Effect of Amylose and Crystallinity Pattern on the Gelatinization Behavior of Cross-Linked Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cross-Linked Starch (CLs)
2.3. X-ray Diffraction (XRD)
2.4. Light Microscopy
2.5. Differential Scanning Calorimetry (DSC)
2.6. Pasting Properties
2.7. Iodine-Binding Capacity (IBC)
2.8. Statistical Analysis
3. Results and Discussion
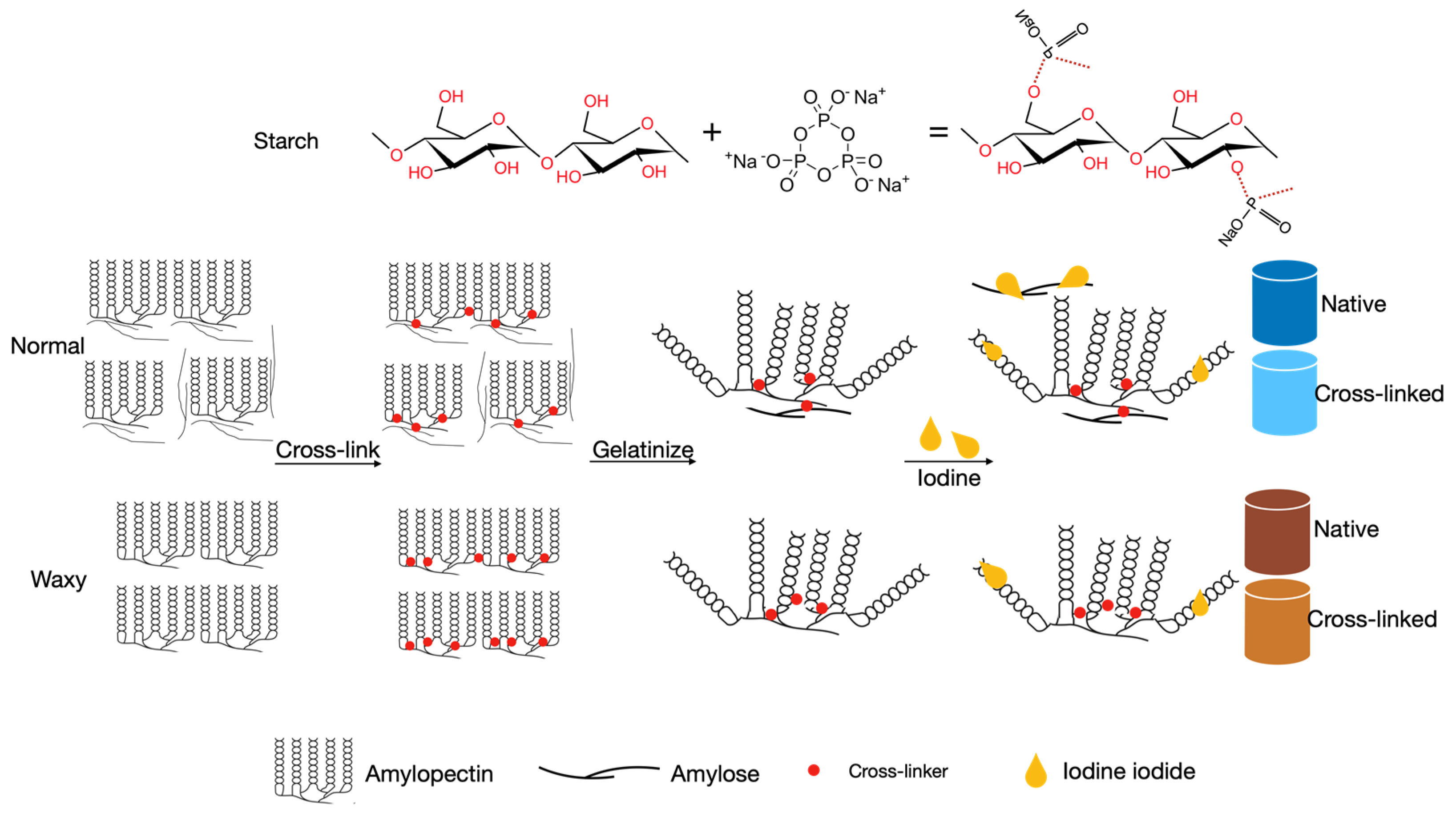
3.1. Cross-Linking Mechanisms of Normal and Waxy Starches
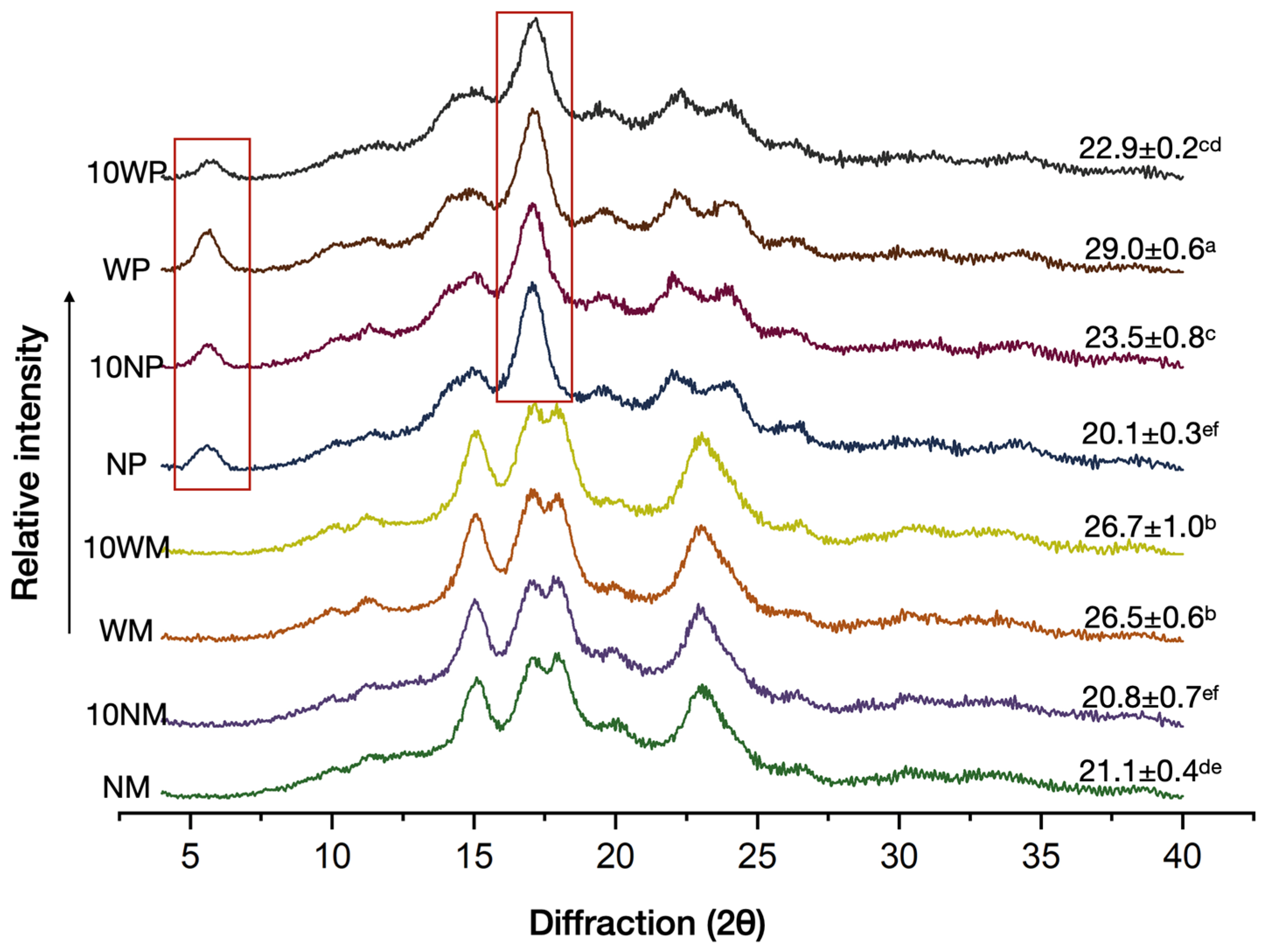
3.2. XRD Pattern
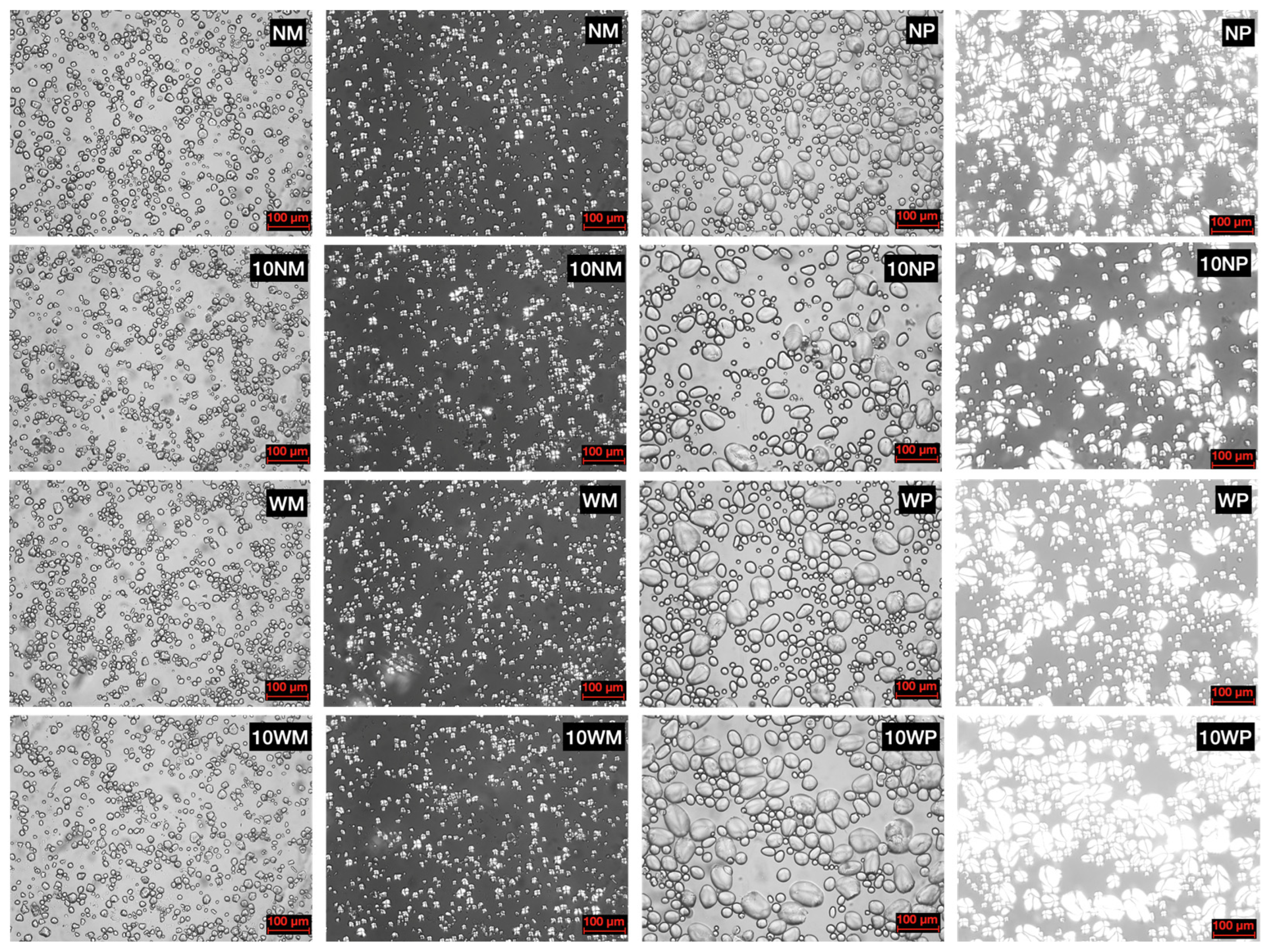
3.3. Morphology of Starch Granules
3.4. Thermal Analysis
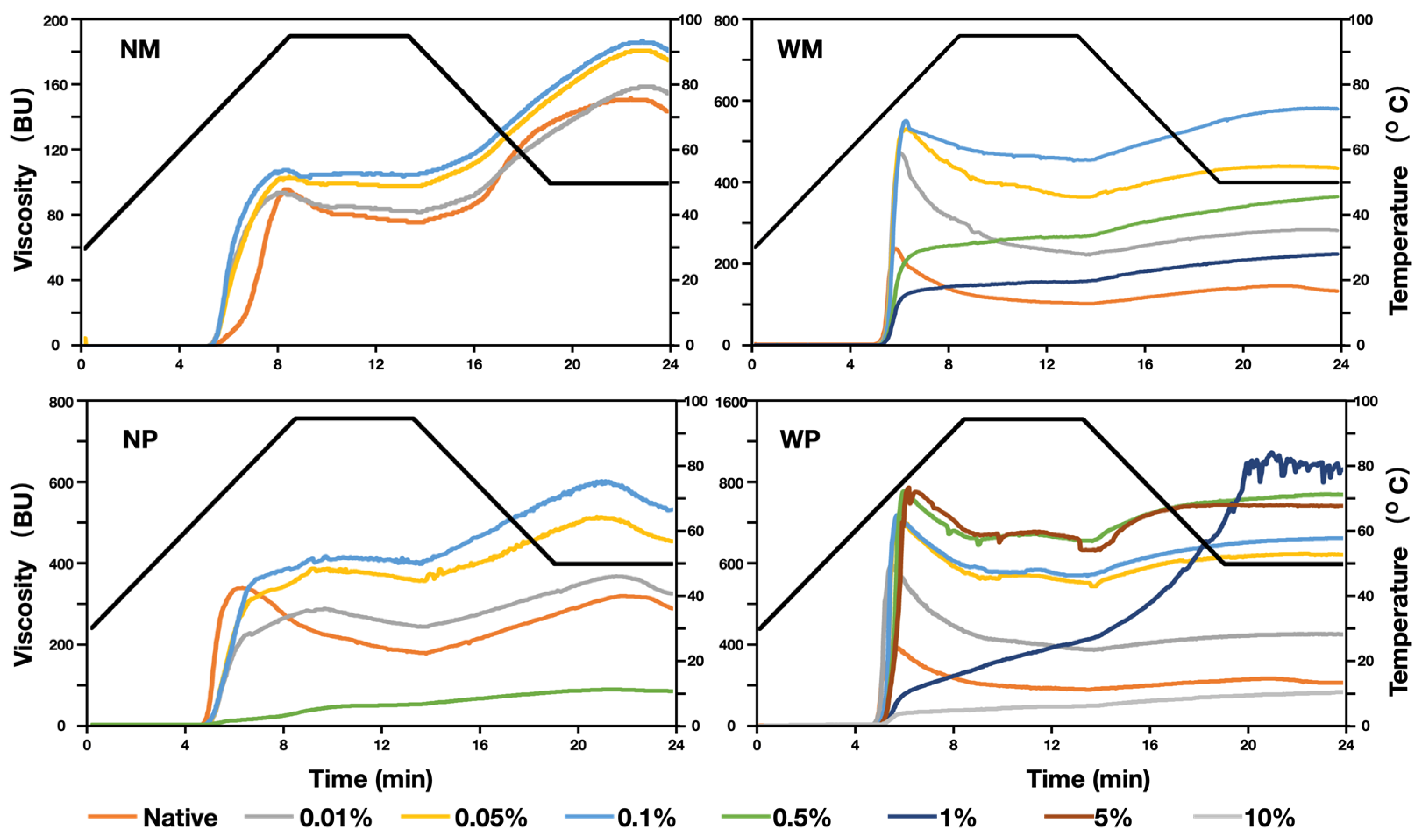
3.5. Pasting Properties
3.6. Iodine-Binding Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Chapter 5—Structural Features of Starch Granules I. In Food Science and Technology, 3rd ed.; BeMiller, J., Whistler, R.B.T.-S., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 149–192. ISBN 978-0-12-746275-2. [Google Scholar]
- Guo, B.; Hu, X.; Deng, F.; Wu, J.; Luo, S.; Chen, R.; Liu, C. Supernatant Starch Fraction of Corn Starch and Its Emulsifying Ability: Effect of the Amylose Content. Food Hydrocoll. 2020, 103, 105711. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Liu, W.; Whaley, J.K.; Shi, Y.C. Structure, Properties, and Potential Applications of Waxy Tapioca Starches—A Review. Trends Food Sci. Technol. 2019, 83, 225–234. [Google Scholar] [CrossRef]
- Kou, T.; Xia, H.; Gao, Q. New Insight into the Determination of Amylose Content for Maize Starches through Digital Image Analysis. Food Hydrocoll. 2018, 83, 438–444. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, T.Z.Z.; Wang, X.; Reimer, M.; Isaak, C.; Ai, Y. Behaviors of Starches Evaluated at High Heating Temperatures Using a New Model of Rapid Visco Analyzer—RVA 4800. Food Hydrocoll. 2019, 94, 217–228. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Recent Advances in Polysaccharides Stabilized Emulsions for Encapsulation and Delivery of Bioactive Food Ingredients: A Review. Carbohydr. Polym. 2020, 242, 116388. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhou, X.-F. Novel Starch Solution Prepared from the Starch in NaOH/Thiourea/Urea Aqueous Solution and Its Application as Surface Sizing Agent. BioResources 2011, 6, 1926–1938. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.; Qu, M.; Zhao, M.; Raj, I. High-Viscosity α-Starch Nanogel Particles to Enhance Oil Recovery. RSC Adv. 2020, 10, 8275–8285. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. High-Amylose Starches to Bridge the “Fiber Gap”: Development, Structure, and Nutritional Functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Dhital, S.; Gilbert, R.G.; Gidley, M.J. High-Amylose Wheat Starch: Structural Basis for Water Absorption and Pasting Properties. Carbohydr. Polym. 2020, 245, 116557. [Google Scholar] [CrossRef]
- Sagnelli, D.; Chessa, S.; Mandalari, G.; Di Martino, M.; Sorndech, W.; Mamone, G.; Vincze, E.; Buillon, G.; Nielsen, D.S.; Wiese, M.; et al. Low Glycaemic Index Foods from Wild Barley and Amylose-Only Barley Lines. J. Funct. Foods 2018, 40, 408–416. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Rincón, S.; García-Gurrola, A.; Zepeda, A.; Martínez-Bustos, F. Preparation and Characterization of Succinylated Nanoparticles from High-Amylose Starch via the Extrusion Process Followed by Ultrasonic Energy. Food Bioprocess Technol. 2019, 12, 1672–1682. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Li, Y.; Huang, Q. Assembly of Pickering Emulsions Using Milled Starch Particles with Different Amylose/Amylopectin Ratios. Food Hydrocoll. 2018, 84, 47–57. [Google Scholar] [CrossRef]
- Faisal, M.; Kou, T.; Zhong, Y.; Blennow, A. High Amylose-Based Bio Composites: Structures, Functions and Applications. Polymers 2022, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Gao, Q. A Study on the Thermal Stability of Amylose-Amylopectin and Amylopectin-Amylopectin in Cross-Linked Starches through Iodine Binding Capacity. Food Hydrocoll. 2019, 88, 86–91. [Google Scholar] [CrossRef]
- Li, H.; Zheng, H.; Tan, Y.J.; Tor, S.B.; Zhou, K. Development of an Ultrastretchable Double-Network Hydrogel for Flexible Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 12814–12823. [Google Scholar] [CrossRef]
- Ayoub, A.S.; Rizvi, S.S.H. An Overview on the Technology of Cross-Linking of Starch for Nonfood Applications. J. Plast. Film Sheeting 2009, 25, 25–45. [Google Scholar] [CrossRef]
- Wei, H.; Ma, J.; Shi, Y.; Cui, D.; Liu, M.; Lu, N.; Wang, N.; Wu, T.; Wujcik, E.K.; Guo, Z. Sustainable Cross-Linked Porous Corn Starch Adsorbents with High Methyl Violet Adsorption. ES Mater. Manuf. 2018, 2, 28–34. [Google Scholar] [CrossRef]
- Lu, H.; Ji, N.; Li, M.; Wang, Y.; Xiong, L.; Zhou, L.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Preparation of Borax Cross-Linked Starch Nanoparticles for Improvement of Mechanical Properties of Maize Starch Films. J. Agric. Food Chem. 2019, 67, 2916–2925. [Google Scholar] [CrossRef]
- Peidayesh, H.; Ahmadi, Z.; Khonakdar, H.A.; Abdouss, M.; Chodák, I. Baked Hydrogel from Corn Starch and Chitosan Blends Cross-Linked by Citric Acid: Preparation and Properties. Polym. Adv. Technol. 2020, 31, 1256–1269. [Google Scholar] [CrossRef]
- Kou, T.; Gao, Q. New Insight in Crosslinking Degree Determination for Crosslinked Starch. Carbohydr. Res. 2018, 458–459, 13–18. [Google Scholar] [CrossRef]
- Ghosh Dastidar, T.; Netravali, A. Cross-Linked Waxy Maize Starch-Based “Green” Composites. ACS Sustain. Chem. Eng. 2013, 1, 1537–1544. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Druzian, J.I. Starch Chemical Modifications Applied to Drug Delivery Systems: From Fundamentals to FDA-Approved Raw Materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Hafizulhaq, F.; Asrofi, M. Properties of Cellulose Nanofiber/Bengkoang Starch Bionanocomposites: Effect of Fiber Loading. LWT 2019, 116, 108554. [Google Scholar] [CrossRef]
- Otache, M.A.; Duru, R.U.; Achugasim, O.; Abayeh, O.J. Advances in the Modification of Starch via Esterification for Enhanced Properties. J. Polym. Environ. 2021, 29, 1365–1379. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of Amylopectin Branch Chain Length and Amylose Content on the Gelatinization and Pasting Properties of Starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- ISO 6647-1:2007; Standards, S. Rice—Determination Of Amylose Content—Part 1: Reference Method. International Organization for Standardization: Geneva, Switzerland, 2007.
- Qin, Y.; Liu, C.; Jiang, S.; Xiong, L.; Sun, Q. Characterization of Starch Nanoparticles Prepared by Nanoprecipitation: Influence of Amylose Content and Starch Type. Ind. Crops Prod. 2016, 87, 182–190. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, Z.; Cai, J.; Xiong, H. Effect of Cross-Linked Waxy Maize Starch on the Quality of Non-Fried Instant Noodles. Starch/Staerke 2015, 67, 1035–1043. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Lin, L.; Zhang, J.; Liu, W.; Xie, J.; Ding, Y. Functional, Physicochemical Properties and Structure of Cross-Linked Oxidized Maize Starch. Food Hydrocoll. 2014, 36, 45–52. [Google Scholar] [CrossRef]
- Park, E.Y.; Ma, J.G.; Kim, J.; Lee, D.H.; Kim, S.Y.; Kwon, D.J.; Kim, J.Y. Effect of Dual Modification of HMT and Crosslinking on Physicochemical Properties and Digestibility of Waxy Maize Starch. Food Hydrocoll. 2018, 75, 33–40. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and Characterization of C-Phycocyanin Coated with STMP/STPP Cross-Linked Starches from Different Botanical Sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Shen, R.; Gao, J.; Li, J. An Efficient Approach to Prepare Water-Redispersible Starch Nanocrystals from Waxy Potato Starch. Polymers 2021, 13, 431. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhang, Y.; Zhu, L.; Gu, Z. Study on Physicochemical Characteristics of Waxy Potato Starch in Comparison with Other Waxy Starches. Starch/Staerke 2011, 63, 754–759. [Google Scholar] [CrossRef]
- Carmona-Garcia, R.; Sanchez-Rivera, M.M.; Méndez-Montealvo, G.; Garza-Montoya, B.; Bello-Pérez, L.A. Effect of the Cross-Linked Reagent Type on Some Morphological, Physicochemical and Functional Characteristics of Banana Starch (Musa Paradisiaca). Carbohydr. Polym. 2009, 76, 117–122. [Google Scholar] [CrossRef]
- Luo, F.X.; Huang, Q.; Fu, X.; Zhang, L.X.; Yu, S.J. Preparation and Characterisation of Crosslinked Waxy Potato Starch. Food Chem. 2009, 115, 563–568. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. The Influence of Amylose on Starch Granule Structure. Int. J. Biol. Macromol. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The Impact of Heat-Moisture Treatment on the Molecular Structure and Physicochemical Properties of Normal and Waxy Potato Starches. Carbohydr. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Demiate, I.M.; Figueroa, A.M.; Zortéa Guidolin, M.E.B.; Rodrigues dos Santos, T.P.; Yangcheng, H.; Chang, F.; Jane, J.L. Physicochemical Characterization of Starches from Dry Beans Cultivated in Brazil. Food Hydrocoll. 2016, 61, 812–820. [Google Scholar] [CrossRef]
- Marques, P.T.; Lima, A.M.F.; Bianco, G.; Laurindo, J.B.; Borsali, R.; Le Meins, J.F.; Soldi, V. Thermal Properties and Stability of Cassava Starch Films Cross-Linked with Tetraethylene Glycol Diacrylate. Polym. Degrad. Stab. 2006, 91, 726–732. [Google Scholar] [CrossRef]
- Kasemsuwam, T.; Jane, J. Location of Amylose in Normal Starch Granules. II. Locations of Phosphodiester Cross-Linking Revealed by Phosphorus-31 Nuclear Magnetic Resonance. Cereal Chem. 1994, 71, 282–287. [Google Scholar]
- McPherson, A.E.; Jane, J. Comparison of Waxy Potato with Other Root and Tuber Starches. Carbohydr. Polym. 1999, 40, 57–70. [Google Scholar] [CrossRef]
- Steeneken, P.A.M. Rheological Properties of Aqueous Suspensions of Swollen Starch Granules. Carbohydr. Polym. 1989, 11, 23–42. [Google Scholar] [CrossRef]
- Chen, B.; Wu, S.; Ye, Q. Fabrication and Characterization of Biodegradable KH560 Crosslinked Chitin Hydrogels with High Toughness and Good Biocompatibility. Carbohydr. Polym. 2021, 259, 117707. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yasui, T.; Matsuki, J. Effect of Amylose Content on Gelatinization, Retrogradation, and Pasting Properties of Starches from Waxy and Nonwaxy Wheat and Their F1 Seeds. Cereal Chem. 2000, 77, 58–63. [Google Scholar] [CrossRef]
- Majzoobi, M.; Sabery, B.; Farahnaky, A.; Karrila, T.T. Physicochemical Properties of Cross-Linked-Annealed Wheat Starch. Iran. Polym. J. 2012, 21, 513–522. [Google Scholar] [CrossRef]
- Jane, J.; Xu, A.; Radosavljevic, M.; Seib, P.A. Location of Amylose in Normal Starch Granules. I. Susceptibility of Amylose and Amylopectin to Cross-Linkin Reagents. Cereal Chem. 1992, 69, 405–409. [Google Scholar]


| Samples | To (°C) | Tp (°C) | Tc (°C) | |
|---|---|---|---|---|
| NM | 68.01 ± 0.93 bc | 72.64 ± 0.89 cde | 78.52 ± 0.16 d | 14.53 ± 0.18 d |
| 10 NM | 71.64 ± 0.71 a | 77.30 ± 0.32 a | 83.45 ± 0.06 b | 16.23 ± 0.55 c |
| WM | 69.13 ± 0.83 b | 75.18 ± 0.08 ab | 86.09 ± 0.85 a | 18.51 ± 0.59 b |
| 10 WM | 67.93 ± 0.39 bc | 74.56 ± 0.98 bc | 81.85 ± 0.71 c | 17.67 ± 0.22 bc |
| NP | 65.69 ± 0.26 d | 71.33 ± 0.86 e | 78.33 ± 0.33 d | 12.47 ± 0.53 e |
| 10 NP | 67.88 ± 0.08 bc | 73.20 ± 0.67 bcde | 79.73 ± 0.62 d | 13.4 ± 0.35 de |
| WP | 65.41 ± 0.03 d | 72.18 ± 0.85 e | 78.72 ± 0.19 d | 21.93 ± 0.60 a |
| 10 WP | 66.55 ± 0.73 cd | 71.76 ± 0.10 e | 78.72 ± 0.04 d | 21.53 ± 0.50 a |
| Samples | Peak η 1/BU | Final η/BU | Break-Down/BU | Set-Back/BU | Samples | Peak η/BU | Final η/BU | Break-Down/BU | Set-Back/BU |
|---|---|---|---|---|---|---|---|---|---|
| NM | 96 ± 2.0 bc | 144 ± 4.5 c | 20 ± 3.0 a | 68 ± 5.5 a | WM | 237 ± 4.0 d | 133 ± 4.5 f | 134 ± 3.0 c | 30 ± 3.5 e |
| 0.01 NM | 94 ± 2.5 c | 155 ± 1.5 b | 11 ± 0.5 b | 72 ± 4.0 a | 0.01 WM | 472 ± 5.5 b | 282 ± 4.5 d | 247 ± 2.0 a | 57 ± 3.0 d |
| 0.05 NM | 104 ± 3.0 ab | 175 ± 4.5 a | 6 ± 2.0 bc | 77 ± 2.0 a | 0.05 WM | 531 ± 7.0 a | 434 ± 7.0 b | 167 ± 3.5 b | 70 ± 2.0 c |
| 0.1 NM | 108 ± 1.0 a | 181 ± 1.5 a | 3 ± 1.0 c | 76 ± 1.0 a | 0.1 WM | 551 ± 13 a | 580 ± 11 a | 95 ± 2.0 d | 124 ± 4.0 a |
| 0.5 WM | 267 ± 8.0 c | 365 ± 2.5 c | 0 | 98 ± 1.5 b | |||||
| 1 WM | 156 ± 2.5 e | 224 ± 3.0 e | 0 | 68 ± 1.0 c | |||||
| NP | 340 ± 7.5 c | 289 ± 3.0 d | 159 ± 1.5 a | 108 ± 4.5 b | WP | 384 ± 8.0 d | 212 ± 5.5 g | 204 ± 4.5 e | 32 ± 1.5 e |
| 0.01 NP | 288 ± 3.0 d | 326 ± 2.5 c | 42 ± 4.5 b | 80 ± 1.5 d | 0.01 WP | 795 ± 12 c | 453 ± 7.0 f | 415 ± 5.0 a | 73 ± 3.5 d |
| 0.05 NP | 388 ± 2.0 b | 455 ± 3.0 b | 29 ± 3.0 bc | 96 ± 2.5 c | 0.05 WP | 1037 ± 9.5 b | 848 ± 5.0 e | 327 ± 8.5 b | 138 ± 2.5 c |
| 0.1 NP | 419 ± 9.0 a | 534 ± 5.0 a | 17 ± 3.5 c | 132 ± 1.5 a | 0.1 WP | 1042 ± 14 b | 929 ± 8.0 d | 296 ± 5.0 c | 183 ± 3.5 b |
| 0.5 NP | 51 ± 1.5 e | 83 ± 2.5 e | 0 d | 32 ± 1.5 e | 0.5 WP | 1163 ± 17 a | 1146 ± 12 b | 246 ± 1.0 d | 229 ± 5.0 a |
| 1 WP | 421 ± 8.0 d | 1270 ± 13 a | nd 2 | nd | |||||
| 5 WP | 1180 ± 18 a | 1090 ± 10 c | 308 ± 7.0 bc | 218 ± 7.5 a | |||||
| 10 WP | 97 ± 4.0 e | 166 ± 3.5 h | 0 | 69 ± 1.0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, T.; Song, J.; Liu, M.; Fang, G. Effect of Amylose and Crystallinity Pattern on the Gelatinization Behavior of Cross-Linked Starches. Polymers 2022, 14, 2870. https://doi.org/10.3390/polym14142870
Kou T, Song J, Liu M, Fang G. Effect of Amylose and Crystallinity Pattern on the Gelatinization Behavior of Cross-Linked Starches. Polymers. 2022; 14(14):2870. https://doi.org/10.3390/polym14142870
Chicago/Turabian StyleKou, Tingting, Jun Song, Mouquan Liu, and Guihong Fang. 2022. "Effect of Amylose and Crystallinity Pattern on the Gelatinization Behavior of Cross-Linked Starches" Polymers 14, no. 14: 2870. https://doi.org/10.3390/polym14142870
APA StyleKou, T., Song, J., Liu, M., & Fang, G. (2022). Effect of Amylose and Crystallinity Pattern on the Gelatinization Behavior of Cross-Linked Starches. Polymers, 14(14), 2870. https://doi.org/10.3390/polym14142870






