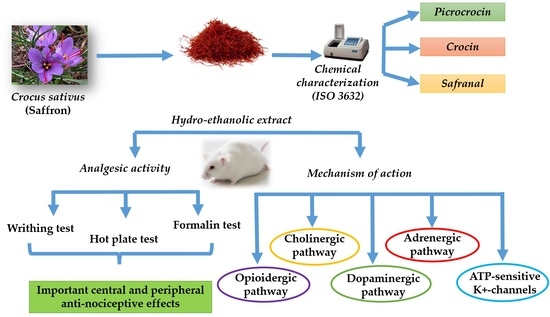
Safety Assessment and Pain Relief Properties of Saffron from Taliouine Region (Morocco)
Abstract
:1. Introduction
2. Results
2.1. CSSE Had No Toxic Effect
2.2. Analgesic Activity
2.2.1. CSSE Reduced the Abdominal Contractions Induced by Intraperitoneal Injection of Acetic Acid in Mice
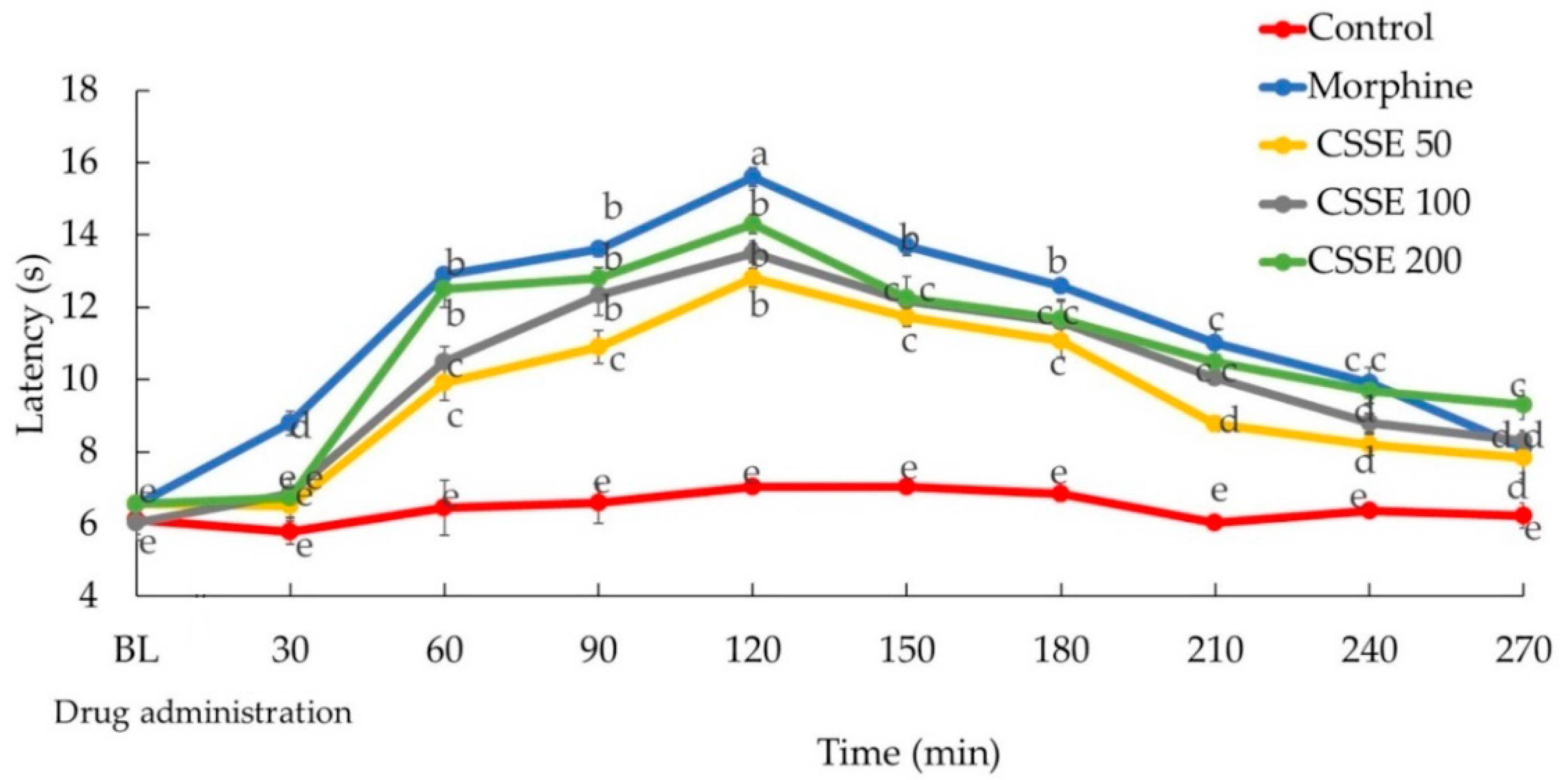
2.2.2. CSSE Increased Mice Latency Time to Respond to Heat-Induced Pain
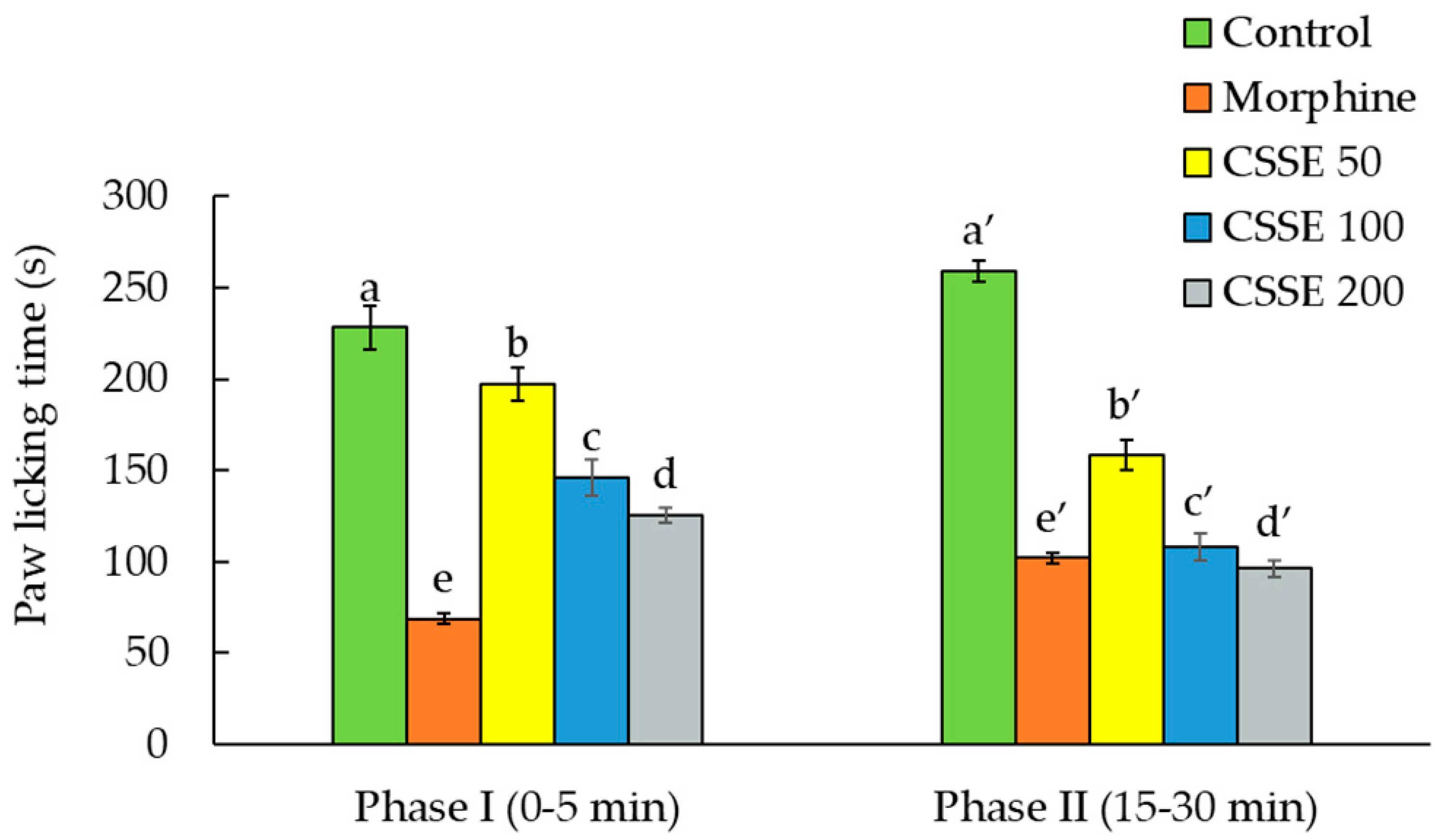
2.2.3. CSSE Inhibited the Pain Induced by Formalin Injection in the Animal Paw
2.3. Mechanistic Studies of CSSE Anti-Nociceptive Effect
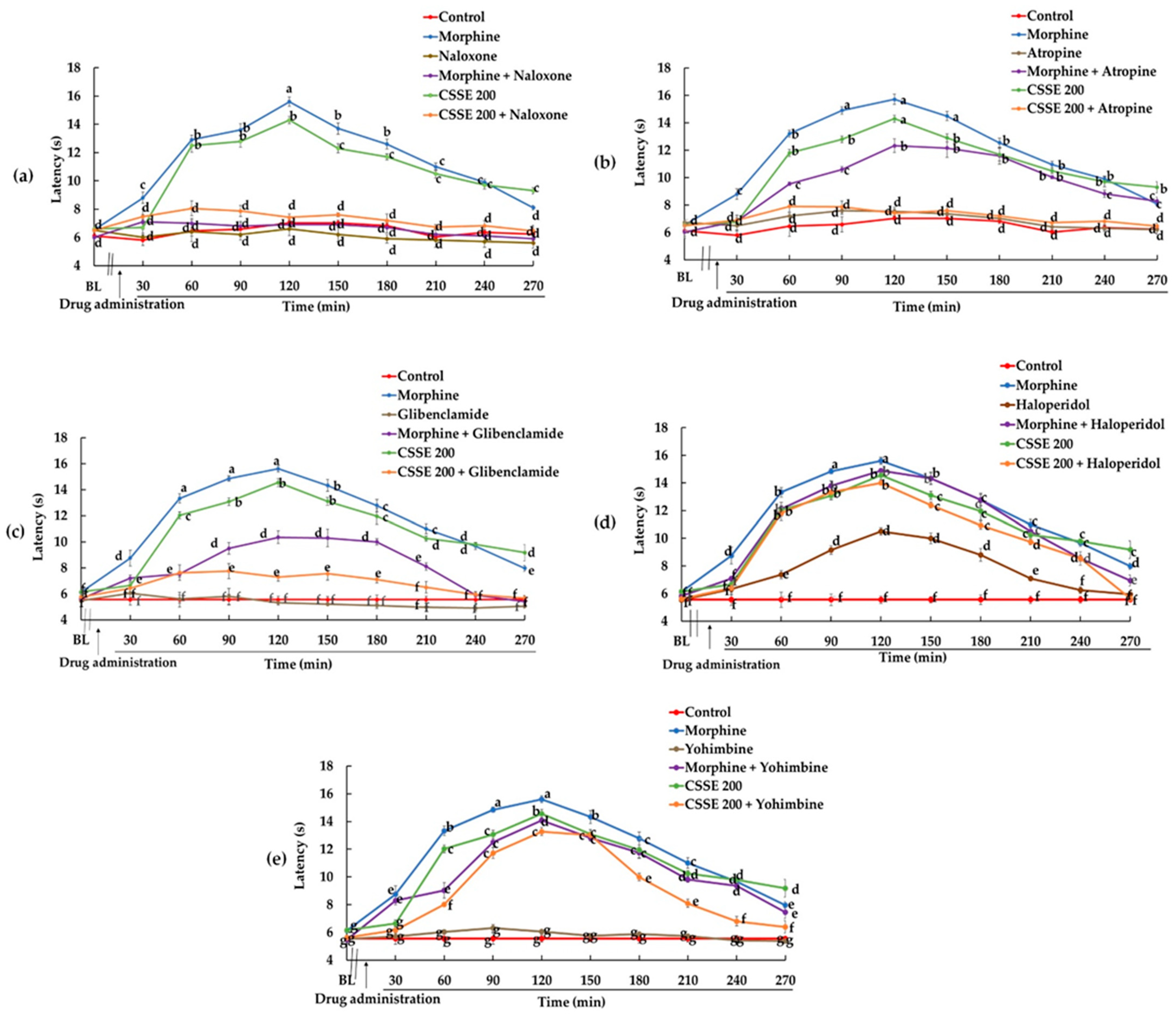
2.3.1. Involvement of the Opioidergic Pathway in CSSE Analgesic Effect
2.3.2. Involvement of the Cholinergic Pathway
2.3.3. Involvement of ATP Channel Blocker Pathway in CSSE Analgesic Effect
2.3.4. Involvement of Dopaminergic Pathway in CSSE Analgesic Effect
2.3.5. Involvement of the Adrenergic Pathway in the Mode of Action of CSSE
2.4. Chemical Characterization of Moroccan Saffron According to ISO 3632 Guidelines
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Standards and Reagents
4.3. Animals
4.4. Preparation of CSSE Extract
4.5. Acute Toxicity Test
4.6. Analgesic Activity
4.6.1. Acetic Acid-Induced Writhing Test
4.6.2. Hot Plate Test
4.6.3. Formalin-Induced Paw-Licking Test
4.7. Mechanistic Studies
4.7.1. The Examination of the Effect of CSSE on Opioidergic System
4.7.2. The Inspection of CSSE Nociceptive Activity on Cholinergic System
4.7.3. The Investigation of the CSSE Effect on K+-ATP Channel Blocker Pathway
4.7.4. The Study of CSSE Analgesic Effect on Dopaminergic Pathway
4.7.5. The Evaluation of CSSE Analgesic Effect on Adrenergic System
4.8. Chemical Characterization of Moroccan Saffron According to ISO 3632 Guidelines
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Sessle, B. Unrelieved pain: A crisis. Pain Res. Manag. 2011, 16, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Droney, J.M.; Gretton, S.K.; Sato, H.; Ross, J.R.; Branford, R.; Welsh, K.I.; Cookson, W.; Riley, J. Analgesia central side-effects: Two separate dimensions of morphine response. Br. J. Clin. Pharmacol. 2013, 75, 1340–1350. [Google Scholar] [CrossRef] [Green Version]
- Vella-Brincat, J.; Macleod, A.D. Adverse effects of opioids on the central nervous systems of palliative care patients. J. Pain Palliat. Care Pharmacother. 2007, 21, 15–25. [Google Scholar] [CrossRef]
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. Side effects of commonly prescribed analgesic medications. Phys. Med. Rehabil. Clin. 2014, 25, 457–470. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Bailliere’s Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Bathaie, S.Z. Historical uses of saffron: Identifying potential new avenues for modern research. Avicenna J. Phytomed. 2011, 1, 57–66. [Google Scholar]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (Crocus sativus L.): Gold of the spices—A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Lage, M.; Melai, B.; Cioni, P.L.; Flamini, G.; Gaboun, F.; Bakhy, K.; Zouahri, A.; Pistelli, L. Phytochemical composition of Moroccan saffron accessions by headspace solid phase microextraction. Am. J. Essent. Oil. 2015, 2, 1–7. [Google Scholar]
- Ministry of Agriculture, Fisheries, Rural Development, Water and Forests, Kingdom of Morocco: Saffron Sector. Available online: https://www.agriculture.gov.ma/fr/filiere/safran (accessed on 28 January 2022).
- Bagur, M.J.; Salinas, G.L.A.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V.; Kontakos, S.; Kontominas, M.G. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur. Food Res. Technol. 2017, 243, 1577–1591. [Google Scholar] [CrossRef]
- Khan, A.; Muhamad, N.A.; Ismail, H.; Nasir, A.; Khalil, A.A.K.; Anwar, Y.; Khan, Z.; Ali, A.; Taha, R.M.; Al-Shara, B.; et al. Potential nutraceutical benefits of in vivo grown saffron (Crocus sativus L.) as analgesic, anti-inflammatory, anticoagulant, and antidepressant in mice. Plants 2020, 9, 1414. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical composition and neuroprotective activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- International Organization for Standardization ISO 3632-2. Épices—Safran (Crocus sativus L.).—Partie 2: Méthodes D’essai; International Organization for Standardization: Geneva, Switzerland, 2010; Available online: https://www.iso.org/fr/standard/44526.html (accessed on 20 December 2019).
- Chaouqi, S.; Moratalla-López, N.; Lage, M.; Lorenzo, C.; Alonso, G.L.; Guedira, T. Effect of drying and storage process on Moroccan saffron quality. Food Biosci. 2018, 22, 146–153. [Google Scholar] [CrossRef]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Avila-Sosa, R. The relation between drying conditions and the development of volatile compounds in saffron (Crocus sativus). Molecules 2021, 26, 6954. [Google Scholar] [CrossRef]
- Tabtabaei, S.; D’Archivio, A.A.; Maggi, M.A.; Brutus, M.; Bajracharya, D.H.; Konakbayeva, D.; Soleimani, A.; Brim, H.; Ashktorab, H. Geographical classification of Iranian and Italian saffron sources based on HPLC analysis and UV–Vis spectra of aqueous extracts. Eur. Food Res. Technol. 2019, 245, 2435–2446. [Google Scholar] [CrossRef]
- Bharate, S.S.; Kumar, V.; Singh, G.; Singh, A.; Gupta, M.; Singh, D.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B. Preclinical development of Crocus sativus-based botanical lead IIIM-141 for Alzheimer’s disease: Chemical standardization, efficacy, formulation development, pharmacokinetics, and safety pharmacology. ACS Omega 2018, 3, 9572–9585. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, A.; Soliman, G.; Mahmoud, S.S.; Nofal, S.M.; Abdel-Rahman, R.F. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J. Saudi Chem. Soc. 2012, 16, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Betti, G.; Hensel, A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007, 157, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Giannoulaki, P.; Kotzakioulafi, E.; Chourdakis, M.; Hatzitolios, A.; Didangelos, T. Impact of Crocus sativus L. on metabolic profile in patients with diabetes mellitus or metabolic syndrome: A systematic review. Nutrients 2020, 12, 1424. [Google Scholar] [CrossRef]
- Rahim, V.B.; Khammar, M.T.; Rakhshandeh, H.; Samzadeh-Kermani, A.; Hosseini, A.; Askari, V.R. Crocin protects cardiomyocytes against LPS-Induced inflammation. Pharmacol. Rep. 2019, 71, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Pitsikas, N. Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules 2016, 21, 303. [Google Scholar] [CrossRef] [Green Version]
- Zandi, N.; Pazoki, B.; Roudsari, N.M.; Lashgari, N.; Jamshidi, V.; Momtaz, S.; Abdolghaffari, A.H.; Akhondzadeh, S. Prospects of saffron and its derivatives in Alzheimer’s disease. Arch. Iran Med. 2021, 24, 233–252. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. Toxicol. 2002, 2, 7. [Google Scholar]
- Azhari, S.; Ahmadi, S.; Rakhshandeh, H.; Jafarzadeh, H.; Mazlom, S.R. Evaluation of the effect of oral saffron capsules on pain intensity during the active phase of labor. Iran. J. Obstet. Gynecol. Infertil. 2014, 17, 1–10. [Google Scholar]
- Safakhah, H.A.; Taghavi, T.; Rashidy-Pour, A.; Vafaei, A.A.; Sokhanvar, M.; Mohebbi, N.; Rezaei-Tavirani, M. Effects of saffron (Crocus sativus L.) stigma extract and its active constituent crocin on neuropathic pain responses in a rat model of chronic constriction injury. Iran. J. Pharm. Sci. 2016, 15, 253. [Google Scholar]
- Mohammadierad, R.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M.; Fazil, F. Effect of saffron with or without date sugar on intensity of pain and anxiety during labor in primiparous females: A randomized, controlled trial. Iran. Red Crescent Med. J. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Saha, D.; Dubyak, P.J.; Anton, S.D. Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. J. Integr. Med. 2013, 11, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiee, M.; Arekhi, S.; Omranzadeh, A.; Sahebkar, A. Saffron in the treatment of depression, anxiety and other mental disorders: Current evidence and potential mechanisms of action. J. Affect. Disord. 2018, 227, 330–337. [Google Scholar] [CrossRef]
- Tóth, B.; Hegyi, P.; Lantos, T.; Szakács, Z.; Kerémi, B.; Varga, G.; Tenk, J.; Pétervári, E.; Balaskó, M.; Rumbus, Z.; et al. The efficacy of saffron in the treatment of mild to moderate depression: A meta-analysis. Planta Med. 2018, 85, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Jelodar, G.; Javid, Z.; Sahraian, A.; Jelodar, S. Saffron improved depression and reduced homocysteine level in patients with major depression: A randomized, double-blind study. Avicenna J. Phytomed. 2018, 8, 43–50. [Google Scholar] [PubMed]
- Kashani, L.; Esalatmanesh, S.; Eftekhari, F.; Salimi, S.; Foroughifar, T.; Etesam, F.; Safiaghdam, H.; Moazen-Zadeh, E.; Akhondzadeh, S. Efficacy of Crocus sativus (saffron) in treatment of major depressive disorder associated with post-menopausal hot flashes: A double-blind, randomized, placebo-controlled trial. Arch. Gynecol. Obstet. 2018, 297, 717–724. [Google Scholar] [CrossRef]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules 2021, 26, 86. [Google Scholar] [CrossRef]
- Gezici, S. Comparative anticancer activity analysis of saffron extracts and a principle component, crocetin for prevention and treatment of human malignancies. J. Food Sci. Technol. 2019, 56, 5435–5443. [Google Scholar] [CrossRef]
- Khorasanchia, Z.; Shafiee, M.; Kermanshahi, F.; Khazaei, M.; Ryzhikov, M.; Parizadeh, M.R.; Kermanshahi, B.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine 2018, 43, 21–27. [Google Scholar] [CrossRef]
- Naeimi, M.; Shafiee, M.; Kermanshahi, F.; Khorasanchi, Z.; Khazaei, M.; Ryzhikov, M.; Avan, A.; Gorji, N.; Hassanian, S.M. Saffron (Crocus sativus) in the treatment of gastrointestinal cancers: Current findings and potential mechanisms of action. J. Cell Biochem. 2019, 120, 16330–16339. [Google Scholar] [CrossRef]
- Shakeri, M.; Tayer, A.H.; Shakeri, H.; Jahromi, A.S.; Moradzadeh, M.; Hojjat-Farsangi, M. Toxicity of saffron extracts on cancer and normal cells: A review article. Asian Pac. J. Cancer Prev. 2020, 21, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gupta, S. Anticancer activity of Crocus sativus: A review. IJRAR 2018, 5, 851–854. [Google Scholar]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Wen, Y.-L.; He, Z.; Hou, D.-X.; Qin, S. Crocetin exerts its anti-inflammatory property in LPS-induced RAW264.7 cells potentially via modulation on the crosstalk between MEK1/JNK/NF-κB/iNOS pathway and Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6631929. [Google Scholar] [CrossRef]
- Akbari-Fakhrabadi, M.; Najafi, M.; Mortazavian, S.; Rasouli, M.; Memari, A.H.; Shidfar, F. Effect of saffron. (Crocus sativus L.) and endurance training on mitochondrial biogenesis, endurance capacity, inflammation, antioxidant, and metabolic biomarkers in Wistar rats. J. Food Biochem. 2019, 43, e12946. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Htar, T.T. Therapeutic properties of saffron and its chemical constituents. J. Nat. Prod. 2014, 7, 5–13. [Google Scholar]
- Siddiqui, M.J.; Saleh, M.S.M.; Basharuddin, S.N.B.; Zamri, S.H.; Mohd Najib, M.H.; Che Ibrahim, M.Z.; Mohd Noor, N.A.; Binti Mazha, H.N.; Hassan, N.M.; Khatib, A. Saffron (Crocus sativus L.): As an antidepressant. J. Pharm. Bioallied Sci. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Nabavi, S.M.; Sancarlo, D.; Greco, A.; Pieretti, S. Crocus sativus L. (Saffron) in Alzheimer’s disease treatment: Bioactive effects on cognitive impairment. Curr. Neuropharm. 2021, 19, 1606–1616. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). LD50 Test Policy. Fed. Regist. 1988, 53, 39650–39651. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). OECD Guidelines for the Testing of Chemicals, Section 4/Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method; Editions OCDE: Paris, France, 2002; pp. 1–14. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharm. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Deng, J.; Han, J.; Chen, J.; Zhang, Y.; Huang, Q.; Wang, Y.; Xiaoxiao, Q.; Zhongqiu, L.; Elaine Lai-Han, L.; Dawei, W.; et al. Comparison of analgesic activities of aconitine in different mice pain models. PLoS ONE 2021, 16, e0249276. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.V.; Canzian, J.; Stefanello, F.V.; Kalueff, A.V.; Rosemberg, D.B. Naloxone prolongs abdominal constriction writhing-like behavior in a zebrafish-based pain model. Neurosci. Lett. 2019, 708, 134336. [Google Scholar] [CrossRef]
- Giorno, T.B.S.; Santos, C.H.C.D.; Carvalho, M.G.D.; Silva, V.C.D.; Sousa, P.T.D.; Fernandes, P.D.; Boylan, F. Study on the antinociceptive activity and mechanism of action of isolated saponins from siolmatra brasiliensis (Cogn.) Baill. Molecules 2019, 24, 4584. [Google Scholar] [CrossRef] [Green Version]
- León-Reyes, M.R.; Castañeda-Hernández, G.; Ortiz, M.I. Pharmacokinetic of diclofenac in the presence and absence of glibenclamide in the rat. J. Pharm. Pharm. Sci. 2009, 12, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.N.; Coetzee, W.A. KATP Channels in the Cardiovascular System. Physiol. Rev. 2016, 96, 177–252. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Liu, L.; Hu, X.; Yu, D.; Dong, S.; Du, J.; Xu, Y.; Liu, B.; Yang, Y.; Gao, F.; et al. KATP Channel Blocker Glibenclamide Prevents Radiation-Induced Lung Injury and Inhibits Radiation-Induced Apoptosis of Vascular Endothelial Cells by Increased Ca2+ Influx and Subsequent PKC Activation. Radiat. Res. 2020, 193, 171–185. [Google Scholar] [CrossRef]
- Becker, A.; Grecksch, G.; Zernig, G.; Ladstaetter, E.; Hiemke, C.; Schmitt, U. Haloperidol and risperidone have specific effects on altered pain sensitivity in the ketamine model of schizophrenia. Psychopharmacology 2009, 202, 579–587. [Google Scholar] [CrossRef]
- Vo, L.; Hood, S.; Drummond, P.D. Involvement of opioid receptors and α2-adrenoceptors in inhibitory pain modulation processes: A double-blind placebo-controlled crossover study. J. Pain 2016, 17, 1164–1173. [Google Scholar] [CrossRef]
- Garcia, M.D.; Fernandez, M.A.; Alvarez, A.; Saenz, M.T. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa varozua (Mirtaceae). J. Ethnopharmacol. 2004, 91, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Pain Medicine: A Comprehensive Review, 2nd ed.; Mosby Publications: St. Louis, MI, USA; Philadelphia, PA, USA, 2003; p. 448. [Google Scholar]
- Bley, K.R.; Hunter, J.C.; Eglen, R.M.; Smith, J.A. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol. Sci. 1998, 19, 141–147. [Google Scholar] [CrossRef]
- Chau, T.T. Analgesic testing in animal’s models. In Pharmacological Methods in the Control of Inflammation; Alan R. Liss, Inc.: New York, NY, USA, 1989; pp. 195–212. [Google Scholar]
- Tsai, H.Y.; Lin, Y.T.; Tsai, C.H.; Chen, Y.F. Effects of paeoniflorin on the formalin-induced nociceptive behavior in mice. J. Ethnopharmacol. 2001, 75, 267–271. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Vilela, F.C.; da Rocha, C.Q.; Dias, D.F.; Cavalcante, G.P.; Freitas, L.A.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J. Ethnopharmacol. 2011, 133, 467–473. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sedighifar, S. Analgesic and anti-inflammatory properties of the hydroalcoholic, polyphenolic and boiled extracts of Stachys lavandulifolia. Res. Pharm. Sci. 2007, 1, 92–98. [Google Scholar]
- Muhammad, N. In-Vivo Models for Management of Pain. Pharmacol. Pharm. 2014, 5, 42226. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, B.L. Opioid receptors: From genes to mice. J. Pain 2000, 1, 45–50. [Google Scholar] [CrossRef]
- Wess, J.; Duttaroy, A.; Gomeza, J.; Zhang, W.; Yamada, M.; Felder, C.C.; Bernardini, N.; Reeh, P.W. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: A review. Life Sci. 2003, 72, 2047–2954. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Roczniak, W.; Wrobel, J.; Dolczak, L.; Nowak, P. Influence of central noradrenergic system lesion on the serotoninergic 5-HT3 receptor mediated analgesia in rats. Adv. Clin. Exp. Med. 2013, 22, 629–638. [Google Scholar]
- Erfanparast, A.; Tamaddonfard, E.; Henareh-Chareh, F. Central H2 histaminergic and alpha-2 adrenergic receptors involvement in crocetin-induced antinociception in orofacial formalin pain in rat. Vet. Res. Forum 2020, 11, 229–234. [Google Scholar] [PubMed]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurotherap. 2008, 8, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Reinig, S.; Driever, W.; Arrenberg, A.B. The descending diencephalic dopamine system is tuned to sensory stimuli. Curr. Biol. 2017, 27, 318–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.A.; Abdul Rahim, M.H.; Roosli, R.A.; Mohd Sani, M.H.; Omar, M.H.; Tohid, S.F.M.; Othman, F.; Ching, S.M.; Abdul Kadir, A. Antinociceptive activity of methanolic extract of Clinacanthus nutans leaves: Possible mechanisms of action involved. Pain Res. Manag. 2018, 2018, 9536406. [Google Scholar] [CrossRef] [Green Version]
- Ettehadi, H.; Mojabi, S.N.; Ranjbaran, M.; Shams, J.; Sahraei, H.; Hedayati, M.; Asefi, F. Aqueous extract of saffron (Crocus sativus) increases brain dopamine and glutamate concentrations in rats. J. Behav. Brain Sci. 2013, 3, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Monchaux De Oliveira, C.; Pourtau, L.; Vancassel, S.; Pouchieu, C.; Capuron, L.; Gaudout, D.; Castanon, N. Saffron extract-induced improvement of depressive-like behavior in mice is associated with modulation of monoaminergic neurotransmission. Nutrients 2021, 13, 904. [Google Scholar] [CrossRef]
- Fuhlendorff, J.; Rorsman, P.; Kofod, H.; Brand, C.L.; Rolin, B.; MacKay, P.; Shymko, R.; Carr, R.D. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes 1998, 47, 345–351. [Google Scholar] [CrossRef]
- Laughlin, T.M.; Tram, K.V.; Wilcox, G.L.; Birnbaum, A.K. Comparison of antiepileptic drugs tiagabine, lamotrigine, and gabapentin in mouse models of acute, prolonged, and chronic nociception. J. Pharmacol. Exp. Ther. 2002, 302, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Kwon, Y.B.; Roh, D.H.; Yoon, S.Y.; Han, H.J.; Kim, K.W.; Lee, J.H. Intrathecal treatment with σ1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br. J. Pharmacol. 2006, 148, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Rangel, R.A.; Marinho, B.G.; Fernandes, P.D.; De Moura, R.S.; Lessa, M.A. Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice. Fundam. Clin. Pharmacol. 2012, 28, 104–113. [Google Scholar] [CrossRef] [Green Version]
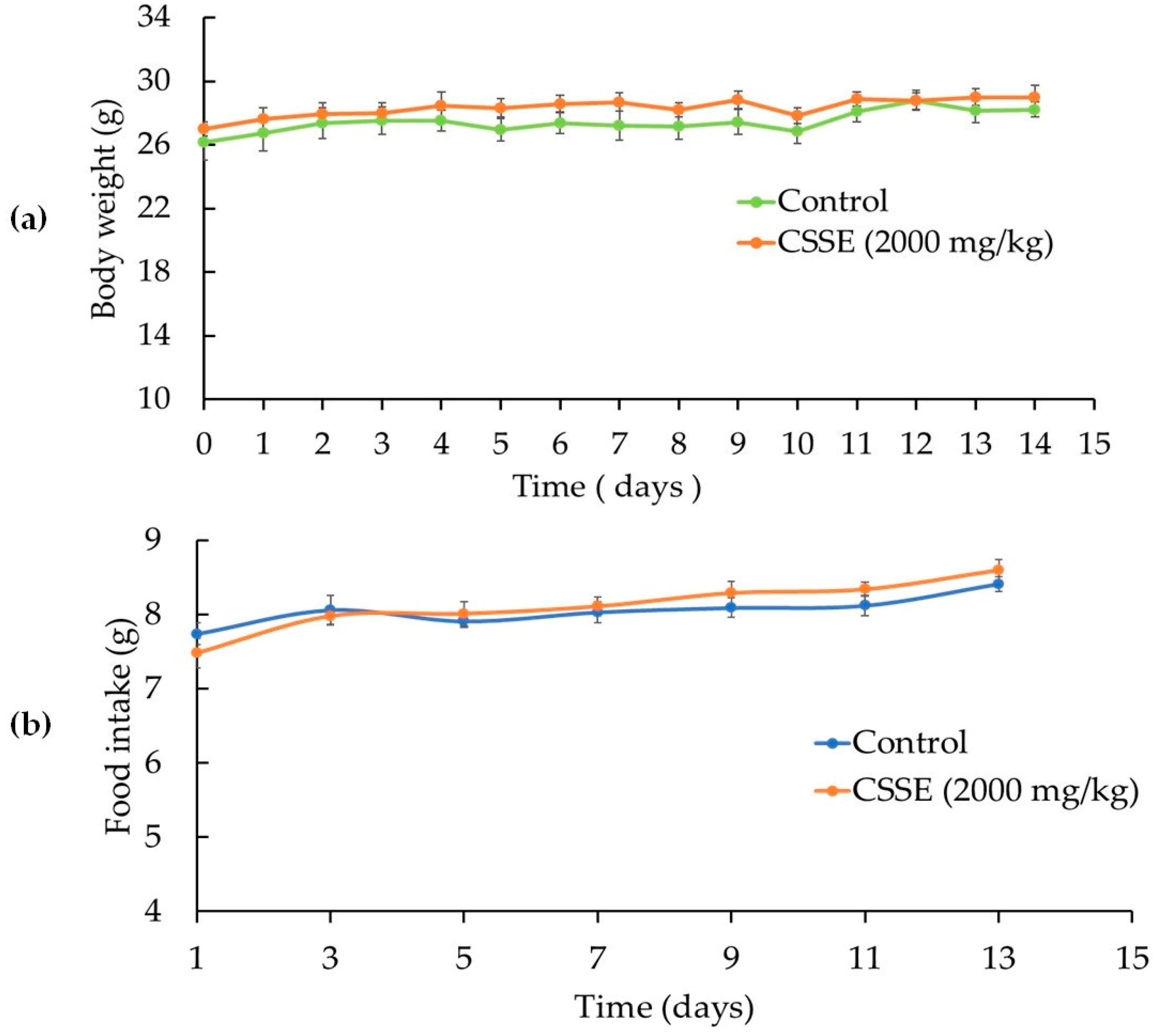
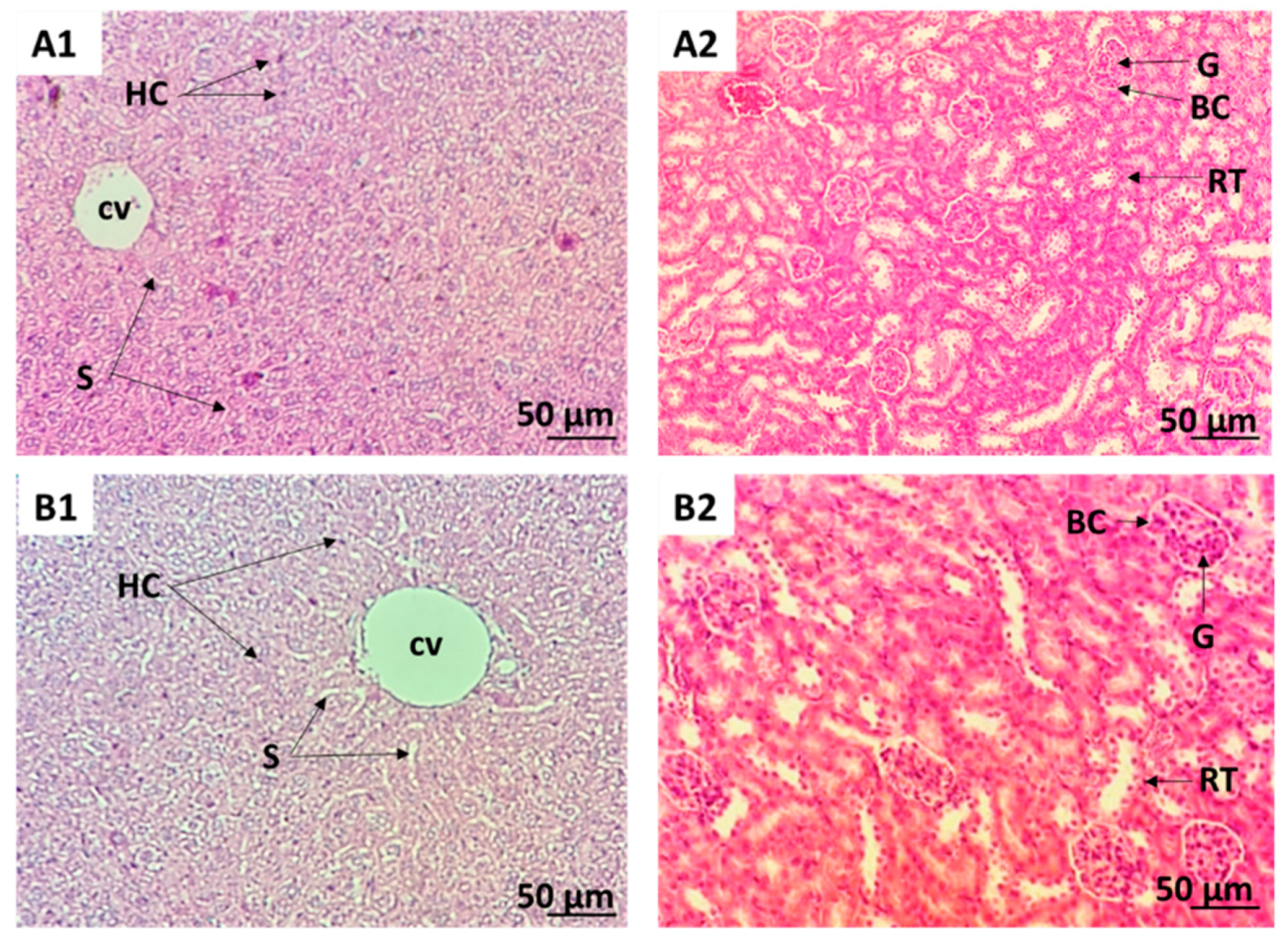

| Group | Dose (mg/kg b.w p.o.) | Sex | D/T | Symptoms |
|---|---|---|---|---|
| Vehicle | 0 | Female | 0/6 | None |
| CSSE | 2000 | Female | 0/6 | None |
| Organ | Relative Organ Weight (g/100 g b.w) | |
|---|---|---|
| Control | CSSE (2000 mg/kg) | |
| Heart | 0.52 ± 0.04 | 0.59 ± 0.04 |
| Lungs | 0.93 ± 0.05 | 0.87 ± 0.08 |
| Liver | 0.06 ± 0.01 | 0.06 ± 0.01 |
| Spleen | 0.43 ± 0.06 | 0.51 ± 0.05 |
| Right kidney | 0.49 ± 0.01 | 0.54 ± 0.05 |
| Left kidney | 0.57 ± 0.01 | 0.56 ± 0.05 |
| Stomach | 0.77 ± 0.05 | 1.08 ± 0.16 |
| Parameters | Unit | Control | CSSE (2000 mg/kg) | |
|---|---|---|---|---|
| Hematological | ||||
| White blood cell count (WBC) | 103/µL | 6.8 ± 0.4 | 6.2 ± 0.8 | |
| Red blood cell count (RBC) | 106/µL | 8.8 ± 0.3 | 8.6 ± 0.4 | |
| Hemoglobin (HGB) | g/dL | 13.5 ± 0.8 | 13.2 ± 1.2 | |
| Hematocrit (HCT) | % | 44.4 ± 2.3 | 42.3 ± 3.6 | |
| Platelets (PLT) | 103/µL | 732 ± 40.3 | 694 ± 73.1 | |
| Mean corpuscular volume (MCV) | fL | 49.7 ± 1.3 | 47.4 ± 2.0 | |
| Mean corpuscular hemoglobin (MCH) | pg | 15.1 ± 0.7 | 16.0 ± 1.3 | |
| Mean corpuscular hemoglobin concentration (MCHC) | g/dL | 30.4 ± 2.1 | 31.7 ± 1.9 | |
| Red cell distribution width (RDW) | % | 22.0 ± 0.3 | 22.9 ± 0.7 | |
| Mean platelet volume (MPV) | fL | 8.0 ± 0.5 | 9.2 ± 0.8 | |
| Neutrophil | % | 10.6 ± 1.2 | 12.2 ± 2.2 | |
| Lymphocyte | % | 84.3 ± 5.9 | 76.9 ± 6.2 | |
| Monocyte | % | 4.7 ± 0.2 | 4.1 ± 0.5 | |
| Biochemical | ||||
| Alanine aminotransferase (ALT) | UI/L | 17 ± 2.00 | 16.25 ± 2.19 | |
| Aspartate aminotransferase (AST) | UI/L | 792 ± 60.50 | 838 ± 75.00 | |
| Creatinine | mg/dL | 3 ± 0.14 | 3 ± 0.09 | |
| Urea | g/L | 0.45 ± 0.04 | 0.50 ± 0.03 | |
| Alkaline phosphatase (ALP) | UI/L | 95.5 ± 7.5 | 89.75 ± 5.38 | |
| Total bilirubin | mg/dL | 0.30 ± 0.05 | 0.33 ± 0.09 | |
| Group | Dose (mg/kg, b.w) | Number of Contortions | Inhibition of Pain (%) | Relative Activity Compared to Morphine (%) |
|---|---|---|---|---|
| Control | - | 34.2 ± 1.5 | - | - |
| Morphine | 1 | 5.3 ± 0.7 *** | 84.5 ± 0.5 | 100 |
| CSSE | 50 | 17.7 ± 0.3 *** | 48.3 ± 0.8 +++ | 57.2 |
| 100 | 13.5 ± 0.4 *** | 60.5 ± 1.0 +++ | 71.6 | |
| 200 | 9.7 ± 0.4 *** | 71.6 ± 0.9 ++ | 84.7 |
| Strength Indicator In: | A1%1cm Measure | Saffron Samples | ISO 3632 Category 1 | Quality Conformity | ||
|---|---|---|---|---|---|---|
| Pigments | Picrocrocin | Flavor | λ 257 | 525.5 ± 0.4 | min. 70 | First Quality Category |
| Crocin | Coloring | λ 440 | 1126.3 ± 0.5 | min. 200 | First Quality Category | |
| Safranal | Aroma | λ 330 | 32.4 ± 0.2 | min. 20; max. 50 | First Quality Category | |
| Moisture content | - | - | 10.3 ± 0.24% | max 12% | Conform | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Tastift, M.; Makbal, R.; Bourhim, T.; Omari, Z.; Isoda, H.; Gadhi, C. Safety Assessment and Pain Relief Properties of Saffron from Taliouine Region (Morocco). Molecules 2022, 27, 3339. https://doi.org/10.3390/molecules27103339
Ait Tastift M, Makbal R, Bourhim T, Omari Z, Isoda H, Gadhi C. Safety Assessment and Pain Relief Properties of Saffron from Taliouine Region (Morocco). Molecules. 2022; 27(10):3339. https://doi.org/10.3390/molecules27103339
Chicago/Turabian StyleAit Tastift, Maroua, Rachida Makbal, Thouria Bourhim, Zineb Omari, Hiroko Isoda, and Chemseddoha Gadhi. 2022. "Safety Assessment and Pain Relief Properties of Saffron from Taliouine Region (Morocco)" Molecules 27, no. 10: 3339. https://doi.org/10.3390/molecules27103339