The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tested Samples
2.2.1. Working Solutions, Calibration Curve, and Quality Control Samples
Evidentiary Samples
Biological Materials
2.2.2. Procedure
Evidentiary Samples
Biological Materials
2.2.3. Chromatographic and Spectrometric Conditions
2.2.4. Validation
Evidentiary Samples
Biological Materials
3. Results
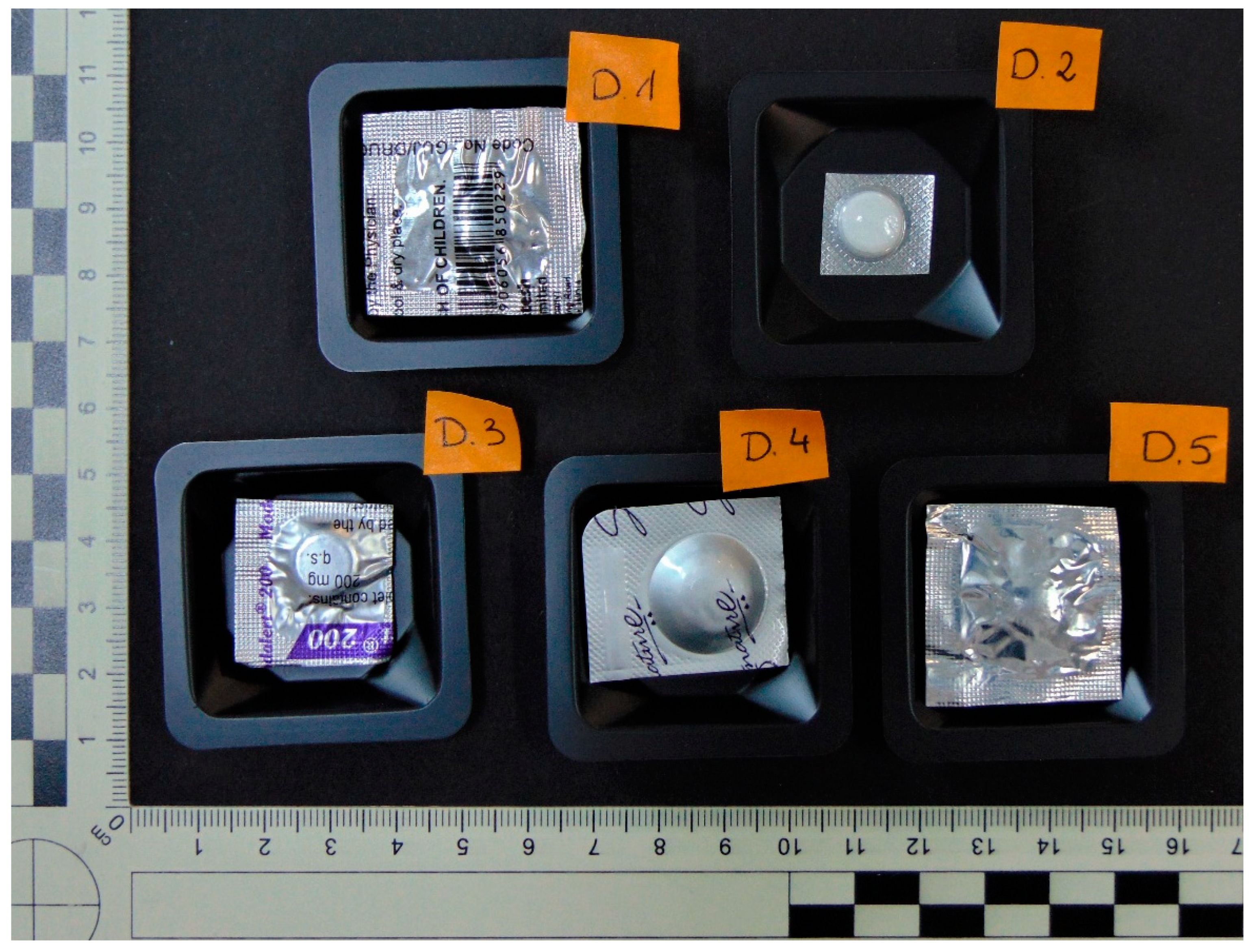
3.1. Evidentiary Samples
3.2. Biological Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Repantis, D.; Schlattmann, P.; Laisney, O.; Heuser, I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 2010, 62, 187–206. [Google Scholar]
- Settineri, S.; Gitto, L.; Conte, F.; Fanara, G.; Mallamace, D.; Mento, C.; Silvestri, R.; Tati, F.; Zoccali, R.; Cordici, F.; et al. Mood and sleep problems in adolescents and young adults: An econometric analysis. J. Ment. Health Policy Econ. 2012, 15, 33–41. [Google Scholar]
- Dresler, M.; Sandberg, A.; Bublitz, C.; Ohla, K.; Trenado, C.; Mroczko-Wasowicz, A.; Kühn, S.; Repantis, D. Hacking the brain: Dimensions of cognitive enhancement. ACS Chem. Neurosci. 2018, 10, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.J.; Illes, J.; Cook-Deegan, R.; Gardner, H.; Kandel, E.; King, P.; Parens, E.; Sahakian, B.; Wolpe, P.R. Neurocognitive enhancement: What can we do and what should we do? Nat. Rev. Neurosci. 2004, 5, 421–425. [Google Scholar] [CrossRef]
- Smith, M.E.; Farah, M.J. Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol. Bull. 2011, 137, 717–741. [Google Scholar] [PubMed]
- Maier, L.J.; Liakoni, E.; Schildmann, J.; Schaub, M.P.; Liechti, M.E. Swiss university students’ attitudes toward pharmacological cognitive enhancement. PLoS ONE 2015, 10, e0144402. [Google Scholar]
- Ferré, S. An update on the mechanisms of the psychostimulant effects of caffeine. J. Neurochem. 2008, 105, 1067–1079. [Google Scholar] [PubMed]
- Battleday, R.M.; Brem, A.K. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. Eur. Neuropsychopharmacol. 2015, 25, 1865–1881. [Google Scholar] [CrossRef]
- Compton, W.M.; Han, B.; Blanco, C.; Johnson, K.; Jones, C.M. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am. J. Psychiatry 2018, 175, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, B.J.; Bruhl, A.B.; Cook, J.; Killikelly, C.; Savulich, G.; Piercy, T.; Hafizi, S.; Perez, J.; Fernandez-Egea, E.; Suckling, J.; et al. The impact of neuroscience on society: Cognitive enhancement in neuropsychiatric disorders and in healthy people. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140214. [Google Scholar] [CrossRef]
- Franke, A.G.; Bonertz, C.; Christmann, M.; Huss, M.; Fellgiebel, A.; Hildt, E.; Lieb, K. Nonmedical use of prescription stimulants and illicit use of stimulants for cognitive enhancement in pupils and students in Germany. Pharmacopsychiatry 2011, 44, 60–66. [Google Scholar] [CrossRef] [PubMed]
- McCabe, S.E.; Knight, J.R.; Teter, C.J.; Wechsler, H. Non-medical use of prescription stimulants among US college students: Prevalence and correlates from a national survey. Addiction 2005, 100, 96–106. [Google Scholar] [CrossRef]
- Wang, Y.; Cottler, L.B.; Striley, C.W. Differentiating patterns of prescription stimulant medical and nonmedical use among youth 10–18 years of age. Drug. Alcohol. Depend. 2015, 157, 83–89. [Google Scholar] [CrossRef]
- Zullig, K.J.; Divin, A.L.; Weiler, R.M.; Haddox, J.D.; Pealer, L.N. Adolescent nonmedical use of prescription pain relievers, stimulants, and depressants, and suicide risk. Subst. Use Misuse 2015, 50, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.; Flory, K.; Humphreys, K.L.; Lee, S.S. Misuse of stimulant medication among college students: A comprehensive review and meta-analysis. Clin. Child. Fam. Psychol. Rev. 2015, 18, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Sumstine, S.; Mendez, J.; Bavarian, N. Health-compromising practices of undergraduate college students: Examining racial/ethnic and gender differences in characteristics of prescription stimulant misuse. Addict. Behav. 2017, 68, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Vrecko, S. Everyday drug diversions: A qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc. Sci. Med. 2015, 131, 297–304. [Google Scholar] [CrossRef]
- Wilens, T.E.; Adler, L.A.; Adams, J.; Sgambati, S.; Rotrosen, J.; Sawtelle, R.; Utzinger, L.; Fusillo, S. Misuse and diversion of stimulants prescribed for ADHD: A systematic review of the literature. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kaloyanides, K.B.; McCabe, S.E.; Cranford, J.A.; Teter, C.J. Prevalence of illicit use and abuse of prescription stimulants, alcohol, and other drugs among college students: Relationship with age at initiation of prescription stimulants. Pharmacotherapy 2007, 27, 666–674. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, A.D.; Webb, E.M.; Noar, S.M. Illicit use of prescription ADHD medications on a college campus: A multimethodological approach. J. Am. Coll. Health 2008, 57, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Rowe, J.B.; Rittman, T.; Lewis, C.; Robbins, T.W.; Sahakian, B.J. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology 2013, 64, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Grebner, S.; Berlowitz, I.; Alvarado, V.; Cassina, M. Stress in Swiss Employees. Associations Between Working Conditions, Person Characteristics, Well-Being, and Health; State Secretary for Economy: Bern, Switzerland, 2010; Available online: https://www.seco.admin.ch/seco/de/home/Publikationen_Dienstleistungen/Publikationen_und_Formulare/Arbeit/Arbeitsbedingungen/Studien_und_Berichte/stressstudie-2010--stress-bei-schweizer-erwerbstaetigen---zusamm.html (accessed on 14 November 2024).
- Compton, W.M.; Thomas, Y.F.; Stinson, F.S.; Grant, B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 2007, 64, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Gavaret, M.; Vidal, C.; Brunel, L.; Riveline, J.-P.; Micoulaud-Franchi, J.-A.; Domenech, P. (Mis)use of prescribed stimulants in the medical student community: Motives and behaviors: A population-based crosssectional study. Medicine 2016, 95, e3366. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.G.; Bagusat, C.; Dietz, P.; Hoffmann, I.; Simon, P.; Ulrich, R.; Lieb, K. Use of illicit and prescription drugs for cognitive or mood enhancement among surgeons. BMC Med. 2013, 11, 102. [Google Scholar] [CrossRef]
- Aikins, R.D. Academic performance enhancement: A qualitative study of the perceptions and habits of prescription stimulant–using college students. J. Coll. Stud. Dev. 2011, 52, 560–576. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Carter, C.S. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmcology 2008, 33, 1477–1502. [Google Scholar] [CrossRef] [PubMed]
- Arria, A.M.; Garnier-Dykstra, L.M.; Caldeira, K.M.; Vincent, K.B.; O’Grady, K.E.; Wish, E.D. Persistent nonmedical use of prescription stimulants among college students: Possible association with ADHD symptoms. J. Atten. Disord. 2011, 15, 347–356. [Google Scholar] [CrossRef]
- Dubljević, V.; Ryan, C.J. Cognitive enhancement with methylphenidate and modafinil: Conceptual advances and societal implications. Neurol. Neurosci. 2015, 4, 25–33. [Google Scholar] [CrossRef]
- Harsh, J.R.; Hayduk, R.; Rosenberg, R.; Wesnes, K.A.; Walsh, J.K.; Arora, S.; Niebler, G.E.; Roth, T. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr. Med. Res. Opin. 2006, 22, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Pigeau, R.; Naitoh, P.; Buguet, A.; McCann, C.; Baranski, J.; Taylor, M.; Mack, I. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature. J. Sleep. Res. 1995, 4, 212–228. [Google Scholar] [CrossRef]
- Sheng, P.; Hou, L.; Wang, X.; Huang, C.; Yu, M.; Han, X.; Dong, Y. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: A systematic review and meta-analysis. PLoS ONE 2013, 8, e81802. [Google Scholar] [CrossRef]
- Ballon, J.S.; Feifel, D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J. Clin. Psychiatry 2006, 67, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Shuman, T.; Wood, S.C.; Anagnostaras, S.G. Modafinil and memory: Effects of modafinil on morris water maze learning and Pavlovian fear conditioning. Behav. Neurosci. 2009, 123, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.A.; Hirotsu, C.; Matos, G.; Alvarenga, T.; Pires, G.N.; Kapczinski, F.; Schröder, N.; Tufik, S.; Andersen, M.L. Modafinil ameliorates cognitive deficits induced by maternal separation and sleep deprivation. Behav. Brain Res. 2013, 253, 274–279. [Google Scholar] [CrossRef]
- Mereu, M.; Bonci, A.; Newman, A.H.; Tanda, G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 2013, 229, 415–434. [Google Scholar] [CrossRef]
- Rugino, T.A.; Copley, T.C. Effects of modafinil in children with attention-deficit/hyperactivity disorder: An open-label study. J. Am. Acad. Child. Adolesc. Psychiatry 2001, 40, 230–235. [Google Scholar] [CrossRef]
- Martinez-Raga, J.; Knecht, C.; Cepeda, S. Modafinil: A useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr. Drug Abus. Rev. 2008, 1, 213–221. [Google Scholar] [CrossRef]
- Shearer, J.; Darke, S.; Rodgers, C.; Slade, T.; van Beek, I.; Lewis, G.; Brady, D.; McKetin, R.; Mattick, R.P.; Wodak, A. A double-blind, placebo controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction 2009, 104, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Approved and investigational uses of modafinil: An evidence-based review. Drugs 2008, 68, 1803–1839. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Sage, J.R.; Shuman, T.; Anagnostaras, S.G. Psychostimulants and cognition: Acontinuum of behavioral and cognitive activation. Pharmacol. Rev. 2013, 66, 193–221. [Google Scholar] [CrossRef] [PubMed]
- de Saint Hilaire, Z.; Orosco, M.; Rouch, C.; Blanc, G.; Nicolaidis, S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: A microdialysis study in rats. Neuroreport 2001, 12, 3533–3537. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Logan, J.; Alexoff, D.; Zhu, W.; Telang, F.; Wang, G.-J.; Jayne, M.; Hooker, J.M.; Wong, C.; et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: Clinical implication. JAMA 2009, 301, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; Antonelli, T.; Tanganelli, S.; O’Connor, W.T.; Perez de la Mora, M.; Mendez-Franco, J.; Rambert, F.A.; Fuxe, K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: Prevention by local GABBA receptor blockade. Neuropsychopharmacology 1999, 20, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Linssen, A.M.; Sambeth, A.; Vuurman, E.F.; Riedel, W.J. Cognitive effects of methylphenidate in healthy volunteers: A review of single dose studies. Int. J. Neuropsychopharmacol. 2014, 17, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.C.; Viswanath, A.; Bharania, P.; Elsabagh, S.M.; Hartley, D.E.; Shneerson, J.M.; File, S.E. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J. Clin. Psychopharmacol. 2005, 25, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Radünz, L.; Reuter, H.; Andresen-Streichert, H. Modafinil in Forensic and Clinical Toxicology—Case Reports, Analytics and Literature. J. Anal. Toxicol. 2018, 42, 353–359. [Google Scholar] [CrossRef]
- Rush, C.R.; Kelly, T.H.; Hays, L.R.; Wooten, A.F. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol. Depend. 2002, 67, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Reinert, J.P.; Dunn, R.L. Management of overdoses of loperamide, gabapentin, and modafinil: A literature review. Expert. Rev. Clin. Pharmacol. 2019, 12, 901–908. [Google Scholar] [CrossRef]
- Şahan, E.; Bölükbaşı, Ö. Modafinil intoxication induced persistent psychosis: Case report. Psychiatr. Danub. 2019, 31, 369–370. [Google Scholar] [CrossRef]
- Neuman, G.; Shehadeh, N.; Pillar, G. Unsuccessful Suicide Attempt of a 15 Year Old Adolescent with Ingestion of 5000 mg Modafinil. J. Clin. Sleep Med. 2009, 5, 372–373. [Google Scholar] [CrossRef]
- Krishnan, R.; Chary, K.V. A rare case modafinil dependence. J. Pharmacol. Pharmacother. 2015, 6, 49–50. [Google Scholar] [CrossRef]
- Kandasamy, R.O.; Kaminskaite, V.; May, F. Hyponatraemia and cerebral oedema due to a modafinil overdose. BMJ Case Rep. 2020, 13, e234530. [Google Scholar] [CrossRef]
- Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man, 10th ed.; Biomedical Publications: Seal Beach, CA, USA, 2014; pp. 1459–1460. [Google Scholar]
- Robertson, P., Jr.; Hellriegel, E.T. Clinical pharmacokinetic profile of modafinil. Clin. Pharmacokinet. 2003, 42, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Malcolm, R.J.; Markowitz, J.S.; DeVane, C.L. Chiral analysis of d- and l-modafinil in human serum: Application to human pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.; Kirby, M.; D’Andrea, D.M.; Yang, R.; Hellriegel, E.T.; Robertson, P., Jr. Pharmacokinetics of armodafinil and modafinil after single and multiple doses in patients with excessive sleepiness associated with treated obstructive sleep apnea: A randomized, open-label, crossover study. Clin. Ther. 2010, 32, 2074–2087. [Google Scholar] [CrossRef]
- Sousa, A.; Dinis-Oliveira, R.J. Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects. Subst. Abus. 2020, 41, 155–173. [Google Scholar] [CrossRef]
- Zhu, H.J.; Wang, J.S.; Donovan, J.L.; Jiang, Y.; Gibson, B.B.; Devane, C.L.; Markowitz, J.S. Interactions of attention-deficit/hyperactivity disorder therapeutic agents with the efflux transporter P-glycoprotein. Eur. J. Pharmacol. 2008, 578, 148–158. [Google Scholar] [CrossRef]
- Xu, P.; Li, H.-D.; Zhang, B.-K.; Xiao, Y.-W.; Yuan, H.-Y.; Zhu, Y.-G. Pharmacokinetics and tolerability of modafinil tablets in Chinese subjects. J. Clin. Pharm. Ther. 2008, 33, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Nowak, K.; Szpot, P. Fatal intoxication with N-ethylpentylone: A case report and method for determining N-ethylpentylone in biological material. Forensic Toxicol. 2020, 38, 255–263. [Google Scholar] [CrossRef]
- Wachełko, O.; Szpot, P.; Tusiewicz, K.; Nowak, K.; Chłopaś-Konowałek, A.; Zawadzki, M. An ultra-sensitive UHPLC-QqQMS/MS method for determination of 54 benzodiazepines (pharmaceutical drugs, NPS and metabolites) and z-drugs in biological samples. Talanta 2023, 251, 123816. [Google Scholar] [CrossRef] [PubMed]
- Tusiewicz, K.; Chłopaś-Konowałek, A.; Wachełko, O.; Zawadzki, M.; Szpot, P. A fatal case involving the highest ever reported 4-CMC concentration. J. Forensic Sci. 2023, 68, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Wachełko, O.; Chłopaś-Konowałek, A.; Szpot, P. Quantification and distribution of 4-fluoroisobutyryl fentanyl (4-FiBF) in postmortem biological samples using UHPLC–QqQ-MS/MS. Forensic Toxicol. 2021, 39, 451–463. [Google Scholar] [CrossRef]
- Szpot, P.; Nowak, K.; Wachełko, O.; Tusiewicz, K.; Chłopaś-Konowałek, A.; Zawadzki, M. Methyl (S)-2-(1-7 (5-fluoropentyl)-1Hindole-3-carboxamido)-3,3-dimethylbutanoate (5F-MDMB-PICA) intoxication in a child with identification of two new metabolites (ultra-high-performance liquid chromatography-tandem mass spectrometry). Forensic Toxicol. 2023, 41, 47–58. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). Guidance for the Validation of Analytical Methodology and Calibration of Equipment Used for Testing of Illicit Drugs in Seized Materials and Biological Specimens. Available online: https://syntheticdrugs.unodc.org/uploads/syntheticdrugs/res/library/forensics_html/Guidance_for_the_Validation_of_Analytical_Methodology_and_Calibration_of_Equipment_used_for_Testing_of_Illicit_Drugs_in_Seized_Materials_and_Biological_Specimens.pdf (accessed on 1 January 2025).
- Scientific Working Group for Forensic Toxicology. Scientific Working Group for Forensic Toxicology (SWGTOX) Standard practices for method validation in forensic toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef]
- Castaldi, S.; Gelatti, U.; Orizio, G.; Hartung, U.; Moreno-Londono, A.M.; Nobile, M.; Schulz, P.J. Use of cognitive enhancement medication among northern Italian university students. J. Addict. Med. 2012, 6, 112–117. [Google Scholar] [CrossRef]
- Dursun, S.; Dunn, M.; McKay, F.H. The availability and acquisition of modafinil on the internet. Drug Alcohol. Rev. 2019, 38, 699–702. [Google Scholar] [CrossRef]
- Pan, H. A non-covalent dimer formed in electrospray ionisation mass spectrometry behaving as a precursor for fragmentations. Rapid Commun. Mass. Spectrom. 2008, 22, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Grocholska, P.; Kowalska, M.; Wieczorek, R.; Bąchor, R. A Non-Covalent Dimer Formation of Quaternary Ammonium Cation with Unusual Charge Neutralization in Electrospray-Ionization Mass Spectrometry. Molecules 2021, 26, 5868. [Google Scholar] [CrossRef] [PubMed]
- Grocholska, P.; Wieczorek, R.; Bąchor, R. Preparation of Deuterium-Labeled Armodafinil by Hydrogen–Deuterium Exchange and Its Application in Quantitative Analysis by LC-MS. Metabolites 2022, 12, 578. [Google Scholar] [CrossRef]
- Assi, S.; Khan, I.; Edwards, A.; Osselton, D.; Al-Obaidi, H. On-spot quantification of modafinil in generic medicines purchased from the Internet using handheld Fourier transform-infrared, near-infrared and Raman spectroscopy. J. Anal. Sci. Technol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Harvanová, M.; Gondová, T. New enantioselective LC method development and validation for the assay of modafinil. J. Pharm. Biomed. Anal. 2017, 138, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Burnat, P.; Robles, F.; Do, B. High-performance liquid chromatographic determination of modafinil and its two metabolites in human plasma using solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1998, 706, 295–304. [Google Scholar] [CrossRef]
- Ramachandra, B. Critical Review of Properties of Modafinil and Analytical, BioAnalytical Methods for Its Determination. Crit. Rev. Anal. Chem. 2016, 46, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.L.; Uralets, V.; Lin, C.-T.; Kuo, F.-H. Detection of modafinil in human urine by gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 1042–1045. [Google Scholar] [CrossRef]
- AL Azzam, K.M.; Saad, B.; Adnan, R.; Saleh, M.I. Enantioselective determination of modafinil in pharmaceutical formulations by capillary electrophoresis, and computational calculation of their inclusion complexes. Microchim. Acta. 2009, 166, 311–317. [Google Scholar] [CrossRef]
- Al Azzam, K.M.; Saad, B.; Aboul-Enein, H.Y. Determination of the binding constants of modafinil enantiomers with sulfated β-cyclodextrin chiral selector by capillary electrophoresis using three different linear plotting methods. Electrophoresis 2010, 31, 2957–2963. [Google Scholar] [CrossRef]
- Warot, D.; Corruble, E.; Payan, C.; Weil, J.S.; Puech, A.J. Subjective effects of modafinil, a new central adrenergic stimulant in healthy volunteers: A comparison with amphetamine, caffeine and placebo. Eur. Psychiatry 1993, 8, 201–208. [Google Scholar] [CrossRef]
- Newman, J.L.; Negus, S.S.; Lozama, A.; Prisinzano, T.E.; Mello, N.K. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp. Clin. Psychopharmacol. 2010, 18, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Arbor, K.; Christ, M. Abstracts of the 2008 North American Congress of Clinical Toxicology Annual Meeting, September 11–16, 2008, Toronto, Canada: Prolonged Delirium in a Pediatric Patient after Modafinil and Escitalopram Ingestion. Clin. Toxicol. 2008, 46, 623–624. [Google Scholar]
- Gresham, C.; Wallace, K.L. Abstracts of the 2008 North American Congress of Clinical Toxicology Annual Meeting, September 11–16, 2008, Toronto, Canada: Challenges in Detection and Confirmation of Modafinil Use. Clin. Toxicol. 2008, 46, 591–645. [Google Scholar]
- Zhou, L.; Wang, X.; Liu, W.; Xiang, P.; Chen, H. Rapid identification of the “smart drug” modafinil in suspicious tablets by DART-HRMS combined with micropunching. Anal. Methods 2020, 12, 1430–1440. [Google Scholar] [CrossRef]
- Kate, N.; Grover, S.; Ghormode, D. Dependence on supratherapeutic doses of modafinil: A case report. Prim. Care Companion CNS Disord. 2012, 14, 11l01333. [Google Scholar] [CrossRef] [PubMed]
- Swapnajeet, S.; Bn, S.; Gourav, G. Modafinil dependence and hypersexuality: A case report and review of the evidence. Clin. Psychopharmacol. Neurosci. 2016, 14, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.; Wu, X.; Bastiampillai, T.; Tibrewal, P. Could modafinil be a drug of dependence? Aust. N. Z. J. Psychiatry 2015, 49, 485–486. [Google Scholar] [CrossRef]
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Drug levels: Therapeutic and toxic serum/plasma concentrations of common drugs. J. Clin. Monit. Comput. 1999, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Bhartiyia, R.; Mishra, S.; Khuroo, A.; Satyanarayana, G.N.V.S. Liquid chromatography tandem mass spectrometry method for the estimation of modafinil in human plasma. Word J. Pharm. Sci. 2014, 2, 1134–1415. [Google Scholar]
- Deventer, K.; Pozo, O.J.; Van Eenoo, P.; Delbeke, F.T. Qualitative detection of diuretics and acidic metabolites of other doping agents in human urine by high-performance liquid chromatography–tandem mass spectrometry. Comparison between liquid–liquid extraction and direct injection. J. Chromatogr. A 2009, 1216, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
- Di Rago, M.; Pantatan, S.; Hargreaves, M.; Wong, K.; Mantinieks, D.; Kotsos, A.; Glowacki, L.; Drummer, O.H.; Gerostamoulos, D. High Throughput Detection of 327 Drugs in Blood by LC-MS-MS with Automated Data Processing. J. Anal. Toxicol. 2021, 45, 154–183. [Google Scholar] [CrossRef]
- Mazzarino, M.; de la Torre, X.; Botre, F.; Gray, N.; Cowan, D. A rapid screening LC-MS/MS method based on conventional HPLC pumps for the analysis of low molecular weight xenobiotics: Application to doping control analysis. Drug Test. Anal. 2010, 2, 311–322. [Google Scholar] [CrossRef]
- Dubey, S.; Ahi, S.; Reddy, I.M.; Kaur, T.; Beotra, A.; Jain, S. A novel study of screening and confirmation of modafinil, adrafinil and their metabolite modafinilic acid under EI-GC-MS and ESI-LC-MS-MS ionization. Indian. J. Pharmacol. 2009, 41, 278–283. [Google Scholar]
- Park, D.; Choi, H.; Jang, M.; Chang, H.; Woo, S.; Yang, W. Simultaneous determination of 18 psychoactive agents and 6 metabolites in plasma using LC-MS/MS and application to actual plasma samples from conscription candidates. Forensic Sci. Int. 2018, 228, 283–290. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, Y.; Luo, L.; Junying, C.; Congcong, W.; Meiling, Z. Determination of modafinil in rat plasma by UPLC-MS/MS and a study of its pharmacokinetics and bioavailability. Acta Chromatogr. 2023, 35, 187–192. [Google Scholar] [CrossRef]
- Phanindra, A.; Kumar, Y.S. Method development and validation of LC-ESI-MS/MS technique for the estimation of modafinil in human plasma; application to pharmacokinetics in healthy rabbits. IJPSR 2020, 11, 1837–1844. [Google Scholar]
- Sim, J.; Kim, E.; Yang, W.; Woo, S.; In, S. An LC-MS/MS method for the simultaneous determination of 15 antipsychotics and two metabolites in hair and its application to rat hair. Forensic Sci. Int. 2017, 274, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.; Roig, M.; Montfort, N.; Sáez, P.; Bergés, R.; Segura, J. High-throughput and sensitive screening by ultra-performance liquid chromatography tandem mass spectrometry of diuretics and other doping agents. Eur. J. Mass Spectrom. 2008, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]

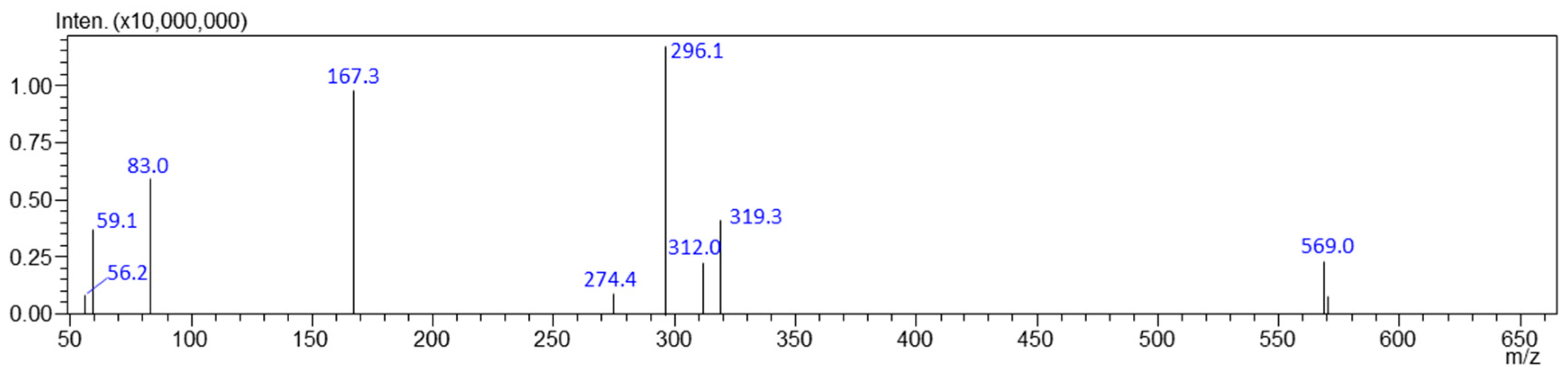

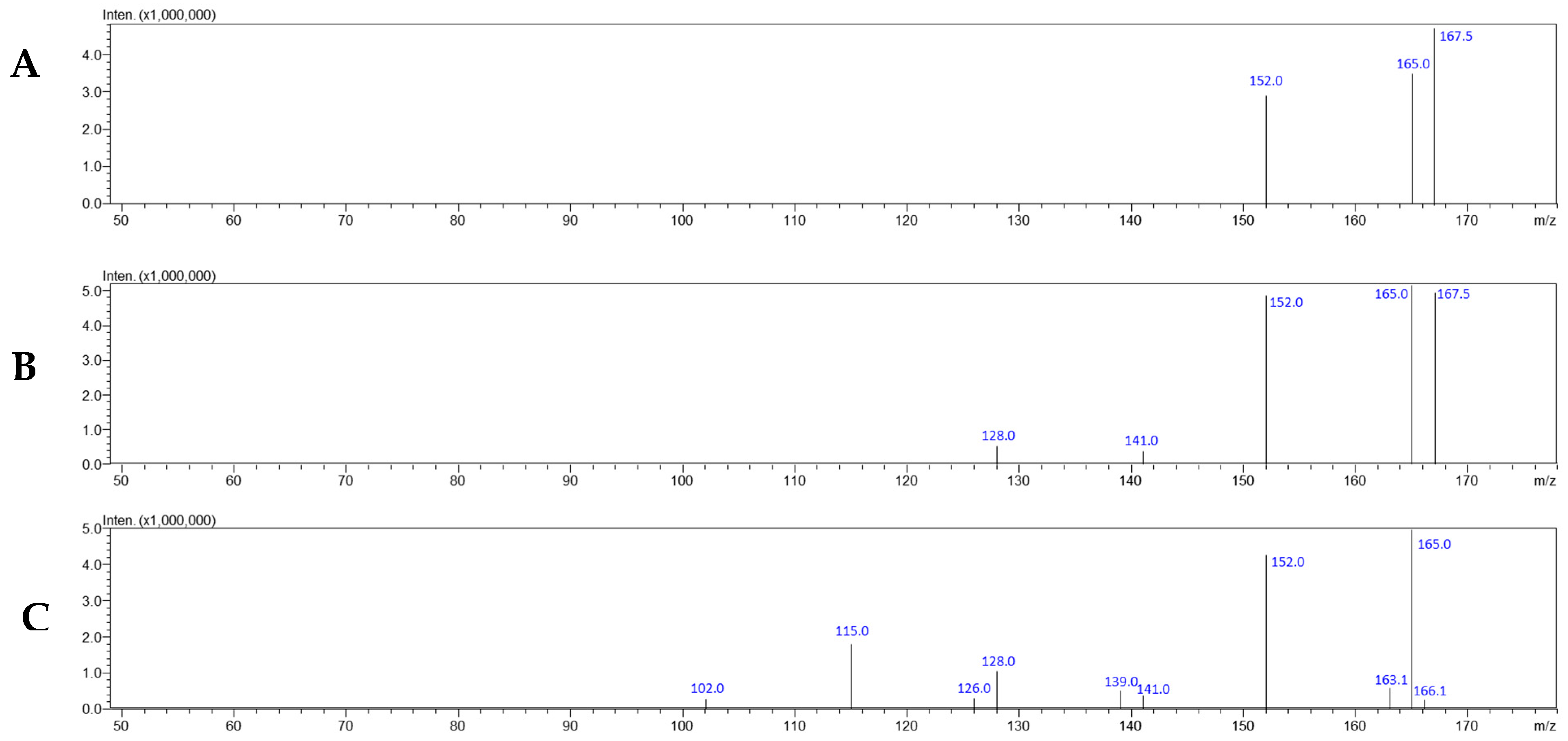
| Compounds | Precursor Ion [m/z] | Product Ion [m/z] | Dwell (msec) | Q1 Pre-Bias [V] | Collision Energy [V] | Q3 Pre-Bias [V] | Retention Time [min] |
|---|---|---|---|---|---|---|---|
| Modafinil | 166.9 | 152.1 * 115.2 | 2.0 | −11 −11 | −22 −40 | −15 −19 | 5.57 |
| Methylphenidate-d9 | 242.9 | 93.1 * 61.1 | 1.0 | −11 −15 | −22 −49 | −15 −10 | 4.42 |
| No. | Determined Substance | Tablet Weight [mg] | Amount of Substance in the Sample [mg] | Percentage of the Total Weight of the Tablet [%] | Probable Dose (Data from the Bister) [mg] | Percentage of the Dose [%] | Presence of Impurity |
|---|---|---|---|---|---|---|---|
| D.1 | Modafinil | 401.0 | 90.7 | 22.5 | 200 * | 45.5 | No |
| D.2 | Modafinil | 269.2 | 120.8 | 44.9 | 150 | 80.5 | No |
| D.3 | Modafinil | 318.4 | 92.0 | 28.9 | 200 | 46.0 | No |
| D.4 | Modafinil | 333.0 | 109.7 | 32.9 | 200 | 54.8 | No |
| D.5 | Modafinil | 341.1 | 105.3 | 30.8 | 200 | 52.6 | No |
| Validation Results | ||
|---|---|---|
| Intra-day precision [%] | 10 ng/mL | 2.3 |
| 100 ng/mL | 14.9 | |
| Intra-day accuracy [%] | 10 ng/mL | 8.6 |
| 100 ng/mL | 5.8 | |
| Iner-day precision [%] | 10 ng/mL | 2.9 |
| 100 ng/mL | 10.0 | |
| Inter-day accuracy [%] | 10 ng/mL | 13.0 |
| 100 ng/mL | 6.3 | |
| Recovery [%] | 10 ng/mL | 111.1 |
| 100 ng/mL | 110.8 | |
| Matrix effect [%] | 10 ng/mL | 105.8 |
| 100 ng/mL | 110.2 | |
| Concentrations of Modafinil [ng/mL a or ng/g b] | |||||||
|---|---|---|---|---|---|---|---|
| Case Number/Sex | Biological Material | ||||||
| Blood | Urine | Vitreous Humor | Putrefaction Fluid | Liver | Kidney | Brain | |
| Case 1/Male | 110 | >3000 | 30 | (−) | 250 | 210 | 410 |
| Case 2/Male | (−) | (−) | (−) | >1000 | (−) | (−) | (−) |
| Case 3/Male | 0 | 14 | (−) | (−) | (−) | (−) | (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, K.; Chłopaś-Konowałek, A.; Szpot, P.; Zawadzki, M. The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples. J. Xenobiot. 2025, 15, 15. https://doi.org/10.3390/jox15010015
Nowak K, Chłopaś-Konowałek A, Szpot P, Zawadzki M. The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples. Journal of Xenobiotics. 2025; 15(1):15. https://doi.org/10.3390/jox15010015
Chicago/Turabian StyleNowak, Karolina, Agnieszka Chłopaś-Konowałek, Paweł Szpot, and Marcin Zawadzki. 2025. "The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples" Journal of Xenobiotics 15, no. 1: 15. https://doi.org/10.3390/jox15010015
APA StyleNowak, K., Chłopaś-Konowałek, A., Szpot, P., & Zawadzki, M. (2025). The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples. Journal of Xenobiotics, 15(1), 15. https://doi.org/10.3390/jox15010015







