3.41 ± 0.2 | 31.85 ± 0.03 | - | 16.09 ± 0.3 | [43] |
| Lepista nuda | - | 6.03 ± 0.02 | 34.37 ± 0.15 | 3.23 ± 0.01 | 56.33 ± 0.15 | [41] |
| Leucoagaricus leucothites | 85.29 ± 1.00 (FW) | 26.46 ± 0.01 | 20.51 ± 0.47 | 1.10 ± 0.15 | 51.93 ± 0.53 | [40] |
| Lycoperdon echinatum | 85.24 ± 0.48 (FW) | 9.43± 0.23 | 23.52 ± 2.20 | 1.22 ± 0.20 | 65.83 ± 2.09 | [46] |
| Lycoperdon umbrinum | 71.98 ± 0.32 (FW) | 33.14 ± 1.06 | 14.53 ± 0.07 | 0.37 ± 0.00 | 51.96 ± 0.70 | [40] |
| Lyophyllum decastes | 87.38 ± 1.40 (FW) | 7.38 ± 0.64 | 25.52 ± 3.49 | 2.10± 0.12 | 64.99 ± 2.96 | [46] |
| Macrolepiota excoriata | 88.92 ± 1.57 (FW) | 28.98 ± 1.11 | 25.28 ± 2.64 | 1.55 ± 0.10 | 44.19 ± 2.14 | [46] |
| Morchella esculenta (as Morchella conica) | - | 14.6 ± 0.30 | 7.5 ± 0.40 | 2.8 ± 0.10 | 75.0 ± 0.40 | [47] |
| Neoboletus erythropus (as Boletus erythropus) | 88.36 ± 1.49 (FW) | 25.90 ± 0.28 | 20.92 ± 0.05 | 0.75 ± 0.02 | 52.44 ± 0.20 | [46] |
| Pleurotus ostreatus | 8.2 ± 0.07 (DW) | 7.1 ± 0.06 | 33.5 ± 0.22 | 2.3 ± 0.07 | 48.9 ± 2.7 | [39] |
| Ramaria aurea | 88.52 ± 0.12 (FW) | 5.68 ± 0.74 | 14.60 ± 0.10 | 2.26 ± 0.05 | 77.47 ± 0.61 | [40] |
| Ramaria largentii | - | 6.67 ± 0.12 | 28.80 ± 0.46 | 5.67 ± 0.12 | 58.87 ± 0.25 | [41] |
| Russula cyanoxantha | 85.44 ± 0.99 (FW) | 7.03 ± 0.87 | 16.80± 0.06 | 1.52 ± 0.52 | 74.65 ±1.01 | [46] |
| Russula delica | - | 5.61 ± 0.03 | 26.10 ± 0.30 | 4.44 ± 0.04 | 63.87 ± 0.31 | [41] |
| Russula olivacea | 84.58 ± 1.01 (FW) | 37.78 ± 5.20 | 16.84 ± 0.05 | 1.99 ± 0.44 | 43.38 ± 3.71 | [46] |
| Schizophyllum commune | 69.8 ± 0.02 (FW) | 6.02 ± 0.6 | 24.42 ± 0.02 | - | 5.31 ± 0.01 | [43] |
| Suillus variegatus | 90.77 ± 0.76 (FW) | 15.36 ± 2.10 | 17.57 ± 0.56 | 3.31 ± 0.49 | 63.76 ± 2.17 | [40] |
| Termitomyces heimii | 81.1 ± 0.02 (FW) | 5.66 ± 0.02 | 60.53 ± 0.01 | - | 22.74 ± 0.01 | [43] |
| Tremella fuciformis | 5.5 ± 0.18 (DW) | 6.5 ± 0.14 | 13.0 ± 0.12 | 2.1 ± 0.08 | 72.9 ± 6.4 | [39] |
| Mushroom Species | Name of Fraction(s) | Bioactivity | Target Cells/Experimental Subjects | References |
|---|---|---|---|---|
| Agaricus bisporus | Agaricus bisporus neutral polysaccharides (Abnp1001 and Abnp1002) and Agaricus bisporus all polysaccharides (Abap1001, and Abap1002) | Hepato-protective activity | CCl4-induced hepatic injury in mice | [7] |
| AlAPS and their three purified fractions (AlAPS-1, AlAPS-2, and AlAPS-3) | Antiaging, antioxidant, and hepatoprotective effects, prevent age-related diseases | Fresh liver and blood samples of male Kunming strain mice | [66] | |
| Mannogalactoglucan polysaccharide | Antitumor activity (lung cancer) | Human hepatocarcinoma cells (HepG2) | [67] | |
| AcAPS and its major purified fractions (AcAPS-1, AcAPS-2 and AcAPS-3) | Antiaging and antioxidant effects | Fresh liver and kidney samples of male Kunming strain mice | [68] | |
| Agaricus bisporus fruiting body polysaccharide (FPS) | Hepato-protective activity | CCl4-induced liver injury in mice | [69] | |
| Glucogalactomanan polysaccharide TJ3 | Immunostimulatory activity | RAW 264.7 cells | [70] | |
| Ganoderma lingzhi/G. sichuanense (as Ganoderma lucidum) | Ganoderma lucidum polysaccharides (GLP) | Immunomodulatory effect | Mice immunized with GLPL/OVA | [71] |
| GLP | Antitumor activity (colorectal cancer) | Colorectal cancer HT29 (p53R273H) and SW480 (p53R273H&P309S) cells | [72] | |
| GLP | Neuroprotective effects | Rat cerebellar granule cells (CGCs) | [73] | |
| GLP | Anticancer activity (prostate cancer) | Human prostate cancer cells LNCaP | [74] | |
| GLP | Antitumor (brain glioma) and immunomodulatory activities | Glioma-bearing rats | [75] | |
| GLP | Hypoglycemic effect | Type 2 diabetes mellitus (T2DM) rats’ blood liver and skeletal muscles | [76] | |
| Degraded Ganoderma lucidum polysaccharides (GLPUD) | Hypolipidemic and antioxidant activities | Blood, heart, spleen, liver and kidney of male Kunming mice | [77] | |
| GLP | Antidiabetic activity | T2DM rats’ blood | [78] | |
| Grifola frondosa | Grifola frondosa polysaccharides (GFP) | Anticancer activity (breast cancer) | MCF-7 and MDA-MB-231 cells, as well as in nude mice bearing MCF-7 tumor xenografts. | [79] |
| GFP | Memory enhancement and antiaging activities | 20-month-old rats | [80] | |
| GFP-N | Hypoglycemic and prebiotic activities | Diabetic mouse livers | [81] | |
| GFP | Hypoglycemic and hypolipidemic activities | Diabetic mice induced by HFD and streptozotocin (STZ) | [82] | |
| Hericium erinaceus | Hydroxyethylated derivative of HEP | Immunomodulatory activities | RAW264.7 macrophages | [83] |
| Selenium derivatives (sHEPs) | Immunostimulant activity | Dendritic cells | [84] | |
| Hericium erinaceus crude polysaccharide (HECP) and Hericium erinaceus refined polysaccharide (HERP) | Gastroprotective activity | Sprague–Dawley rats’ stomach | [85] | |
| Novel Hericium erinaceus polysaccharide HEPN | Gastroprotective activity | Human gastric epithelium (GES-1) cells | [86] | |
| Hericium erinaceus fruiting body polysaccharide (HEFP)-2b | Anticancer activity (colon cancer) | Colon cancer cells (HCT-116) | [87] | |
| Enzymatic hydrolysis of Hericium erinaceus polysaccharide (EHEP) | Immune-enhancement activity | Female Balb/c mice | [88] | |
| Lentinula edodes | Mannogalactoglucan-type polysaccharides (WPLE-N-2 and WPLE-A0.5-2) | Anticancer and immunomodulating activities | Sarcoma 180-bearing mice | [64] |
| Myeloid-derived suppressor cells | Immunosuppressive effects | Immortalized myeloid immune suppressor cell line (MSC2) | [89] | |
| Lentinula edodes polysaccharide (LEP)1 | Antitumor activity | Human cervical carcinoma HeLa cells | [90] | |
| Residue polysaccharide (RPS) and its enzymatic-RPS (ERPS) | Antioxidant and anti-inflammatory activities | LPS-induced sepsis in mice | [91] | |
| LEP | Anticancer (colon cancer) | HT-29 colon cancer cells | [85] | |
| Acidic spent mushroom compost polysaccharides (ASMCP) | Antioxidant, anti-inflammatory and renoprotective effects | LPS-induced KI in mice | [92] | |
| Polysaccharide fractions (F1, F2 and F3) | Immunomodulatory effects | Female BALB/c mice | [93] | |
| Ophiocordyceps sinensis (as Cordyceps sinensis) | Cordyceps sinensis polysaccharide (CSP1-2) | Antihypertensive effect | Spontaneously hypertensive rats (SHR) | [94] |
| CPS-A | Protective effect | L02 cells | [95] | |
| CSP | Prebiotics | Cyclophosphamide (Cy)-induced intestinal mucosal immunosuppression and microbial dysbiosis in mice | [96,97] | |
| CSP | Anti-obesity | High-fat diet (HFD)-feeding C57BL/6J mice | [98] | |
| Docetaxel-loaded acetic acid conjugated Cordyceps sinensis polysaccharide (DTX-AA-CSP) | Drug carrier and anticancer (liver and colon cancers) | Human umbilical vein endothelial cells; human liver HepG2; colon cancer cells SW480 | [99] | |
| CSP | Anticancer activity (colon cancer) | Colon cancer cell line HCT116 | [100] | |
| Pleurotus eryngii | Pleurotus eryngii polysaccharides PEP-1 and PEP-2 | Antitumor | Human hepatoblastoma HepG-2 cells | [101] |
| Pleurotus eryngii polysaccharide (PEP) | Hypolipidemic and hypoglycemic activities | KK-Ay mice | [102] | |
| water-soluble polysaccharide EPA-1 | Immunoregulatory activity | RAW 264.7 cells | [103] | |
| PEP | Hypolipidemic effect | Mice with hyperlipidemia | [104] | |
| PEP | Neuroprotective effect | β-amyloid-induced neurotoxicity in cultured rat pheochromocytoma (PC12) cells | [105] | |
| Pleurotus ostreatus | Pleurotus ostreatus polysaccharide (POP) | Regulating dyslipidemia effect | STZ-induced diabetic rats | [106] |
| POP | Anticancer activity | Sarcoma 180 tumor cells | [107] | |
| POP | Regulating dyslipidemia effect | Fat-emulsion-induced hyperlipidemia rats | [108] | |
| POP | Anticancer (lymphoid cancer) | Murine lymphoid cancer cell line | [109] | |
| Selenium polysaccharide fraction (Se-POP-3) | Antitumor activity | Human cancer cell lines HepG2, MCF-7, SKOV3, HeLa, and PC-3 | [110] | |
| Phosphorylated Pleurotus ostreatus polysaccharide (PPOP) | Hepatoprotective effect | Carbon tetrachloride-induced liver injury in mice | [111] | |
| Trametes versicolor | Polysaccharopeptides PSPs-EH80 | Antioxidative effect | HaCaT cells | [112] |
| Trametes versicolor polysaccharide (TVP) | Anti-proliferative and anti-invasive effects | LoVo and HT-29 human colon cancer cells | [113] | |
| Intracellular polysaccharide extract of Trametes versicolor (IPTV) and extracellular polysaccharide extracts of T. versicolor (EPTV) | Antihyperlipidemic effects | HFD-induced hyperlipidemic mice | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. https://doi.org/10.3390/jof7050397
Niego AG, Rapior S, Thongklang N, Raspé O, Jaidee W, Lumyong S, Hyde KD. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. Journal of Fungi. 2021; 7(5):397. https://doi.org/10.3390/jof7050397
Chicago/Turabian StyleNiego, Allen Grace, Sylvie Rapior, Naritsada Thongklang, Olivier Raspé, Wuttichai Jaidee, Saisamorn Lumyong, and Kevin D. Hyde. 2021. "Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value" Journal of Fungi 7, no. 5: 397. https://doi.org/10.3390/jof7050397
APA StyleNiego, A. G., Rapior, S., Thongklang, N., Raspé, O., Jaidee, W., Lumyong, S., & Hyde, K. D. (2021). Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. Journal of Fungi, 7(5), 397. https://doi.org/10.3390/jof7050397








