Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine
Abstract
:1. Introduction
2. Polymer Synthesis
3. Adhesion
4. Mechanical Properties
5. Antibacterial and Antifouling Properties
6. Optical Photothermal and Conductive Properties
7. Cancer
8. Tissue Engineering
9. Wound Healing
10. Structure-Property Correlation
10.1. Synthesis
- Synthesis, Reaction, Polymerization, and Crosslinking: The advancements in bioinspired polymers have been facilitated by breakthroughs in synthesis, reaction, polymerization, and crosslinking methods [13]. These techniques allow researchers to precisely control the molecular architecture and chemical composition of the polymers, which directly impacts their final properties. Tailoring the polymer structure at the molecular level enables the development of materials with unique functionalities for specific biomedical applications.
- Dual Functional Coatings, Biohybrid Nanoreactors, Shell- and Core-Crosslinked Nanoparticles: The use of dual functional coatings, biohybrid nanoreactors, and shell- and core-crosslinked nanoparticles has shown promise in preventing biofilm formation, combating infections, enhancing drug release, and improving stability [6,12,13]. These design strategies impart specific chemical and physical characteristics to the bioinspired polymers, making them suitable for targeted biomedical applications.
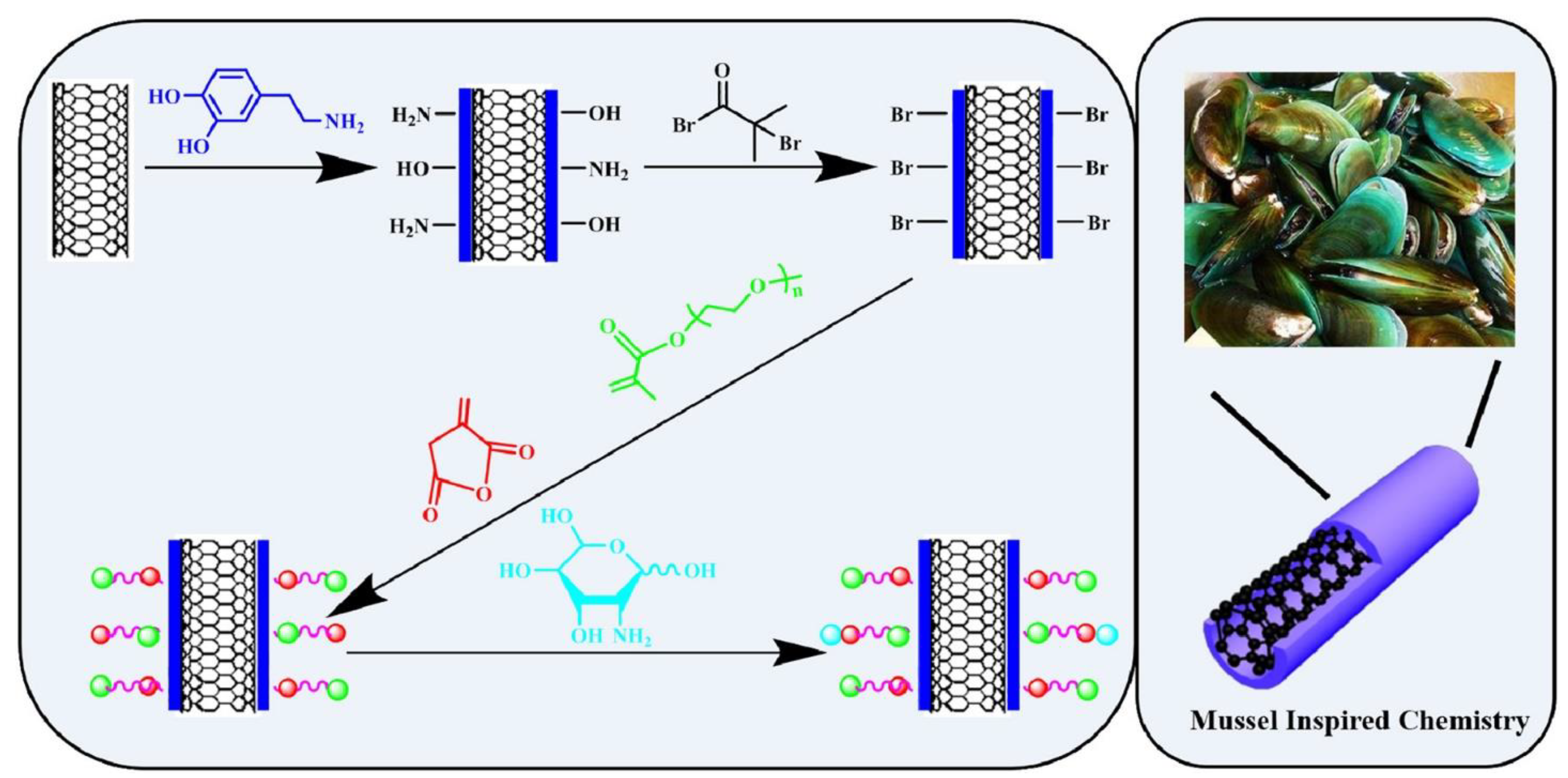
- Mussel-Inspired Chemistry and Catechol Chemistry: Mussel-inspired chemistry and catechol chemistry have been utilized in bioinspired polymer design. These approaches mimic the adhesive properties found in mussel proteins, leading to enhanced surface adhesion, biocompatibility, and biodegradability of the polymers. This enables their use in tissue engineering, wound healing, and drug delivery applications [14,15,16].
- Tannic Acid-Based Surface Modification: Tannic acid-based surface modification has emerged as an effective method for modifying bioinspired polymers. It imparts antifouling properties to the materials, making them ideal for marine and biomedical applications, where inhibiting fouling is crucial for device performance [17,31].
- Applications in Biomedical Materials and Healthcare: The mentioned bioinspired polymers find applications in various biomedical fields, such as implantable biomedical devices, tissue engineering, wound healing, drug delivery, and biosensors. Their specific structures contribute to improved biocompatibility, controlled drug release, and targeted therapies, thus advancing targeted and personalized medicine [13,14,16,29,31,32,39,41,51].
- Hydrophilic Magnetic Nanorods, Amphiphilic Copolymer Micelles, Biomimetic Superlubricated Nanoparticles, MoS2-Based Polymer Nanocomposites: Further advancements in bioinspired polymers have led to the development of hydrophilic magnetic nanorods, amphiphilic copolymer micelles, biomimetic superlubricated nanoparticles, and MoS2-based polymer nanocomposites [77,78,79,80,81]. These novel structures offer potential applications in drug pollution removal, drug carriers, osteoarthritis treatment, and photothermal cancer treatment, expanding the possibilities for various biomedical interventions.
10.2. Adhesion
- Catechol-Functionalized Patches for Transdermal Drug Delivery: Catechol-functionalized patches demonstrate excellent adhesion, making them promising candidates for transdermal drug delivery systems [5]. The incorporation of catechol groups enables strong adhesion to the skin, enhancing the efficiency of drug delivery through this route. The specific structure of the catechol-functionalized polymers plays a crucial role in their adhesive properties.
- Nature-Inspired Dual Functional Coatings for Implantable Biomedical Devices: Nature-inspired dual functional coatings show potential in improving the performance of implantable biomedical devices [6]. These coatings can prevent biofilm formation and infections, which are directly related to their chemical composition and surface structure. The design of these polymers is crucial in achieving effective adhesion and long-term performance in biomedical applications.
- Mussel-Inspired Catechol-Modified Biomaterials in Tissue Engineering: Mussel-inspired catechol-modified biomaterials find applications in tissue engineering and regenerative medicine [7]. Their adhesive properties facilitate tissue regeneration and drug delivery to the targeted site. The molecular structure of the catechol-modified biomaterials influences their adhesion to biological tissues, making them suitable for specific biomedical applications.
- Silver-Releasing Hydrogels with Mussel-Inspired Catechol Moieties: Silver-releasing hydrogels containing mussel-inspired catechol moieties possess antibacterial properties, contributing to their suitability for various biomedical applications [8]. The presence of catechol moieties affects the hydrogel’s adhesive properties and enhances its antimicrobial effectiveness.
- Mussel-Inspired Ionoelastomers with Exceptional Adhesion: Mussel-inspired ionoelastomers exhibit exceptional adhesion, self-healing, and sensing capabilities [33]. Their unique structure and chemical composition enables strong adhesion to various surfaces, making them versatile in coatings, adhesives, drug delivery, and biomedical engineering applications.
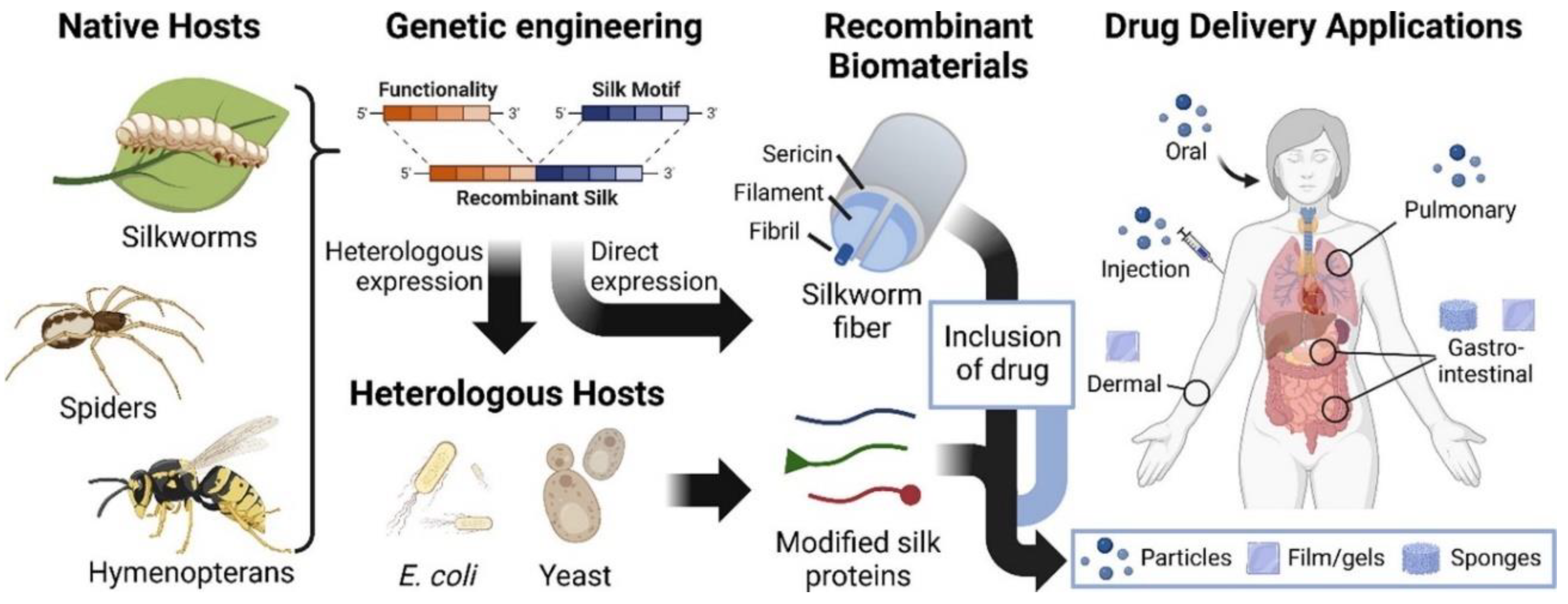
- Recombinant Silk-Based Drug Delivery Systems: Recombinant silk-based drug delivery systems are designed to improve the loading, release, and stability of drugs, ensuring efficient and safe medical treatments [11]. The silk-based polymer’s structure and functional groups are crucial in controlling drug release and enhancing adhesive interactions at the target site.
- Tannin-Inspired Gelatin Bioadhesives for Wound Closure: Tannin-inspired gelatin bioadhesives offer wet tissue adhesion, degradation control, and cost-effectiveness, making them suitable for wound closure and tissue sealant applications [9]. The specific structure of the gelatin-based polymer plays a key role in its adhesion properties and biocompatibility.
- Progress in Other Bioinspired Polymers and Technologies: The advancements in helical microfiber composite membranes, gecko-inspired medical adhesives, polydopamine chemistry for oral medicine, chemically defined surface coatings for stem cell culture, tannic acid-based surfaces for marine applications, and mPEG-DOPA3 for biofouling issues contribute to progress in regenerative medicine, marine coatings, and biomedical devices [17,39,40,41,42,43]. The structural characteristics of these bioinspired polymers are crucial in determining their adhesion, biocompatibility, and performance in various applications.
10.3. Mechanical Properties
- Fmoc-RGD/Chitosan Composite Hydrogel for Tissue Engineering and Regenerative Medicine: Fmoc-RGD/chitosan composite hydrogel exhibits potential in tissue engineering, regenerative medicine, antimicrobial applications, and nanotechnology, with its mechanical properties, antibacterial activity, and silver nanoparticle production expanding its impact [18]. The specific composition and structure of this composite hydrogel influences its mechanical strength, biocompatibility, and antimicrobial properties, making it valuable for various biomedical applications.
- Polymer-Colloid Composites for Advanced Materials with Specific Structures and Properties: Polymer-colloid composites provide control over aggregate formation, benefiting advanced materials with specific structures and properties, thereby advancing non-equilibrium self-assembly and complex systems understanding [82]. The specific composition and arrangement of polymer-colloid composites allow for precise control over their mechanical properties and self-assembly behavior, making them versatile in designing complex materials.
- Functionally Graded Multilayers for Enhanced Dental Restorations: Functionally graded multilayers inspired by the dentin-enamel junction enhance the mechanical performance and longevity of dental restorations, optimizing multilayered systems for improved clinical outcomes [55]. The design of these multilayers allows for gradual variations in mechanical properties, replicating the natural tooth structure and enhancing the durability of dental restorations.
- Stimuli-Responsive SP Hydrogels for Intestinal Drug Delivery and Suturing Small Vessels: Stimuli-responsive SP hydrogels show promise in intestinal drug delivery and suturing small vessels, with their pH and calcium responsiveness, thermoplasticity, and self-healing ability making them valuable for biomedical applications [51]. The responsiveness of these hydrogels to pH and calcium ions influences their drug release behavior and their suitability for specific medical applications.
- Liquid-Free Ionoelastomers Inspired by Mussels for Versatile Applications: Liquid-free ionoelastomers inspired by mussels find applications in coatings, adhesives, and biomedical engineering, offering opportunities in various fields [33]. The specific structure and composition of these ionoelastomers enhance their adhesion properties and make them suitable for versatile applications, including biomedical devices.
- Silk-Based Drug Delivery Systems for Controlled Release and Biocompatibility: Silk-based drug delivery systems protect and control drug release, advancing effective drug delivery systems and biomaterials in healthcare [11]. The structure of silk-based polymers allows for controlled drug release, protecting the encapsulated drug and ensuring biocompatibility with the body.
- Bio-Inspired Silica-Collagen Materials for Enhanced Bioactivity and Drug Delivery: Bio-inspired silica-collagen materials enhance bioactivity, biocompatibility, and drug delivery capabilities in biomedical devices, contributing to the field of biomedicine [62]. The specific incorporation of silica and collagen influences the bioactivity and drug-loading capacity of these materials, making them valuable for biomedical applications.
- Kneading-Dough-Inspired Method for Tunable Properties and Self-Healing Hydrogel Networks: The kneading-dough-inspired method for hydrogel networks benefits drug delivery systems and biosensors, with the PEI/PAM composite hydrogel offering tunable properties and self-healing ability [20]. The specific method of preparation allows for precise control over the mechanical properties of hydrogel networks, making them valuable in drug delivery and biosensing applications.
- Citrate-Based Tissue Adhesives (POEC-d) for Strong Wet Adhesion and Biodegradability: Citrate-based tissue adhesives (POEC-d) with strong wet adhesion, biodegradability, and desirable mechanical properties advance surgical procedures and have potential as tissue adhesives and sealants [36]. The specific citrate-based composition enhances the adhesive properties and biocompatibility of these tissue adhesives, making them suitable for various biomedical applications.
- Stable Alginate Hydrogel Fibers for Tissue Engineering and Biomedical Applications: Stable alginate hydrogel fibers enable tissue engineering and biomedical applications, advancing tissue engineering techniques [15]. The specific structure and mechanical stability of these alginate hydrogel fibers make them suitable for scaffold fabrication and tissue regeneration.
- Anisotropic PEG-Based Hydrogels for Heart Valve Tissue Engineering: Anisotropic PEG-based hydrogels hold promise for heart valve tissue engineering, contributing to scaffold design for improved regenerative therapies [19]. The anisotropic design of these hydrogels allows for tissue-specific organization and function, making them valuable in heart valve tissue engineering.
10.4. Antifouling and Antibacterial Properties
- Poly(oxonorbornene)-based SMAMPs for Antibacterial Applications: Poly(oxonorbornene)-based SMAMPs (Synthetic Mimics of Antimicrobial Peptides) demonstrate potential as antimicrobial agents, addressing antibiotic resistance [52]. The specific structure of these SMAMPs plays a crucial role in their antibacterial activity, allowing them to effectively target and disrupt bacterial membranes.
- Bioinspired Dual Functional Coatings for Biomedical Devices: Bioinspired dual functional coatings improve the biocompatibility and long-term performance of implantable biomedical devices by reducing bacterial adhesion and protein adsorption [53]. The design of these coatings, inspired by natural systems, influences their surface properties, making them resistant to bacterial attachment and biofilm formation.
- Glucose-Responsive Nanoreactors with Antimicrobial Enzymes: Glucose-responsive nanoreactors loaded with antimicrobial enzymes show promise for therapeutic applications against drug-resistant bacterial infections [12]. The structure of these nanoreactors enables them to respond to specific environmental cues (e.g., glucose concentration) and release antimicrobial enzymes at the target site.
- Fmoc-RGD/Chitosan Composite Hydrogel for Tissue Engineering: Fmoc-RGD/chitosan composite hydrogel serves as a versatile scaffold for tissue engineering, axonal regeneration, and drug delivery, offering a multifunctional platform for biomedical applications [18]. The composition and structural characteristics of this composite hydrogel influence its mechanical properties, biocompatibility, and drug-loading capacity.
- Phenylalanine-Based Metal-Biomolecule Complexes and Cu(mDF) Coordination Polymer for Water Purification: Phenylalanine-based metal-biomolecule complexes and Cu(mDF) coordination polymer address water purification challenges by detecting and removing organic pollutants [21]. The coordination and complexation of metals with the phenylalanine-based biomolecules play a key role in the removal of pollutants, making them effective water purification agents.
- Titanium-Coated Dendritic Material for Implant-Associated Infection Treatment: Titanium-coated dendritic material enhances implant-associated infection treatment by concentrating and activating antibiotic prodrugs locally [83]. The specific structure of the dendritic material and the titanium coating contributes to its ability to release antibiotics in a controlled manner, improving antibiotic therapy effectiveness while minimizing toxicity.
- Virus-Inspired Nanodrugs (VNDs) for Enhanced Antibiotic Delivery: Virus-inspired nanodrugs (VNDs) provide a biomimetic approach to enhance antibiotic delivery and efficacy against bacteria [84]. The structure of VNDs mimics virus-like particles, allowing them to efficiently deliver antibiotics to bacterial cells and address challenges related to bioavailability and antibiotic resistance.
10.5. Optical Photothermal and Conductive Properties
- Encapsulation of Semiconducting Polymer Nanoparticles (SPNs) with Polydopamine (PDA) for theranostic. The encapsulation of semiconducting polymer nanoparticles with polydopamine offers a versatile platform for the development of functionalized nanoparticles with enhanced stability and bioconjugation capabilities, advancing theranostic [54]. The specific structure of the SPNs and the polydopamine shell influences their stability and surface chemistry, allowing for efficient bioconjugation and targeted therapeutic and diagnostic applications.
- Bio-Inspired Polymers in Drug Delivery Systems for Diabetes Management: Bio-inspired polymers in drug delivery systems contribute to advancements in the targeted management of diabetes and its complications [85]. The design of these polymers plays a crucial role in their drug-loading and release capacities, enabling specific and controlled drug delivery against diabetes-related conditions.
- Functionally Graded Multilayers for Enhanced Dental Restorations: Functionally graded multilayers that mimic the dentin-enamel junction provide enhanced mechanical performance for dental materials and restorative dentistry [55]. The specific composition and structure of these multilayers allow them to replicate the natural tooth structure, resulting in improved mechanical properties and longevity of dental restorations.
- Polydopamine Surface Modification for Nanomedicine Applications: Polydopamine surface modification of nanosystems enables targeted drug delivery, photothermal therapy, and tumor imaging, advancing nanomedicine and personalized medicine [56]. The polydopamine layer plays a critical role in facilitating targeted drug delivery and enhancing the therapeutic and imaging capabilities of nanosystems.
- Surface-Modified SiO2-poly(PEGMA-IA-DA) Nanoparticles for Multifunctional Biomedical Applications: Surface-modified SiO2-poly(PEGMA-IA-DA) nanoparticles exhibit desirable properties for targeted drug delivery, imaging, and therapeutics, advancing multifunctional biomedical applications [57]. The specific surface modifications and polymer structure enable these nanoparticles to carry out multiple functions, making them versatile tools in various biomedical applications.
- Poly(dopamine) (PDA) Coatings for Cell Interfacing, Drug Delivery, and Biosensing: Poly(dopamine) (PDA) coatings find diverse applications in cell interfacing, drug delivery, and biosensing, transforming bioengineering and healthcare systems [86]. The structure of the PDA coatings plays a key role in their biocompatibility, drug-loading capacity, and sensing capabilities.
- Electrochemical Biosensor for Neurobiology and Drug Discovery: The developed electrochemical biosensor offers a low-cost, sensitive, and selective approach for detecting acetylcholinesterase (AChE) activity and screening potential inhibitors, impacting drug discovery and neurobiology [26]. The design of the biosensor’s sensing elements and the electrode surface influences its sensitivity and specificity in detecting AChE activity, making it valuable in neurobiological research and drug development.
10.6. Cancer
- Eco-Friendly Chitosan/Copper Oxide Nanocomposites for Anticancer Therapy: Eco-friendly synthesis of chitosan/copper oxide nanocomposites using rutin shows potential as an anticancer agent, contributing to the advancement of nanomedicine [87]. The specific structure and composition of these nanocomposites influence their biocompatibility, drug-loading capacity, and anticancer activity.
- Nanoscale Coordination Polymers for Breast Cancer Drug Delivery: Nanoscale coordination polymers offer promising applications in anticancer drug delivery, improving chemotherapy outcomes specifically in breast cancer treatment [88]. The coordination and composition of these polymers play a crucial role in their drug-loading capacity and targeted delivery to breast cancer cells.
- pH-Responsive Polydopamine (PDA) Capsules for Controlled Drug Release: pH-responsive polydopamine (PDA) capsules provide a controlled drug release mechanism, enhancing anticancer drug delivery and stimuli-responsive systems [89]. The pH-sensitive nature of PDA capsules allows them to release drugs in response to specific acidic conditions in the tumor microenvironment, improving therapeutic efficacy.
- Nanocarriers Modified with Polydopamine and Sensitive to Near-Infrared Light for Chemo-Photothermal Therapy and Imaging: Nanocarriers modified with polydopamine and sensitive to near-infrared light have applications in chemo-photothermal therapy and tumor imaging, advancing nanosystems with diagnostic and therapeutic capabilities [56]. The combination of polydopamine modification and light sensitivity enables targeted drug delivery and localized photothermal therapy for cancer treatment.
- Silk-Based Drug Delivery Systems for Controlled and Targeted Delivery: Silk-based drug delivery systems offer controlled and targeted drug delivery with reduced side effects, impacting biomedical applications [11]. The silk-based polymer’s structure allows for controlled drug release, improving the efficiency of drug delivery and reducing unwanted side effects.
- Functional Polymers Integrated with Lipid-Based Drug Delivery Systems for Tumor-Specific Delivery: Functional polymers integrated with lipid-based drug delivery systems enable tumor-specific delivery and controlled drug release, improving cancer therapy [59]. The design of these polymers and lipid carriers facilitates specific targeting to tumor cells and controlled release of therapeutic agents.
- Rod-Like Nanocarriers for Enhanced Drug Delivery Efficiency: Rod-like nanocarriers enhance drug delivery efficiency and therapeutic efficacy against cancer, impacting future clinical applications [60]. The unique rod-like structure of these nanocarriers allows for increased drug-loading capacity and efficient cellular uptake, leading to improved therapeutic outcomes.
- Innovative Strategies for Targeted Drug Delivery: Advancements in cancer therapy include innovative strategies such as Targetin conjugated with a folate group for enhanced targeted delivery and guanidylated chitosan-copper chelates for drug-resistant lung cancer treatment [27,90]. The specific modifications and conjugations in these strategies enable precise targeting of cancer cells and overcoming drug resistance.
- CuO-NiO@PDA-PTX/FA Nanocarriers for Breast Cancer Therapy: The development of CuO-NiO@PDA-PTX/FA nanocarriers offers tumor-targeting capability and sustained drug release for breast cancer therapy, improving treatment outcomes [91]. The combination of metal nanoparticles, PDA coating, and folate targeting enhances drug delivery specificity and sustained release, leading to improved therapeutic effects.
- Light-Controlled “Trojan Horse” Strategy for Efficient Drug Delivery: The light-controlled “Trojan horse” strategy emerges as a powerful approach for efficient and targeted drug delivery, advancing drug delivery systems in cancer therapy [92]. The design of this strategy allows for controlled drug release upon light activation, enabling precise targeting and delivery to cancer cells.
- Dopamine-Based Molecular Imprinting Polymers for Drug Analysis and Diagnostics: Dopamine-based molecular imprinting polymers provide a selective electrochemical sensor for drug analysis and clinical diagnostics [30]. The specific molecular imprinting in these polymers allows for highly selective detection of drugs, making them valuable tools for drug analysis and diagnostics.
10.7. Tissue Engineering
- HA-Based Bioink for Cartilage Tissue Engineering in 3D Bioprinting: The use of HA-based bioink in 3D bioprinting shows promise for cartilage tissue engineering, providing an optimal niche for chondrocyte growth and potential treatment for osteochondral disorders [61]. The structure of the HA-based bioink influences its biocompatibility, mechanical properties, and ability to support chondrocyte growth and cartilage regeneration.
- Functionalized and Electroconductive Scaffolds for Bone Tissue Engineering: Functionalized and electroconductive scaffolds offer improved cell-scaffold interactions, making them promising for bone tissue engineering and bone regeneration [58]. The specific functional groups and conductive properties integrated into the scaffolds enhance cellular attachment, proliferation, and osteogenic differentiation.
- Elastin-Like Polypeptides (ELPs) for Spheroid Fabrication: Elastin-like polypeptides (ELPs) provide a versatile and cost-effective platform for spheroid fabrication, advancing drug discovery, stem cell research, and tumor biology [28]. The structure of ELPs allows for controlled self-assembly into spheroids, making them valuable tools in various biomedical applications.
- Bio-Inspired Silica-Collagen Materials for Controlled Drug Delivery: Bio-inspired silica-collagen materials with controlled drug delivery capabilities are suitable for innovative biomedical devices [62]. The structure and composition of these materials allow for the controlled release of therapeutic agents, making them useful in various tissue engineering applications.
- Engineering Scaffolds with Cues from the Embryonic Tendon Microenvironment for Tendon Regeneration: Engineering scaffolds with cues from the embryonic tendon microenvironment enhances tendon regeneration, contributing to advancements in regenerative medicine [93]. The specific cues integrated into the scaffolds influence cellular responses and promote tendon tissue regeneration.
- Polydopamine-Based Surface Modification Techniques for Tissue Engineering Applications: Polydopamine-based surface modification techniques enhance tissue responses, promoting repair and immune processes in tissue engineering applications [63]. The structure of the polydopamine coating plays a key role in promoting tissue integration and regeneration.
- Collagen-Based Biocomposites for Bone and Cartilage Tissue Regeneration: Collagen-based biocomposites improve mechanical properties and osteoinductivity, promoting bone/cartilage tissue regeneration and therapeutic applications [64]. The composition and structure of these biocomposites enhance their mechanical strength and ability to support tissue regeneration.
- Mussel-Inspired Bioactive Scaffolds for Diabetic Wound Healing: The use of mussel-inspired bioactive scaffolds accelerates diabetic wound healing through antioxidant properties and inflammation regulation, addressing the challenges of non-healing diabetic wounds [65]. The mussel-inspired bioactive components contribute to improved wound healing and tissue repair.
- “Switchable Surfaces” of Microfibrous Scaffolds for Targeted Cell Recruitment: “Switchable surfaces” of microfibrous scaffolds enable targeted cell recruitment, advancing regenerative medicine and tissue engineering [48]. The design of these switchable surfaces allows for controlled release of bioactive factors, attracting specific cell types for tissue repair and regeneration.
- Anisotropic PEG Hydrogels for Heart Valve Tissue Engineering: Anisotropic PEG hydrogels with biomimetic design features offer transformative approaches for heart valve tissue engineering, modulating cell behavior and tissue development [19]. The anisotropic design of these hydrogels mimics native heart valve structure, promoting tissue-specific organization and function.
10.8. Wound Healing
- Oriented Antibacterial Sericin Microneedles for Wound Healing: Oriented antibacterial sericin microneedles demonstrate their potential in promoting wound healing in infected wounds through efficient penetration, directional traction, antibacterial activity, and skin repair [66]. The specific structure and composition of these microneedles play a crucial role in their antibacterial properties, mechanical strength, and ability to promote wound closure.
- pH and Calcium Ion Responsive SP Hydrogels for Intestinal Drug Delivery: pH and calcium ion responsive SP hydrogels hold promise as biodevices for intestinal drug delivery and injectable fillers for suturing small vessels [51]. The responsiveness of these hydrogels to changes in pH and calcium ion concentration influences their drug release behavior and their suitability for specific medical applications.
- Silk-Based Drug Delivery Systems for Targeted and Controlled Release: Silk-based drug delivery systems offer targeted and controlled drug release with customizable design and reduced side effects [11]. The structure of silk-based polymers allows for controlled drug release, making them valuable tools for delivering therapeutics with precision and minimizing side effects.
- MXene-Based Microneedle Dressing for Wound Healing and Soft Robotics: MXene-based microneedle dressing with biomimetic structure and conductive pathways advances wound healing and finds applications in artificial tendons and soft robotics [67]. The specific structure of MXene-based microneedles enables efficient drug delivery, electrical conduction, and tissue regeneration.
- Alginate-Based Helical Microfiber Composite Membranes for Joint Wound Dressings: Alginate-based helical microfiber composite membranes serve as versatile joint wound dressings, promoting healing, controlled drug release, and motion monitoring [39]. The unique helical structure of these composite membranes enhances their mechanical properties and drug-loading capacity, making them suitable for joint wound dressings.
- Mussel-Inspired Bioactive Scaffold CHS-PDA-2@EGF for Chronic Diabetic Wound Healing: Mussel-inspired bioactive scaffold CHS-PDA-2@EGF accelerates chronic diabetic wound healing by reducing inflammation and promoting tissue repair [65]. The incorporation of mussel-inspired bioactive components contributes to the scaffold’s bioactivity and ability to enhance wound healing processes.
11. Perspective
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, H.; Hwang, I.; Sung, M.; Lee, D.; Kim, J.H.; Kang, S.M.; Bae, W.G.; Jeong, H.E. Bio-inspired adhesive systems for next-generation green manufacturing. Int. J. Precis. Eng. Manuf.-Green Technol. 2014, 1, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Tian, Y.; Erickson, J.S.; Puthoff, J.; Autumn, K.; Pesika, N.S. Design and fabrication of Gecko-inspired adhesives. Langmuir 2012, 28, 5737–5742. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Gao, Q.; Zhang, W.; Zhou, W.; Shi, S.Q.; Xia, C.; Li, J. Bioinspired design by gecko structure and mussel chemistry for bio-based adhesive system through incorporating natural fibers. J. Clean. Prod. 2019, 236, 117591. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Mussel-inspired poly(hydroxyethyl acrylate-co-itaconic acid)-catechol/hyaluronic acid drug-in-adhesive patches for transdermal delivery of ketoprofen. Int. J. Pharm. 2022, 629, 12. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Barros, N.R.; Chen, Y.; Hosseini, V.; Wang, W.Y.; Nasiri, R.; Mahmoodi, M.; Yalcintas, E.P.; Haghniaz, R.; Mecwan, M.M.; Karamikamkar, S.; et al. Recent developments in mussel-inspired materials for biomedical applications. Biomater. Sci. 2021, 9, 6653–6672. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, D.E.; Rivera, J.G.; Gong, Y.K.; Lau, K.H.; He, L.; Varshney, R.; Messersmith, P.B. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials 2012, 33, 3783–3791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Gonzalez-Obeso, C.; Hartzell, E.J.; Scheel, R.A.; Kaplan, D.L. Delivering on the promise of recombinant silk-inspired proteins for drug delivery. Adv. Drug Deliv. Rev. 2023, 192, 19. [Google Scholar] [CrossRef]
- Blackman, L.D.; Oo, Z.Y.; Qu, Y.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. Antimicrobial Honey-Inspired Glucose-Responsive Nanoreactors by Polymerization-Induced Self-Assembly. ACS Appl. Mater. Interfaces 2020, 12, 11353–11362. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Strategies toward well-defined polymer nanoparticles inspired by nature: Chemistry versus versatility. J. Polym. Sci. Pol. Chem. 2012, 50, 1869–1880. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, M.; Xu, D.; Mao, L.; Huang, Q.; Deng, F.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile fabrication of glycosylated and PEGylated carbon nanotubes through the combination of mussel inspired chemistry and surface-initiated ATRP. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110157. [Google Scholar] [CrossRef] [PubMed]
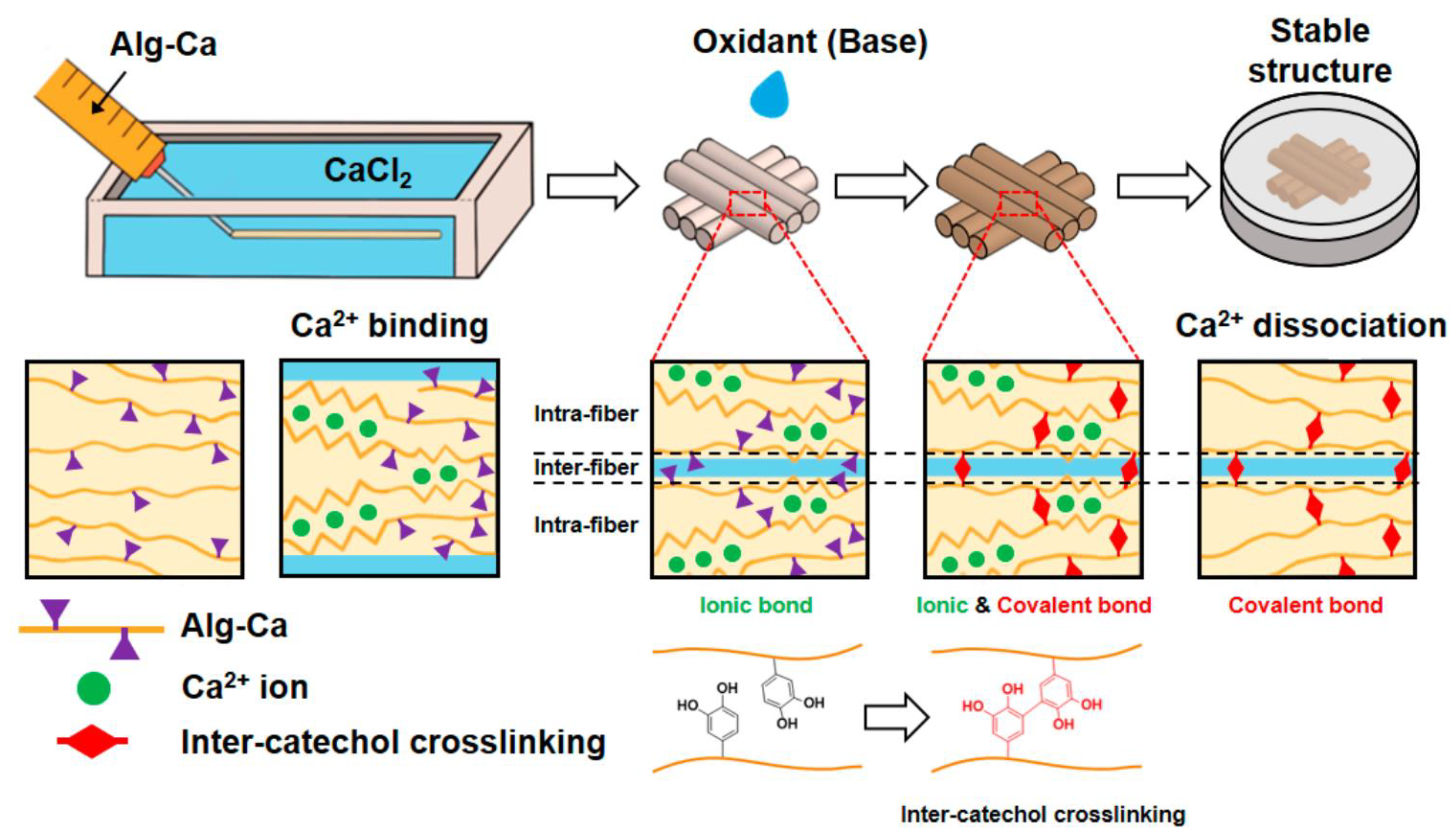
- Kim, K.; Choi, J.H.; Shin, M. Mechanical Stabilization of Alginate Hydrogel Fiber and 3D Constructs by Mussel-Inspired Catechol Modification. Polymers 2021, 13, 892. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Liu, X.; Chen, F.; Fu, Q. Mussel-inspired chemistry: A promising strategy for natural polysaccharides in biomedical applications. Prog. Polym. Sci. 2021, 123, 77. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.G.; Kang, E.T.; Ng, Y.X.; Teo, S.L. Tea stains-inspired initiator primer for surface grafting of antifouling and antimicrobial polymer brush coatings. Biomacromolecules 2015, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Ghosh, M.; Schnaider, L.; Adadi, N.; Ji, W.; Bychenko, D.; Dvir, T.; Adler-Abramovich, L.; Gazit, E. Composite of Peptide-Supramolecular Polymer and Covalent Polymer Comprises a New Multifunctional, Bio-Inspired Soft Material. Macromol. Rapid Commun. 2019, 40, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Puperi, D.S.; Yonezawa, A.L.; Wu, Y.; Tseng, H.; Cuchiara, M.L.; West, J.L.; Grande-Allen, K.J. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015, 14, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, Y.; Liu, P.; Li, J.; Song, M.; Cui, J.; Wei, L.; Yan, Y.; Liu, J. Kneading-Dough-Inspired Quickly Dispersing of Hydrophobic Particles into Aqueous Solutions for Designing Functional Hydrogels. Gels 2023, 9, 242. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Lin, Z.; Chen, J.; Zhang, Y.; Wang, C.; Qing, G.; Sun, Y.; Chi, Z. A methylation-inspired mesoporous coordination polymer for identification and removal of organic pollutants in aqueous solutions. J. Mater. Chem. B 2021, 9, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Dvoretckaia, A.; Egorova, T.; Dzhuzha, A.; Levit, M.; Sivtsov, E.; Demyanova, E.; Korzhikova-Vlakh, E. Polymyxin B Conjugates with Bio-Inspired Synthetic Polymers of Different Nature. Int. J. Mol. Sci. 2023, 24, 1832. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Cheng, C.; Ma, L.; Deng, J.; Zhao, C. Mussel-Inspired Antibacterial and Biocompatible Silver-Carbon Nanotube Composites: Green and Universal Nanointerfacial Functionalization. Langmuir 2016, 32, 5955–5965. [Google Scholar] [CrossRef] [PubMed]
- Kuksenok, O.; Singh, A.; Balazs, A.C. Designing polymer gels and composites that undergo bio-inspired phototactic reconfiguration and motion. Bioinspir. Biomim. 2018, 13, 035004. [Google Scholar] [CrossRef]
- He, C.; Ji, H.F.; Qian, Y.H.; Wang, Q.; Liu, X.L.; Zhao, W.F.; Zhao, C.S. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mat. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.; Wu, D.; Ma, S.; Wang, J.; Rao, J.; Xu, L.; Xu, H.; Shao, H.; Guo, Z.; et al. Protein-mimicking nanowire-inspired electro-catalytic biosensor for probing acetylcholinesterase activity and its inhibitors. Talanta 2018, 183, 258–267. [Google Scholar] [CrossRef]
- Naik, P.K.; Lopus, M.; Aneja, R.; Vangapandu, S.N.; Joshi, H.C. In silico inspired design and synthesis of a novel tubulin-binding anti-cancer drug: Folate conjugated noscapine (Targetin). J. Comput. Aided Mol. Des. 2012, 26, 233–247. [Google Scholar] [CrossRef]
- Goel, R.; Gulwani, D.; Upadhyay, P.; Sarangthem, V.; Singh, T.D. Unsung versatility of elastin-like polypeptide inspired spheroid fabrication: A review. Int. J. Biol. Macromol. 2023, 234, 13. [Google Scholar] [CrossRef]
- Niu, J.Q.; Wang, H.H.; Chen, J.; Chen, X.Q.; Han, X.; Liu, H.L. Bio-inspired zwitterionic copolymers for antifouling surface and oil-water separation. Colloid. Surf. A-Physicochem. Eng. Asp. 2021, 626, 10. [Google Scholar] [CrossRef]
- Zaidi, S.A. Effective imprinting of an anticancer drug, 6-thioguanine, via mussel-inspired self-polymerization of dopamine over reduced graphene oxide. Analyst 2019, 144, 2345–2352. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Wolski, K.; Grzes, G.; Isse, A.A.; Gennaro, A.; Zapotoczny, S.; Sobkowiak, A. Tannic Acid-Inspired Star-Like Macromolecules via Temporally Controlled Multi-Step Potential Electrolysis. Macromol. Chem. Phys. 2019, 220, 8. [Google Scholar] [CrossRef]
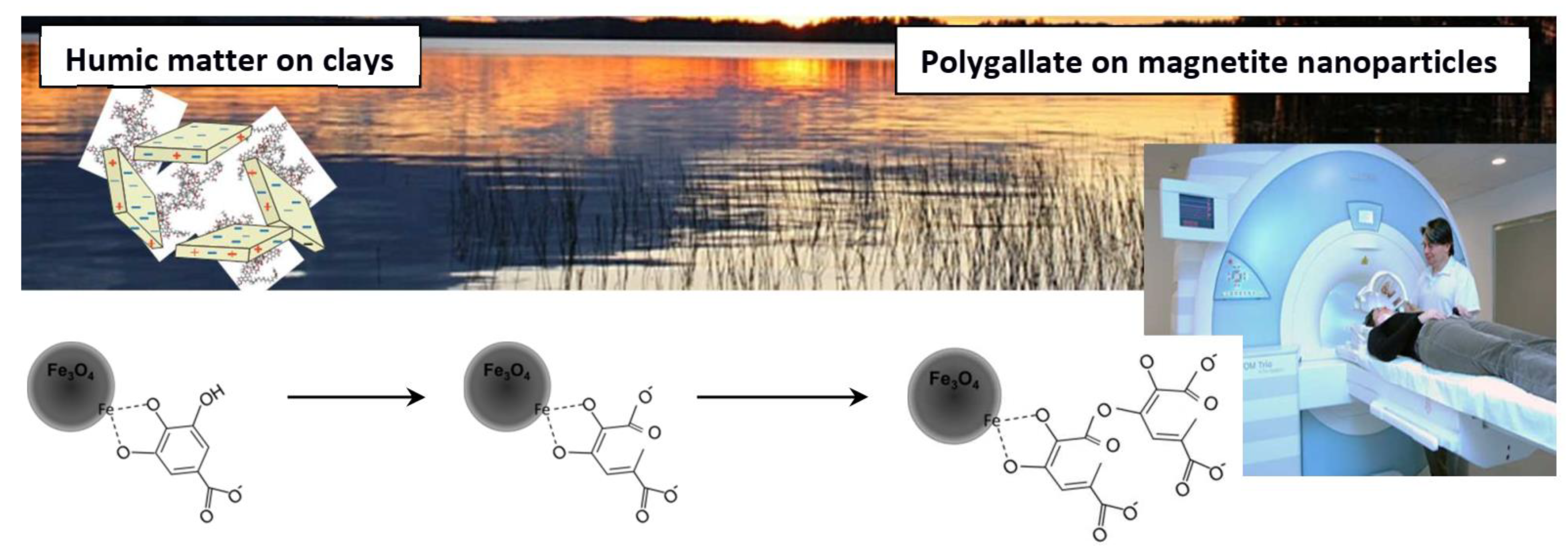
- Toth, I.Y.; Szekeres, M.; Turcu, R.; Saringer, S.; Illes, E.; Nesztor, D.; Tombacz, E. Mechanism of in Situ Surface Polymerization of Gallic Acid in an Environmental-Inspired Preparation of Carboxylated Core Shell Magnetite Nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.X.; Zhang, Q.; Wu, B.; Gao, X.D.; Liu, Z.Y.; Yang, H.Y.; Yuan, J.K.; Huang, J.J. Mussel-Inspired, Underwater Self-Healing Ionoelastomers Based on alpha-Lipoic Acid for Iontronics. Small 2023, 16, 2207334. [Google Scholar] [CrossRef]
- Hafner, D.; Ziegler, L.; Ichwan, M.; Zhang, T.; Schneider, M.; Schiffmann, M.; Thomas, C.; Hinrichs, K.; Jordan, R.; Amin, I. Mussel-Inspired Polymer Carpets: Direct Photografting of Polymer Brushes on Polydopamine Nanosheets for Controlled Cell Adhesion. Adv. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Jawadi, Z.; Yang, C.; Haidar, Z.S.; Santa Maria, P.L.; Massa, S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers 2022, 14, 5459. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ji, T.; Liang, K.; Zhu, L. Mussel-inspired soft-tissue adhesive based on poly(diol citrate) with catechol functionality. J. Mater. Sci. Mater. Med. 2016, 27, 30. [Google Scholar] [CrossRef]
- Kang, T.; Banquy, X.; Heo, J.; Lim, C.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.K.; Hong, Y.K.; Hwang, D.S.; et al. Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties. ACS Nano 2016, 10, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, H.; Hong, S. TAPE: A Biodegradable Hemostatic Glue Inspired by a Ubiquitous Compound in Plants for Surgical Application. J. Vis. Exp. 2016, 6, e53930. [Google Scholar] [CrossRef]
- Ma, W.; Ling, S.; Liu, Y.; Chen, Z.; Xu, J. Bio-Inspired Low-Cost Fabrication of Stretchable, Adhesive, Transparent, and Multi-Functionalized Joint Wound Dressings. ACS Appl. Mater. Interfaces 2023, 15, 22915–22928. [Google Scholar] [CrossRef]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.D.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E.; et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef]
- Owji, N.; Mandakhbayar, N.; Gregory, D.A.; Marcello, B.; Kim, H.W.; Roy, I.; Knowles, J.C. Mussel Inspired Chemistry and Bacteria Derived Polymers for Oral Mucosal Adhesion and Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yang, K.; Kim, M.J.; Jang, J.; Lee, M.; Kim, D.W.; Lee, H.; Cho, S.W. Bio-inspired oligovitronectin-grafted surface for enhanced self-renewal and long-term maintenance of human pluripotent stem cells under feeder-free conditions. Biomaterials 2015, 50, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Statz, A.; Finlay, J.; Dalsin, J.; Callow, M.; Callow, J.A.; Messersmith, P.B. Algal antifouling and fouling-release properties of metal surfaces coated with a polymer inspired by marine mussels. Biofouling 2006, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X.; Shen, S.G. Mussel-Inspired Polydopamine Coating: A General Strategy to Enhance Osteogenic Differentiation and Osseointegration for Diverse Implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef]
- Wang, Y.T.; Shang, L.R.; Chen, G.P.; Shao, C.M.; Liu, Y.X.; Lu, P.H.; Rong, F.; Zhao, Y.J. Pollen-inspired microparticles with strong adhesion for drug delivery. Appl. Mater. Today 2018, 13, 303–309. [Google Scholar] [CrossRef]
- Wolfel, A.; Euti, E.M.; Picchio, M.L.; Romero, M.R.; Josa, V.M.G.; Martinelli, M.; Minari, R.J.; Igarzabal, C.I.A. Unraveling the gallol-driven assembly mechanism of thermoreversible supramolecular hydrogels inspired by ascidians. Polym. Chem. 2020, 11, 7185–7198. [Google Scholar] [CrossRef]
- Xu, J.; Soliman, G.M.; Barralet, J.; Cerruti, M. Mollusk glue inspired mucoadhesives for biomedical applications. Langmuir 2012, 28, 14010–14017. [Google Scholar] [CrossRef]
- Zhang, J.G.; Chen, W.; Yu, L.X.; Li, M.J.; Neumann, F.; Li, W.Z.; Haag, R. Selective Endothelial Cell Adhesion via Mussel-Inspired Hybrid Microfibrous Scaffold. ACS Appl. Nano Mater. 2018, 1, 1513–1521. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, F.L.; Song, Y.Y.; Fan, J.B.; Wang, S.T. Recent Progress of Mussel-Inspired Underwater Adhesives. Chin. J. Chem. 2017, 35, 811–820. [Google Scholar] [CrossRef]
- Zhang, T.; Qu, Y.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S.; Blackman, L.D. Honey-inspired antimicrobial hydrogels resist bacterial colonization through twin synergistic mechanisms. Sci. Rep. 2020, 10, 15796. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Li, Y.; Xu, B.; Cao, Z.; Liu, W. Sea Cucumber-Inspired Autolytic Hydrogels Exhibiting Tunable High Mechanical Performances, Repairability, and Reusability. ACS Appl. Mater. Interfaces 2016, 8, 8956–8966. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Laird, D.; Zou, P.; Tomakidi, P.; Steinberg, T.; Lienkamp, K. Nature-inspired antimicrobial polymers--assessment of their potential for biomedical applications. PLoS ONE 2013, 8, e73812. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, G.; Xu, K.; Wang, L.; Yu, L.; Xing, M.M.Q.; Qiu, X. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 379–386. [Google Scholar] [CrossRef]
- Bao, B.; Tong, L.; Xu, Y.; Zhang, J.; Zhai, X.; Su, P.; Weng, L.; Wang, L. Mussel-inspired functionalization of semiconducting polymer nanoparticles for amplified photoacoustic imaging and photothermal therapy. Nanoscale 2019, 11, 14727–14733. [Google Scholar] [CrossRef]
- Du, J.; Niu, X.; Rahbar, N.; Soboyejo, W. Bio-inspired dental multilayers: Effects of layer architecture on the contact-induced deformation. Acta Biomater. 2013, 9, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Thomas, S. Functionalized theranostic nanocarriers with bio-inspired polydopamine for tumor imaging and chemo-photothermal therapy. J. Control. Release 2019, 309, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.N.; Liu, M.Y.; Wang, P.F.; Wang, K.; Zheng, X.Y.; Fan, D.D.; Hui, J.F.; Zhang, X.Y.; Wei, Y. Preparation of silica nanoparticles based multifunctional therapeutic systems via one-step mussel inspired modification. Chem. Eng. J. 2016, 296, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, F.; Ghalandari, B.; Khan, A.L.; Li, D.; Zamanian, A.; Yu, B. Decoration of electrical conductive polyurethane-polyaniline/polyvinyl alcohol matrixes with mussel-inspired polydopamine for bone tissue engineering. Biotechnol. Prog. 2020, 36, e3043. [Google Scholar] [CrossRef]
- Kim, M.W.; Kwon, S.H.; Choi, J.H.; Lee, A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Tang, Z.M.; Gao, Y.Q.; Sun, H.L.; Zhou, S.B. A Bio-Inspired Rod-Shaped Nanoplatform for Strongly Infecting Tumor Cells and Enhancing the Delivery Efficiency of Anticancer Drugs. Adv. Funct. Mater. 2016, 26, 66–79. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jimenez, G.; Chocarro, C.; Carrillo, E.; Montanez, E.; Galvez-Martin, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-inspired silica-collagen materials: Applications and perspectives in the medical field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Perikamana, S.K.M.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef]
- Qin, D.; Wang, N.; You, X.G.; Zhang, A.D.; Chen, X.G.; Liu, Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: Ongoing research and perspectives. Biomater. Sci. 2022, 10, 318–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Ren, D.Y.; Feng, Z.X.; Zhang, L.Y.; Zhong, Y.F.; Jin, M.Y.; Xu, F.W.; Feng, C.Y.; Du, Y.Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Bio 2022, 15, 15. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, C.; Zhu, Y.Y.; Liu, W.Y.; Li, H.L.; Wang, L.P.; Chen, W.; Wang, Z.; Wang, L. Lamprey-Teeth-Inspired Oriented Antibacterial Sericin Microneedles for Infected Wound Healing Improvement. Nano Lett. 2022, 22, 2702–2711. [Google Scholar] [CrossRef]
- Lu, H.H.; Shao, W.Y.; Gao, B.B.; Zheng, S.Y.; He, B.F. Intestine-inspire d wrinkle d MXene micronee dle dressings for smart wound management. Acta Biomater. 2023, 159, 201–210. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- de Espinosa, L.M.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired Polymer Systems with Stimuli-Responsive Mechanical Properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef] [Green Version]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Chinnu Sabu, C.; Rejo, C.; Kotta, S.; Pramod, K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J. Control. Release 2018, 287, 142–155. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Zhao, W.; Han, Y.; Luo, J.; Zhao, X.; Zhang, H. Bioinspired Polymeric Coating with Self-Adhesion, Lubrication, and Drug Release for Synergistic Bacteriostatic and Bactericidal Performance. Adv. Mater. Interfaces 2022, 9, 2200561. [Google Scholar] [CrossRef]
- Yan, C.; Jiang, P.; Jiac, X.; Wang, X. 3D printing of bioinspired textured surfaces with superamphiphobicity. Nanoscale 2020, 12, 2924–2938. [Google Scholar] [CrossRef]
- Hasan, M.N.; Shahriar, S.; Mondal, J.; Nurunnabi, M.; Lee, Y.K. Chapter SIX—Bioinspired and biomimetic materials for oral drug delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 89–104. [Google Scholar]
- Neubi, G.M.N.; Opoku-Damoah, Y.; Gu, X.; Han, Y.; Zhou, J.P.; Ding, Y. Bio-inspired drug delivery systems: An emerging platform for targeted cancer therapy. Biomater. Sci. 2018, 6, 958–973. [Google Scholar] [CrossRef]
- Ma, P.; Zhou, Z.P.; Dai, J.D.; Qin, L.; Ye, X.B.; Chen, X.; He, J.S.; Xie, A.T.; Yan, Y.S.; Li, C.X. A biomimetic Setaria viridis-inspired imprinted nanoadsorbent: Green synthesis and application to the highly selective and fast removal of sulfamethazine. RSC Adv. 2016, 6, 9619–9630. [Google Scholar] [CrossRef]
- Manna, K.; Patra, P.; Roy, A.; Roy, R.K.; Sunka, K.C.; Dhara, S.; Patra, N.; Pal, S. Amino Acid Inspired Alginate-Based pH Sensitive Polymeric Micelles via Reversible Addition-Fragmentation Chain Transfer Polymerization. ACS Appl. Polym. Mater. 2022, 4, 4432–4444. [Google Scholar] [CrossRef]
- Yan, Y.F.; Sun, T.; Zhang, H.B.; Ji, X.L.; Sun, Y.L.; Zhao, X.; Deng, L.F.; Qi, J.; Cui, W.G.; Santos, H.A.; et al. Euryale Ferox Seed-Inspired Superlubricated Nanoparticles for Treatment of Osteoarthritis. Adv. Funct. Mater. 2019, 29, 14. [Google Scholar] [CrossRef]
- Zeng, G.J.; Chen, T.T.; Huang, L.; Liu, M.Y.; Jiang, R.M.; Wang, Q.; Dai, Y.F.; Wen, Y.Q.; Zhang, X.Y.; Wei, Y. Surface modification and drug delivery applications of MoS2 nanosheets with polymers through the combination of mussel inspired chemistry and SET-LRP. J. Taiwan Inst. Chem. Eng. 2018, 82, 205–213. [Google Scholar] [CrossRef]
- Zeng, G.J.; Liu, M.Y.; Liu, X.H.; Huang, Q.; Xu, D.Z.; Mao, L.C.; Huang, H.Y.; Deng, F.J.; Zhang, X.Y.; Wei, Y. Mussel inspired preparation of MoS2 based polymer nanocomposites: The case of polyPEGMA. Appl. Surf. Sci. 2016, 387, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Fallah, M.A.; Huck, V.; Angerer, J.I.; Reininger, A.J.; Schneider, S.W.; Schneider, M.F.; Alexander-Katz, A. Blood-clotting-inspired reversible polymer-colloid composite assembly in flow. Nat. Commun. 2013, 4, 1333. [Google Scholar] [CrossRef] [Green Version]
- Czuban, M.; Kulka, M.W.; Wang, L.; Koliszak, A.; Achazi, K.; Schlaich, C.; Donskyi, I.S.; Di Luca, M.; Mejia Oneto, J.M.; Royzen, M.; et al. Titanium coating with mussel inspired polymer and bio-orthogonal chemistry enhances antimicrobial activity against Staphylococcus aureus. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111109. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.F.R.; Li, Y.C.; Liang, X.Y.; Shen, C.; Zeng, Z.N.; Xu, X.H. Virus-inspired nanoparticles as versatile antibacterial carriers for antibiotic delivery against Gram-negative and Gram-positive bacteria. Chin. Chem. Lett. 2022, 33, 1619–1622. [Google Scholar] [CrossRef]
- Choudhary, S.; Kalra, V.; Kumar, M.; Tiwary, A.K.; Sood, J.; Silakari, O. Bio-Inspired Strategies against Diabetes and Associated Complications: A Review. Recent. Pat. Drug Deliv. Formul. 2019, 13, 273–282. [Google Scholar] [CrossRef]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Stadler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Lai, J.J.; Huang, C.J. Bio-Inspired Amphoteric Polymer for Triggered-Release Drug Delivery on Breast Cancer Cells Based on Metal Coordination. ACS Appl. Mater. Interfaces 2021, 13, 25663–25673. [Google Scholar] [CrossRef]
- Cui, J.; Yan, Y.; Such, G.K.; Liang, K.; Ochs, C.J.; Postma, A.; Caruso, F. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules. Biomacromolecules 2012, 13, 2225–2228. [Google Scholar] [CrossRef]
- Pandit, A.; Khare, L.; Jahagirdar, D.; Srivastav, A.; Jain, R.; Dandekar, P. Probing synergistic interplay between bio-inspired peptidomimetic chitosan-copper complexes and doxorubicin. Int. J. Biol. Macromol. 2020, 161, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Exploration of polydopamine capped bimetallic oxide (CuO-NiO) nanoparticles inspired by mussels for enhanced and targeted paclitaxel delivery for synergistic breast cancer therapy. Appl. Surf. Sci. 2023, 626, 13. [Google Scholar] [CrossRef]
- Wang, Z.D.; Peng, C.; Zheng, Y.; Zhang, Y.W.; Xu, T.R.; Yan, J.Y.; Zhou, Y.Q.; Liu, Y.; Xie, H.X.; Wang, C.; et al. Enzyme-Inspired Nanoreactor Containing Light-Controlled “Trojan Horse” for Redox-Hypersensitive Drug Delivery. Adv. Funct. Mater. 2023, 12, 2214899. [Google Scholar] [CrossRef]
- Marturano, J.E.; Schiele, N.R.; Schiller, Z.A.; Galassi, T.V.; Stoppato, M.; Kuo, C.K. Embryonically inspired scaffolds regulate tenogenically differentiating cells. J. Biomech. 2016, 49, 3281–3288. [Google Scholar] [CrossRef] [PubMed]


| Application | Bioinspired Polymer | Key Findings | Ref. |
|---|---|---|---|
| Adhesion | DMA-MPC copolymers with antimicrobial silver nanoparticles | Catechol-containing copolymers reduce bacterial adhesion and resist BSA adsorption. Successful surface modification with antimicrobial silver nanoparticles. | [6] |
| Poly(hydroxyethyl acrylate-co-itaconic acid)-catechol (PHI-cat) adhesive | PHI-cat adhesive exhibits superior adhesion to human skin. Good stability, drug-polymer interaction, and adhesive performance. | [5] | |
| Catechol-containing hydrogel | The developed hydrogel covalently crosslinks with simultaneous silver reduction, enabling sustained silver release for at least two weeks. It exhibits antibacterial properties without significant effect on mammalian cell viability. The hydrogel films show resistance to bacterial and mammalian cell attachment. | [8] | |
| Recombinant silk biomaterials for drug delivery systems | The design and formulation of recombinant silk biomaterials for drug delivery systems (DDSs). It emphasizes the versatility of silk-based DDSs in various formats and highlights their properties and advantages, including processability, drug loading capacity, and degradability. | [11] | |
| Tannin-inspired gelatin bioadhesives | The tannin-inspired gelatin bioadhesives demonstrate excellent wet tissue adhesion strength and controllable degradation. They exhibit good cytocompatibility and possess inherent antibacterial and antifungal properties through the inclusion of tannic acid and silver nanoparticles. | [9] | |
| Functional polymer brushes on SS surface | The cationic polymer brushes exhibit bactericidal properties, while the zwitterionic coatings resist bacterial adhesion. The functional polymer-grafted surfaces show significant reduction in microalgal attachment (microfouling) and barnacle cyprid settlement (macrofouling) compared to the pristine SS surface. | [17] | |
| Poly(hydroxyethyl acrylate-co-itaconic acid)-catechol (PHI-cat) adhesive | PHI-cat adhesive exhibits superior adhesion to human skin. Good stability, drug-polymer interaction, and adhesive performance. | [5] | |
| Catechol-containing hydrogel | The developed hydrogel covalently crosslinks with simultaneous silver reduction, enabling sustained silver release for at least two weeks. It exhibits antibacterial properties without significant effect on mammalian cell viability. The hydrogel films show resistance to bacterial and mammalian cell attachment. | [8] | |
| Polydopamine (PDA) nanosheets | The 2D mussel-inspired PDA nanosheets and their polymer-brush derivatives show lateral integrity and robustness, promoting cell growth and attachment. A PDA-based poly(3-sulfopropyl methacrylate) carpet exhibits nonfouling behavior. | [34] | |
| Citrate-based tissue adhesives (POEC-d) | POEC-d adhesives with PEO to 1,8-octanediol mole ratio of 70% exhibited water solubility. They showed rubber-like behavior, a degradation rate of 1–2 weeks, and lap shear adhesion strength surpassing commercial fibrin glue. Low cytotoxicity was observed. | [36] | |
| Synthetic polymer-based dry adhesives | Adhesives show anisotropic adhesion properties similar to gecko adhesive system. Adhesion forces increase with lower tilt angles. High anisotropy in frictional adhesion behavior is observed. | [2] | |
| Triblock copolymers with catechol-anchoring groups | Robustly anchored triblock copolymers with loop conformations exhibit excellent lubricating properties, with an extremely low friction coefficient. These polymers also inhibit cell adhesion and proliferation compared to other conformations. | [37] | |
| TAPE (Tannic Acid-Poly(Ethylene) glycol adhesive) | TAPE demonstrates superior water-resistant adhesion compared to commercial fibrin glue. It is biodegradable under physiological conditions. | [38] | |
| Alginate-based helical fiber composite membranes | The composite membrane design incorporating alginate-based helical fibers and PAM-Gel achieved low-cost preparation, excellent mechanical properties, wound healing promotion, controlled drug release, and motion monitoring ability. The membranes demonstrated good biocompatibility and potential for application in joint wound dressings. | [39] | |
| Gecko-inspired tissue adhesive with nanoscale pillars | The adhesive demonstrates increased interfacial adhesion strength on tissue surfaces. Optimization of pillar dimensions and dextran coating enhance performance. | [40] | |
| Polyhydroxyalkanoates (PHA) films with polydopamine coating | Polydopamine coating enhances adhesive properties and promotes cell attachment and proliferation. In vivo biocompatibility is improved with reduced inflammation and neovascularization. | [41] | |
| Functionalization of polymer substrates with VN-dimer/pDA | The surface coating supports adhesion, proliferation, and colony formation of hPSCs. Enhanced focal adhesion and cell-cell interaction promote self-renewal and pluripotency of hPSCs. | [42] | |
| mPEG-DOPA3 modified titanium surfaces | The mPEG-DOPA3 modified titanium surfaces significantly decrease the attachment of diatom cells and algal zoospores compared to control surfaces. The modified surfaces show the highest detachment of attached cells under flow conditions, demonstrating their potential effectiveness in marine antifouling and fouling-release applications. | [43] | |
| PDA-coated implant surfaces | PDA coating improves hydrophilicity, enhances adhesion, proliferation, and osteogenic differentiation of bone marrow stem cells. | [44] | |
| Polymer microparticles with surface textures | The fabricated microparticles exhibit controllable surface roughness and strong adhesion to intestinal mucosa. Effective drug delivery is achieved. | [45] | |
| PVA/GA supramolecular hydrogels | The deacetylation degree of PVA affects gelation kinetics and properties. Interactions between GA and PVA chains enhance stability and elastic modulus. | [46] | |
| Chitosan mixed hydrogels with catechol compounds | Incorporation of HCA significantly enhances mucoadhesion. pH-dependent swelling behavior and controlled release of catechol compounds are observed. | [47] | |
| Thermoswitchable microfibrous scaffold (cRGD-PNIPAM) | The microfibrous scaffold exhibited accelerated endothelial cell adhesion and spreading through integrin-cRGD interaction below the LCST (25 degrees C), and rapid detachment at 37 degrees C as the cRGD became concealed. This reversible switchable property enables precise control over cell adhesion and detachment. | [48] | |
| Mussel-inspired underwater adhesive polymers | Recent studies show design progress in catechol-functional polymers for enhanced underwater adhesion. Properties and applications of these polymers reviewed. | [49] | |
| Biohybrid composite materials | Hydrogels with tunable mechanical properties, long-term enzymatic activities, low-fouling properties, and antimicrobial activity (>7-log reduction in bacterial adherence and viability) are potential biomedical substrates. | [50] | |
| Mechanical Properties | Fmoc-RGD/chitosan composite hydrogel | The composite hydrogel shows enhanced gelation rate, improved mechanical properties, and increased durability in cell culture. It serves as an effective scaffold for 2D and 3D cell cultures and displays notable antibacterial activity and silver nanoparticle production. | [18] |
| PEI/PAM composite hydrogel | The kneading-dough-inspired method disperses hydrophobic particles into water, forming stable suspensions. PEI/PAM composite hydrogel shows improved mechanical properties and stabilization mechanism. | [20] | |
| Anisotropic PEG hydrogels | PEG hydrogels with anisotropic mechanical properties were achieved via photolithographic patterning, exhibiting higher tensile modulus parallel to the stripes. RGDS and PQ peptide incorporation influenced VICs’ behavior and hydrogel degradation for heart valve tissue engineering. | [19] | |
| Mussel-inspired catechol crosslinked alginate hydrogel fibers | Catechol-tethered alginate fibers with inter-catechol crosslinking showed enhanced mechanical strength. The gluing capability of catechol stabilized fiber interfaces, maintaining shape fidelity of 3D constructs and facilitating cell encapsulation during culture. | [15] | |
| pH and calcium ion-responsive SP hydrogels | SP hydrogels doped with calcium ions exhibited excellent mechanical properties, transitioning between stiff and stable states and soft, highly swollen states with autolysis based on pH and calcium ion concentration. They also demonstrated thermoplasticity, self-healability, and biocompatibility for potential use in various applications. | [51] | |
| Antibacterial and antifouling properties | Glucose oxidase-loaded nanoreactors | The biohybrid nanomaterials exhibit antimicrobial activity against Gram-negative and Gram-positive bacterial pathogens, including drug-resistant strains. Their toxicity is optimized for safe use under physiological glucose concentrations. | [12] |
| Catechol-containing hydrogel | The developed hydrogel covalently crosslinks with simultaneous silver reduction, enabling sustained silver release for at least two weeks. It exhibits antibacterial properties without significant effect on mammalian cell viability. The hydrogel films show resistance to bacterial and mammalian cell attachment. | [8] | |
| Fmoc-RGD/chitosan composite hydrogel | The composite hydrogel shows enhanced gelation rate, improved mechanical properties, and increased durability in cell culture. It serves as an effective scaffold for 2D and 3D cell cultures and displays notable antibacterial activity and silver nanoparticle production. | [18] | |
| Phenylalanine-based metal-biomolecule complexes | The phenylalanine-based metal-biomolecule complexes demonstrate subtle sequence-dependent assembly behaviors and possess a mesoporous structure with high surface area and color-shifting feature for organic pollutant detection. They also exhibit antimicrobial properties. | [21] | |
| Polymeric conjugates of PMX B | Different polymeric conjugates of PMX B are synthesized, and PMAG demonstrates the most promising carrier properties for delivering PMX B, preserving its antimicrobial properties and enabling controlled drug release. Conjugate with deferoxamine shows the lowest MIC against Pseudomonas aeruginosa. | [22] | |
| SMAMPs (Synthetic Multifunctional Antimicrobial Polymers) | Certain hydrophilic SMAMPs demonstrated ‘doubly selective’ antimicrobial activity, targeting bacteria over mammalian cells and Gram-positive over Gram-negative bacteria. One SMAMP showed improved broad-band activity. Transmission electron studies revealed damage to bacterial membranes. | [52] | |
| AgNPs/PDA hydrogel | AgNPs/PDA hydrogel exhibits significant antibacterial activity, promotes bone generation, and efficiently repairs maxillary bone defects in vivo. | [53] | |
| Optical photothermal and conductive properties | Polydopamine-encapsulated semiconducting polymer | Polydopamine (PDA) encapsulation enables uniform-sized semiconducting polymer nanoparticles (SPNs) with enhanced structural stability, increased photothermal brightness, and improved photothermal therapy (PTT) efficacy for tumor ablation. | [54] |
| Contact-induced stresses in bio-inspired dental multilayers | Finite element modeling and experimental analysis demonstrate the influence of thickness and architecture on contact-induced stresses in bio-inspired dental multilayers. The multilayer structure significantly affects the stress distribution, with loading rate dependence accurately predicted. | [55] | |
| Polydopamine surface modification of nanocarriers | Polydopamine (PDA) serves as a versatile polymer for surface modification of nanocarriers, enabling chemo-photothermal therapy (PTT) and tumor imaging. PDA-coated nanosystems based on various materials provide enhanced functionalities and theranostic capabilities. | [56] | |
| SiO2-poly(PEGMA-IA-DA) nanoparticles | A biomimetic strategy successfully coated SiO2 NPs with hydrophilic polymers (poly(PEGMA-IA-DA)). The resulting nanoparticles exhibited high water dispersibility, low cytotoxicity, and controlled drug loading and release behavior, showing potential for biomedical applications. | [57] | |
| PVA/PU-PANI electroconductive scaffolds | Homogeneous decoration of the matrixes with PDA improved tensile strength and Young’s modulus. PDA modification enhanced hydrophilicity and supported hydroxyapatite-like crystal formation. Modified scaffolds showed suitable cell viability and enhanced osteogenic markers. | [58] | |
| Heparin-based and heparin-inspired hydrogels | The review categorizes different forms of heparin-based hydrogels and summarizes fabrication strategies, including covalent bonding and physical conjugation methods. Biomedical applications such as implantation, tissue engineering, biosensors, and drug release are discussed. | [25] | |
| Cancer | Polydopamine surface modification of nanocarriers | Polydopamine (PDA) serves as a versatile polymer for surface modification of nanocarriers, enabling chemo-photothermal therapy (PTT) and tumor imaging. PDA-coated nanosystems based on various materials provide enhanced functionalities and theranostic capabilities. | [56] |
| SiO2-poly(PEGMA-IA-DA) nanoparticles | A biomimetic strategy successfully coated SiO2 NPs with hydrophilic polymers (poly(PEGMA-IA-DA)). The resulting nanoparticles exhibited high water dispersibility, low cytotoxicity, and controlled drug loading and release behavior, showing potential for biomedical applications. | [57] | |
| Ligands and lipid-based drug delivery systems (LBDDSs) | The review analyzes ligands used for tumor-specific delivery, strategies for controlled drug release in tumor microenvironments, and recent designs of LBDDSs. It highlights the potential applications of functionalized LBDDSs in cancer therapy, contributing to improved treatment outcomes. | [59] | |
| Rod-like biodegradable polymer micellar system | Rod-shaped micelles, with dimensions of approximately 40 nm in diameter and 600 nm in length, exhibit minimal uptake by the reticuloendothelial system (RES), prolonged blood circulation half-lives, and improved delivery efficiency compared to spherical micelles. They demonstrate superior potency and efficacy against artificial solid tumors. | [60] | |
| Folate-conjugated noscapine derivative (Targetin) | Targetin, a folate-conjugated noscapine derivative, shows a strong binding affinity to tubulin and alters microtubule assembly dynamics. It exhibits enhanced sensitivity compared to noscapine in various cancer cell lines, particularly ovarian cancer cells that overexpress FRalpha. | [27] | |
| Tissue engineering | PVA/PU-PANI electroconductive scaffolds | Homogeneous decoration of the matrixes with PDA improved tensile strength and Young’s modulus. PDA modification enhanced hydrophilicity and supported hydroxyapatite-like crystal formation. Modified scaffolds showed suitable cell viability and enhanced osteogenic markers. | [58] |
| Bio-inspired silica-collagen | Bio-inspired silica-collagen materials exhibit diverse structures and properties. Polymer self-assembly and inorganic condensation interplay elucidated. Biocompatible with controlled drug delivery. | [62] | |
| Polydopamine-modified lyophilized collagen hyaluronic acid | The CHS-PDA-2@EGF wound dressing exhibited favorable physical and chemical properties, antioxidant effects, inflammation regulation, resistance to ROS, and promoted chronic wound regeneration in diabetic rats. It also displayed excellent swelling ability, coagulation effect, and reasonable degradation. | [65] | |
| Wound healing | Oriented antibacterial sericin microneedles | The designed microneedles efficiently penetrate and provide directional traction for wound closure. Sericin promotes skin repair through hair follicle regeneration and angiogenesis. Zinc oxide integration enhances the microneedles’ antibacterial activity. | [66] |
| MXene-based microneedle dressing | The MXene-based microneedle dressing offers ductility, biocompatibility, high conductivity, controllable drug delivery, and facilitates wound healing in animal models. Its biomimetic structure, controllable drug release, and conductive pathways contribute to intelligent wound management and other biomedical applications. | [67] | |
| Polydopamine-modified lyophilized collagen hyaluronic acid | The CHS-PDA-2@EGF wound dressing exhibited favorable physical and chemical properties, antioxidant effects, inflammation regulation, resistance to ROS, and promoted chronic wound regeneration in diabetic rats. It also displayed excellent swelling ability, coagulation effect, and reasonable degradation. | [65] | |
| Alginate-based helical fiber composite membranes | The composite membrane design incorporating alginate-based helical fibers and PAM-Gel achieved low-cost preparation, excellent mechanical properties, wound healing promotion, controlled drug release, and motion monitoring ability. The membranes demonstrated good biocompatibility and potential for application in joint wound dressings. | [39] | |
| pH and calcium ion-responsive SP hydrogels | SP hydrogels doped with calcium ions exhibited excellent mechanical properties, transitioning between stiff and stable states and soft, highly swollen states with autolysis based on pH and calcium ion concentration. They also demonstrated thermoplasticity, self-healability, and biocompatibility for potential use in various applications. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Wilson, R.L.; Babanejad, N. Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine. Life 2023, 13, 1673. https://doi.org/10.3390/life13081673
Omidian H, Wilson RL, Babanejad N. Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine. Life. 2023; 13(8):1673. https://doi.org/10.3390/life13081673
Chicago/Turabian StyleOmidian, Hossein, Renae L. Wilson, and Niloofar Babanejad. 2023. "Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine" Life 13, no. 8: 1673. https://doi.org/10.3390/life13081673
APA StyleOmidian, H., Wilson, R. L., & Babanejad, N. (2023). Bioinspired Polymers: Transformative Applications in Biomedicine and Regenerative Medicine. Life, 13(8), 1673. https://doi.org/10.3390/life13081673






