Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts
Abstract
:1. Introduction
2. Review Methodology
- Scilit (scilit.net).
- Web of Science (scholar.google.com).
- Scopus (scopus.com).
- Wiley (onlinelibrary.wiley.com).
- Web of Science (webofknowledge.com).
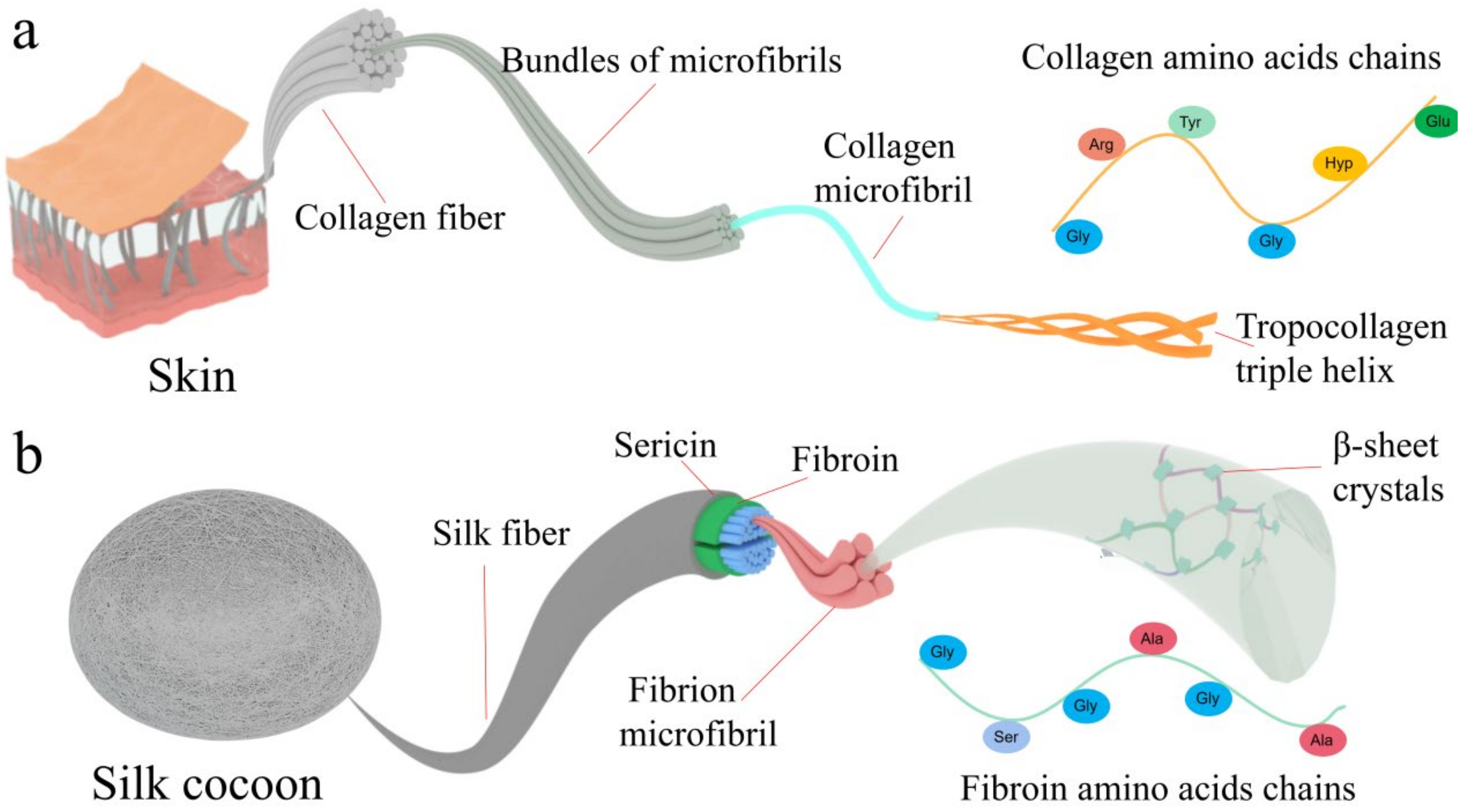
3. The Structural Advantages of Collagen and Silk Fibroin for Constructing Catalysts
4. The Processing Methods of Collagen and Silk Fibroin
5. Catalysts by Using Collagen and Silk Fibroin as Carriers
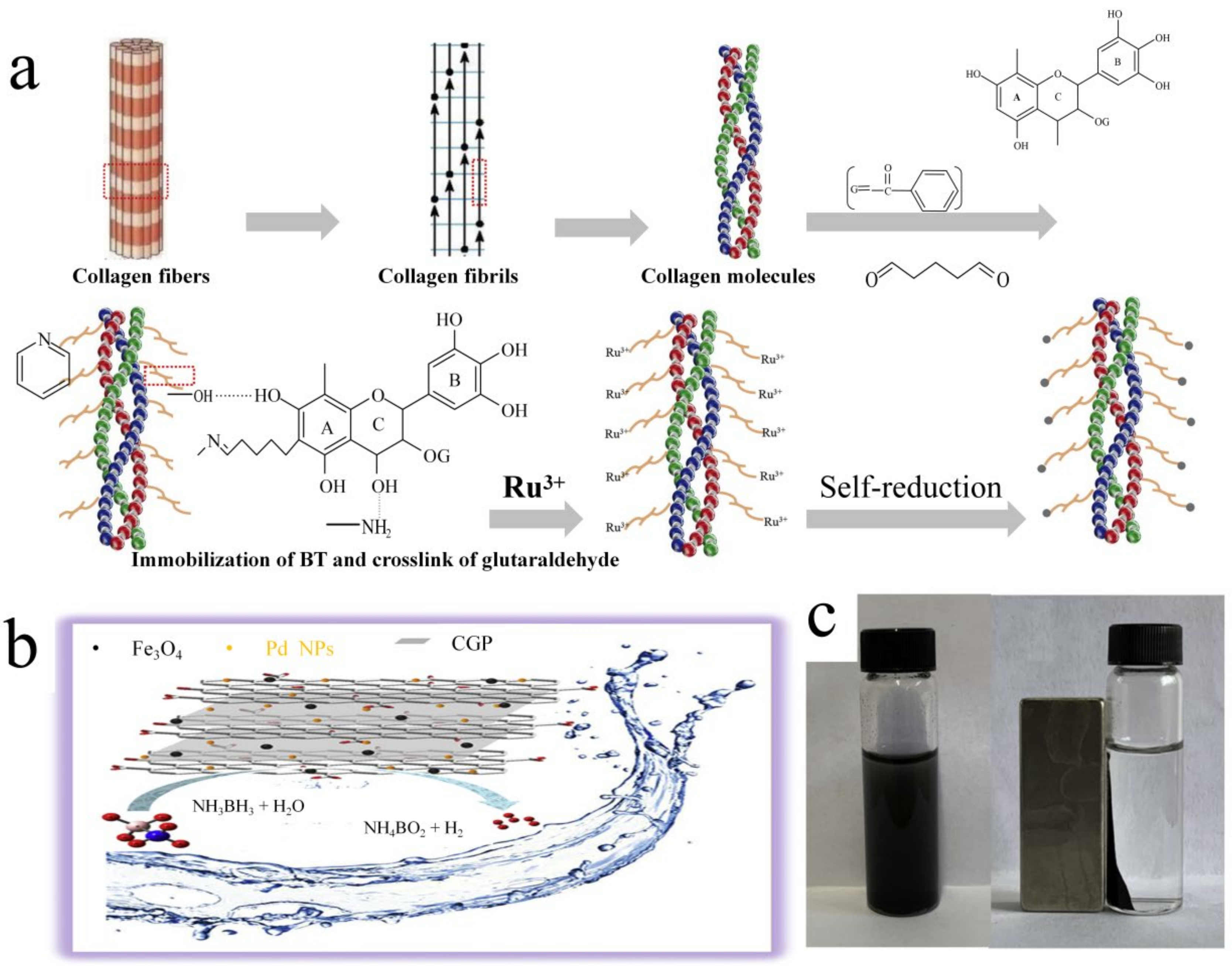
5.1. Collagen-Based Catalysts
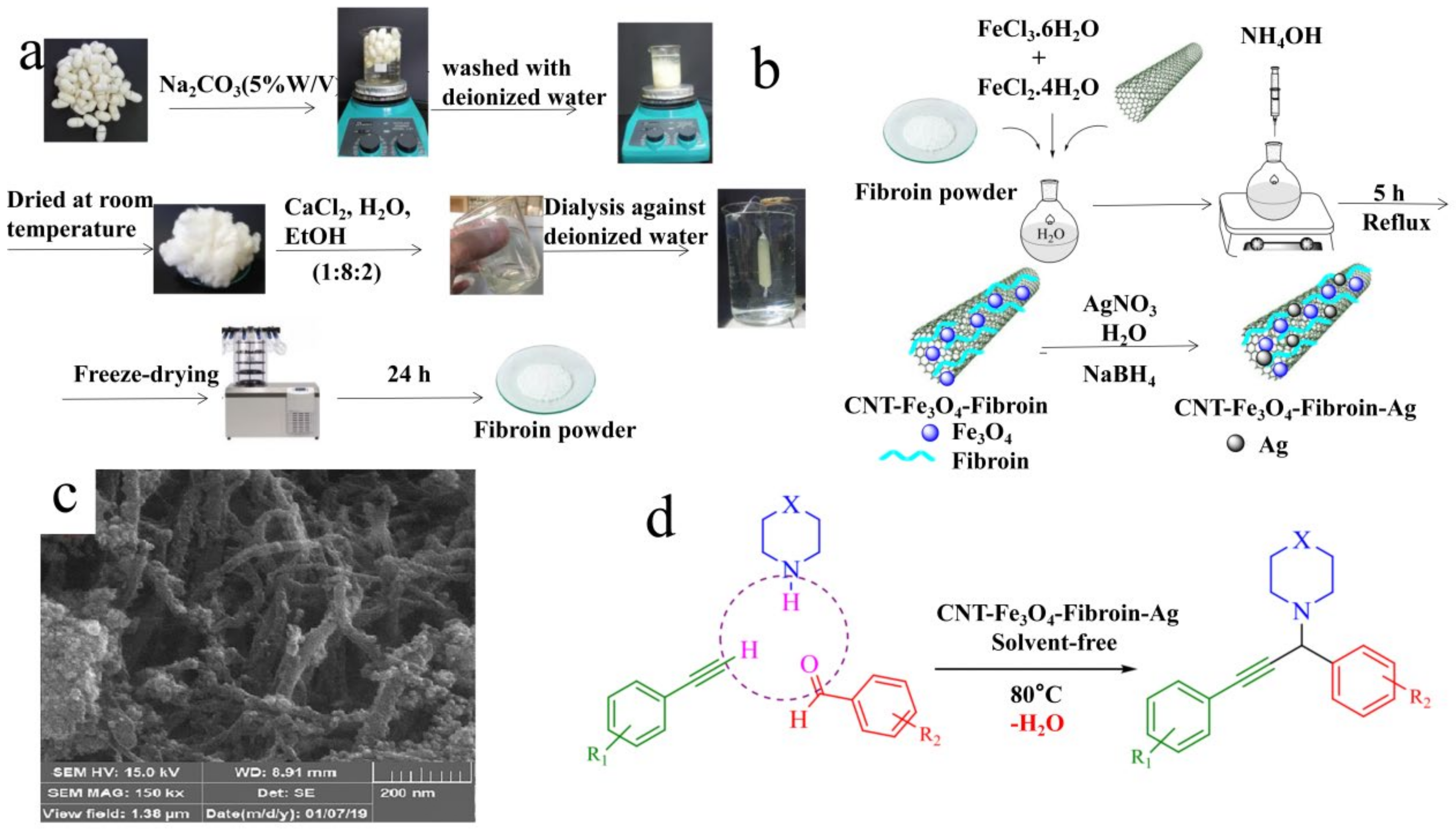
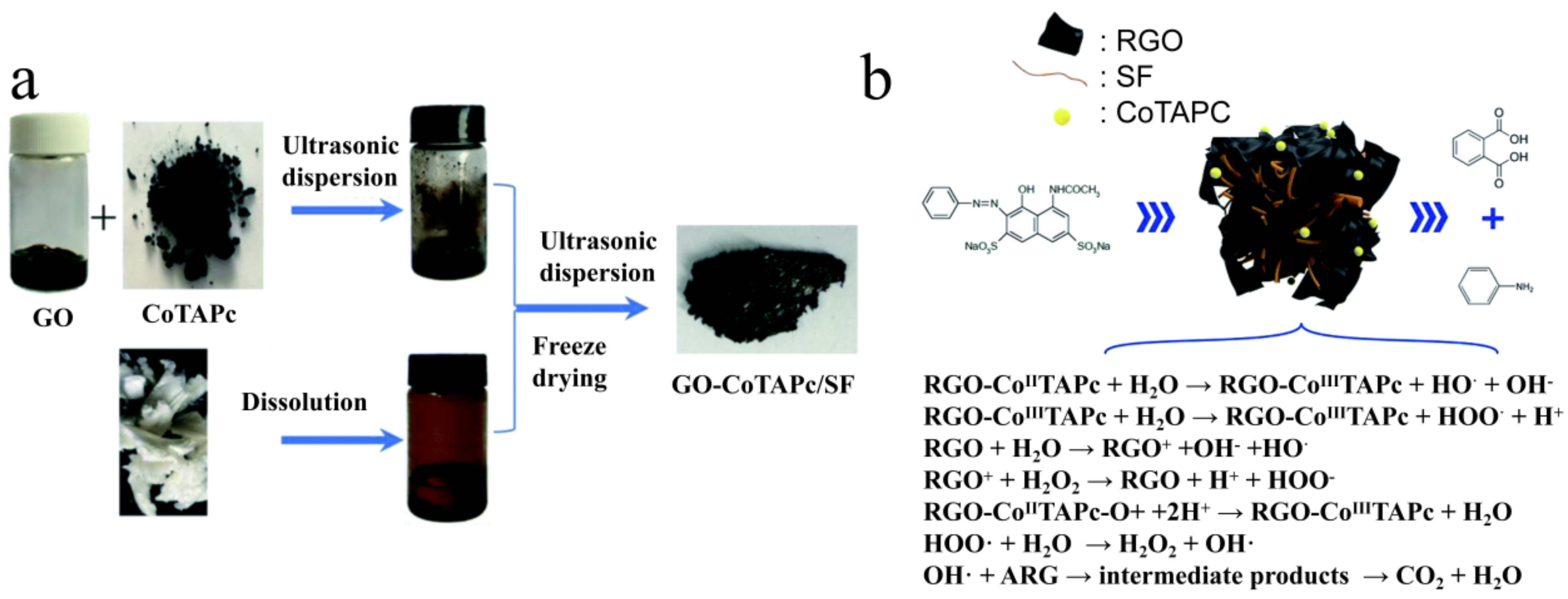
5.2. Silk Fibroin-Based Catalysts
6. Catalysts by Using Collagen and Silk Fibroin as Precursors
6.1. Collagen-Derived Carbon Catalysts
6.2. Silk Fibroin-Derived Carbon Catalysts
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augugliaro, V.; Palmisano, G.; Palmisano, L.; Soria, J. Heterogeneous photocatalysis and catalysis: An overview of their distinctive features. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose Supported Metal Nanoparticles as Green and Sustainable Catalysis for Organic Synthesis. Cellulose 2021, 28, 4545–4574. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-atom Sites. Chem. Rev. 2019, 120, 623–682. [Google Scholar] [CrossRef] [PubMed]
- Fehl, C.; Amanda, G.; Jarvis, A.G.; Palm-Espling, M.; Davis, B.; Kamer, P.C.J. Outperforming Nature’s Catalysts: Designing Metalloenzymes for Chemical Synthesis. In Modern Developments in Catalysis; Hutchings, G.J., Davidson, M.G., Catlow, R.C.A., Hardacre, C., Turner, N.J., Collier, P., Eds.; World Scientific: Singapore, 2017; pp. 89–122. [Google Scholar] [CrossRef]
- Fechete, I.; Wang, Y.; Védrine, J.C. The Past, Present and Future of Heterogeneous Catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental Catalysis: Present and Future. ChemCatChem 2018, 11, 18–38. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging Homogeneous and Heterogeneous Catalysis by Heterogeneous Single-metal-site Catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Liu, D.; He, Q.; Ding, S.; Song, L. Structural Regulation and Support Coupling Effect of Single-Atom Catalysts for Heterogeneous Catalysis. Adv. Energy Mater. 2020, 10, 2001482. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of Metal-support Interactions in Heterogeneous Catalysts to enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.R.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.L.; Zhu, H.Y.; Zhao, C.L.; Huang, M.-Y.; Jiang, Y.Y. Asymmetric Hydrogenation of Furfuryl Alcohol Catalyzed by a Biopolymer–metal Complex, Silica-supported Alginic Acid–Amino Acid–Pt Complex. React. Funct. Polym. 2004, 59, 33–39. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S.; Soleimani, F. Recent Progresses in the Application of Cellulose, Starch, Alginate, Gum, Pectin, Chitin and Chitosan based (nano) Catalysts in Sustainable and Selective Oxidation Reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Veisi, H.; Aliani, M.H.; Mohammadi, P.; Karmakar, B. Alginate Modified Magnetic Nanoparticles to Immobilization of Gold Nanoparticles as an Efficient Magnetic Nanocatalyst for Reduction of 4-nitrophenol in Water. J. Mol. Liq. 2021, 327, 114868. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, N.S.; Reddy, P.S.; Sreedhar, B.; Kantam, M.L. Cellulose Supported Palladium (0) Catalyst for Heck and Sonogashira Coupling Reactions. J. Mol. Catal. A Chem. 2006, 252, 12–16. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, R.; Yang, Z.; Chen, J.; Deng, J.; Fakhri, A.; Gupta, V.K. Synthesis of Co3S4-SnO2/polyvinylpyrrolidone-Cellulose Heterojunction as Highly Performance Catalyst for Photocatalytic and Antimicrobial Properties under Ultra-violet Irradiation. Int. J. Biol. Macromol. 2020, 162, 220–228. [Google Scholar] [CrossRef]
- Huang, K.; Xue, L.; Hu, Y.C.; Huang, M.Y.; Jiang, Y.Y. Catalytic Behaviors of Silica-supported Starch–polysulfosiloxane–Pt Complexes in Asymmetric Hydrogenation of 4-methyl-2-Pentanone. React. Funct. Polym. 2002, 50, 199–203. [Google Scholar] [CrossRef]
- Xiong, Z.B.; Li, Z.Z.; Li, C.X.; Wang, W.; Lu, W.; Du, Y.P. Green synthesis of Tungsten-Doped CeO2 Catalyst for Selective Catalytic Reduction of NOx with NH3 Using Starch Bio-Template. Appl. Surf. Sci. 2021, 536, 147719. [Google Scholar] [CrossRef]
- Wu, H.; Wu, C.; He, Q.; Liao, X.; Shi, B. Collagen Fiber with Surface-grafted Polyphenol as A Novel Support for Pd (0) Nanoparticles: Synthesis, Characterization and Catalytic Application. Mater. Sci. Eng. C 2010, 30, 770–776. [Google Scholar] [CrossRef]
- Sharma, B.; Malik, P.; Jain, P. Biopolymer Reinforced Nanocomposites: A Comprehensive Review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Agnieray, H.; Glasson, J.L.; Chen, Q.; Kaur, M.; Domigan, L.J. Recent Developments in Sustainably Sourced Protein-based Biomaterials. Biochem. Soc. Trans. 2021, 49, 953–964. [Google Scholar] [CrossRef]
- Sozer, N.; Nordlund, E.; Ercili Cura, D.; Poutanen, K. Cereal Side-Streams as Alternative Protein Sources. Cereal Foods World 2017, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Ajayan, P.M. Materials Science Perspective of Multifunctional Materials Derived from Collagen. Int. Mater. Rev. 2020, 66, 160–187. [Google Scholar] [CrossRef]
- Ocak, B. Development of Novel Collagen Hydrolysate Bio-Nanocomposite Films Extracted from Hide Trimming Wastes Reinforced with Chitosan Nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 35145–35156. [Google Scholar] [CrossRef]
- Al.Sakkari, E.G.; Elozeiri, A.A.; Abdeldayem, O.M.; Likozar, B.; Boffito, D.C. Chapter 7—Fish and Animal Waste as Catalysts for Biodiesel Synthesis. In Waste and Biodiesel; Singh, B., Guldhe, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–136. [Google Scholar] [CrossRef]
- Lamsal, B.; Wang, H.; Pinsirodom, P.; Dossey, A.T. Applications of Insect-Derived Protein Ingredients in Food and Feed Industry. J. Am. Oil Chem. Soc. 2019, 96, 105–123. [Google Scholar] [CrossRef]
- Babu, K.M. Silk Fibres—Structure, Properties and Applications. In Handbook of Natural Fibres, 2nd ed.; Ryszard, M.K., Talarczyk, M., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2020; pp. 385–416. [Google Scholar] [CrossRef]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen Fibrils: Nanoscale Ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent Advances of Collagen-Based Biomaterials: Multi-Hierarchical Structure, Modification and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Liu, B.; Song, Y.W.; Jin, L.; Wang, Z.J.; Pu, D.Y.; Lin, S.Q.; Zhou, C.; You, H.J.; Ma, Y.; Li, J.M. Silk Structure and Degradation. Colloids Surf. B 2015, 131, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Shao, R.; Liu, M.; Zan, Y.; Dou, M.; Liu, J.; Zhang, Z.; Huang, Y.; Wang, F. Porous Carbons Derived from Collagen-Enriched Biomass: Tailored Design, Synthesis, and Application in Electrochemical Energy Storage and Conversion. Adv. Funct. Mater. 2019, 29, 1905095. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-Alloy Fuel Cell Catalyst Activity and Durability Via Nitrogen-Modified Carbon Supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Wong, W.Y.; Daud, W.R.W.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S.; Majlan, E.H. Recent Progress in Nitrogen-Doped Carbon and Its Composites as Electrocatalysts for Fuel Cell Applications. Int. J. Hydrog. Energy 2013, 38, 9370–9386. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, Y.F.; Chang, K.H.; Hu, C.C. Synthesis of N-Doped Carbon Nanosheets from Collagen for Electrochemical Energy Storage/Conversion Systems. Electrochem. Commun. 2011, 13, 50–53. [Google Scholar] [CrossRef]
- Lu, R.; Sam, D.K.; Wang, W.; Gong, S.; Liu, J.; Durairaj, A.; Li, M.; Lv, X. Boron, Nitrogen Co-Doped Biomass-Derived Carbon Aerogel Embedded Nickel-Cobalt-Iron Nanoparticles as a Promising Electrocatalyst for Oxygen Evolution Reaction. J. Colloid Interface Sci. 2022, 613, 126–135. [Google Scholar] [CrossRef]
- Ueda, E.K.M.; Gout, P.W.; Morganti, L. Current and Prospective Applications of Metal Ion–Protein Binding. J. Chromatogr. A 2003, 988, 1–23. [Google Scholar] [CrossRef]
- Kumar, G.; Tibbitts, L.; Newell, J.; Panthi, B.; Mukhopadhyay, A.; Rioux, R.M.; Pursell, C.J.; Janik, M.; Chandler, B.D. Evaluating Differences in the Active-Site Electronics of Supported Au Nanoparticle Catalysts Using Hammett and DFT Studies. Nat. Chem. 2018, 10, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, I.S.; Viana, L.; Verona, C.; Fallavena, V.L.V.; Azevedo, C.M.N.; Pires, M. Alkydic Resin Wastewaters Treatment by Fenton and Photo-Fenton Processes. J. Hazard. Mater. 2007, 146, 564–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Mehta, M.; Mansel, B.W.; Ng, H.W.; Liu, Y.; Holmes, G.; Le Ru, E.C.; Prabakar, S. Anion-Regulated Binding Selectivity of Cr (III) in Collagen. Biopolymers 2020, 111, e23406. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, Z.; Javanshir, S.; Bazgir, A. Isinglass–Palladium as Collagen Peptide–Metal Complex: A Highly Efficient Heterogeneous Biocatalyst for Suzuki Cross-Coupling Reaction in Water. J. Iran. Chem. Soc. 2019, 16, 1473–1481. [Google Scholar] [CrossRef]
- Ferrari, R.P. Metal Binding Sites of Oxovanadium (IV) in Native and Modified Soluble Collagen. Inorg. Chim. Acta 1990, 176, 83–86. [Google Scholar] [CrossRef]
- Tang, R.; Liao, X.-P.; Liu, X.; Shi, B. Collagen Fiber Immobilized Fe (III): A Novel Catalyst for Photo-Assisted Degradation of Dyes. Chem. Commun. 2005, 41, 5882–5884. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, R.; He, Q.; Liao, X.; Shi, B. Fe (III)-Loaded Collagen Fiber as a Heterogeneous Catalyst for the Photo-Assisted Decomposition of Malachite Green. J. Hazard. Mater. 2010, 174, 687–693. [Google Scholar] [CrossRef]
- Liu, X.; Tang, R.; He, Q.; Liao, X.; Shi, B. Fe (III)-Immobilized Collagen fiber: A Renewable Heterogeneous Catalyst for the Photoassisted Decomposition of Orange II. Ind. Eng. Chem. Res. 2009, 48, 1458–1463. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J.; et al. Recent Advances in Functional Materials through Cellulose Nanofiber Templating. Adv. Mater. 2021, 33, e2005538. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Tao, S.; Yue, D.; Zeng, Q.; Chen, W.; Yang, B. Recent Advances in Energy Conversion Applications of Carbon Dots: From Optoelectronic Devices to Electrocatalysis. Small 2020, 16, e2001295. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Li, H.; Zan, Y.; Li, X.; Zhu, Y.; Dou, M.; Wang, F. Sustainable Carbonaceous Materials Derived from Biomass as Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, e1805718. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Xiao, G.; Lin, Y.; Lin, H.; Dai, M.; Chen, L.; Jiang, X.; Cao, X.; Afewerki, S.; Wang, Y.; Zhang, W. Bioinspired Self-Assembled Fe/Cu-Phenolic Building Blocks of Hierarchical Porous Biomass-Derived Carbon Aerogels for Enhanced Electrocatalytic Oxygen Reduction. Colloids Surf. A 2022, 648, 128932. [Google Scholar] [CrossRef]
- Kotula, M.; Kubiak, A.; Leśniewski, B.; Pajewska-Szmyt, M. Carbonization of Selected Biological Materials, Trends, and Perspectives. Lett. Appl. NanoBioSci. 2023, 12, 68. [Google Scholar] [CrossRef]
- Iwazaki, T.; Yang, H.; Obinata, R.; Sugimoto, W.; Takasu, Y. Oxygen-Reduction Activity of Silk-Derived Carbons. J. Power Sources 2010, 195, 5840–5847. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, Y.; Wang, Y.; Chen, X.; Xia, Y.; Shao, Z. Graphene/Silk Fibroin Based Carbon Nanocomposites for High Performance Supercapacitors. J. Mater. Chem. A 2015, 3, 773–781. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Wang, P.; Hu, D. Preparation of Sponge-Cake-Like N-Doped Porous Carbon Materials Derived from Silk Fibroin by Chemical Activation. Microporous Mesoporous Mater. 2021, 317, 110998. [Google Scholar] [CrossRef]
- Rebelo, A.L.; Bizeau, J.; Russo, L.; Pandit, A. Glycan-Functionalized Collagen Hydrogels Modulate the Glycoenvironment of a Neuronal Primary Culture. Biomacromolecules 2020, 21, 2681–2694. [Google Scholar] [CrossRef]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital Light Processing 3D Printed Silk Sibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Geanaliu-Nicolae, R.E.; Andronescu, E. Blended Natural Support Materials-Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Rojo, R.D.; López-Cervantes, J.; Sánchez-Machado, D.I.; Escárcega-Galaz, A.A.; del Rosario Martínez-Macias, M. Antibacterial, Mechanical and Physical Properties of Collagen—Chitosan Sponges from Aquatic Source. Sustain. Chem. Pharm. 2020, 15, 100218. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and Antibacterial Properties of Thin Films Based on Collagen and Thymol. Mater. Today Commun. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Andonegi, M.; Penalba, M.; de la Caba, K.; Guerrero, P. ZnO Nanoparticle-Incorporated Native Collagen Films with Electro-Conductive Properties. Mater. Sci. Eng. C 2020, 108, 110394. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer Nanofibrils: Structure, Modeling, Preparation, and Applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Xiao, Y.; Liu, Z.; Pei, Y.; Wang, G.; Tang, K. Dissolution of Collagen Fibers from Tannery Solid Wastes in Salt Aqueous Solutions: Hofmeister Series Evaluation. J. Chem. Technol. Biotechnol. 2020, 95, 1225–1233. [Google Scholar] [CrossRef]
- Pei, Y.; Chu, S.; Zheng, Y.; Zhang, J.; Liu, H.; Zheng, X.; Tang, K. Dissolution of Collagen Fibers from Tannery Solid Wastes in 1-Allyl-3-methylimidazolium Chloride and Modulation of Regenerative Morphology. ACS Sustain. Chem. Eng. 2018, 7, 2530–2537. [Google Scholar] [CrossRef]
- Gobeaux, F.; Belamie, E.; Mosser, G.; Davidson, P.; Asnacios, S. Power Law Rheology and Strain-Induced Yielding in Acidic Solutions of Type I-Collagen. Soft Matter. 2010, 6, 3769–3777. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, B.; Li, Z.; Qin, X.; Wen, Y.; Shi, D.; Lu, Q.; Yang, M.; Zhou, H.; Liu, Y. Biomimetic Organization of a Ruthenium-Doped Collagen-Based Carbon Scaffold for Hydrogen Evolution. J. Mater. Chem. A 2018, 6, 2311–2317. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Ramakrishna, S. Recent Developments in Regenerated Silk Fiber. J. Nanosci. Nanotechnol. 2017, 17, 8667–8682. [Google Scholar] [CrossRef]
- Chen, L.; Sun, L.; Yao, J.; Zhao, B.; Shao, Z.; Chen, X. Robust Silk Protein Hydrogels Made by a Facile One-Step Method and Their Multiple Applications. ACS Appl. Bio. Mater. 2022, 5, 3086–3094. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Zhou, L.; Ye, C.; Omenetto, F.G.; Kaplan, D.L.; Ling, S. Design, Fabrication, and Function of Silk-Based Nanomaterials. Adv. Funct. Mater. 2018, 28, 1805305. [Google Scholar] [CrossRef]
- Ma, Y.; Teng, A.; Zhao, K.; Zhang, K.; Zhao, H.; Duan, S.; Li, S.; Guo, Y.; Wang, W. A Top-Down Approach to Improve Collagen Film’s Performance: The Comparisons of Macro, Micro and Nano Sized Fibers. Food Chem. 2020, 309, 125624. [Google Scholar] [CrossRef]
- Pei, Y.; Jordan, K.E.; Xiang, N.; Parker, R.N.; Mu, X.; Zhang, L.; Feng, Z.; Chen, Y.; Li, C.; Guo, C.; et al. Liquid-Exfoliated Mesostructured Collagen from the Bovine Achilles Tendon as Building Blocks of Collagen Membranes. ACS Appl. Mater. Interfaces 2021, 13, 3186–3198. [Google Scholar] [CrossRef]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical Characterization of Collagen Fibers and Scaffolds for Tissue Engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, L.; Tang, K.; Kaplan, D.L. Biopolymer Nanoscale Assemblies as Building Blocks for New Materials: A Review. Adv. Funct. Mater. 2021, 31, 2008552. [Google Scholar] [CrossRef]
- Zheng, K.; Zhong, J.; Qi, Z.; Ling, S.; Kaplan, D.L. Isolation of Silk Mesostructures for Electronic and Environmental Applications. Adv. Funct. Mater. 2018, 28, 1806380. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, W.; Mu, T. Controllable Exfoliation of Natural Silk Fibers into Nanofibrils by Protein Denaturant Deep Eutectic Solvent: Nanofibrous Strategy for Multifunctional Membranes. Green Chem. 2018, 20, 3625–3633. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Du, W.; Mu, T. Top-Down Extraction of Silk Protein Nanofibers by Natural Deep Eutectic Solvents and Application in Dispersion of Multiwalled Carbon Nanotubes for Wearable Sensing. ChemSusChem 2020, 13, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, R.; Wang, J.; Xiang, J.; Chen, C.; Liu, G.; Liao, X. Hierarchical Collagen Fibers Complexed with Tannic Acid and Fe3+ as a Heterogeneous Catalyst for Enhancing Sulfate Radical-Based Advanced Oxidation Process. Environ. Sci. Pollut. Res. 2022, 29, 58675–58684. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Jameela, S.R. Glutaraldehyde as a Fixative in Bioprostheses and Drug Delivery Matrices. Biomaterials 1996, 17, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of Collagen with a Natural Cross-Linker, Procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Wierenga, P.A.; Gruppen, H. Antioxidative Activity and Emulsifying Properties of Cuttlefish Skin Gelatin–Tannic Acid Complex as Influenced by Types of Interaction. Innov. Food Sci. Emerg. Technol. 2010, 11, 712–720. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, Q.; Sun, G.T.; Chen, C.Z.; Zhu, M.Q.; Huang, X.-H. The Synthesis of Tannin-Based Graphene Aerogel by Hydrothermal Treatment for Removal of Heavy Metal Ions. Ind. Crops Prod. 2022, 176, 114304. [Google Scholar] [CrossRef]
- Wu, H.; Zhuo, L.; He, Q.; Liao, X.; Shi, B. Heterogeneous Hydrogenation of Nitrobenzenes over Recyclable Pd (0) Nanoparticle Catalysts Stabilized by Polyphenol-Grafted Collagen Fibers. Appl. Catal. A 2009, 366, 44–56. [Google Scholar] [CrossRef]
- Mao, H.; Chen, C.; Liao, X.; Shi, B. Catalytic Hydrogenation of Quinoline over Recyclable Palladium Nanoparticles Supported on Tannin Grafted Collagen Fibers. J. Mol. Catal. A Chem. 2011, 341, 51–56. [Google Scholar] [CrossRef]
- Fu, L.; Cai, L. Ru Nanoparticles Loaded on Tannin Immobilized Collagen Fibers for Catalytic Hydrolysis of Ammonia Borane. Int. J. Hydrog. Energy 2021, 46, 10749–10762. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, J.; Wang, T.; Zeng, J.; Liu, L.; Jian, J.; Zhou, Z.; Zeng, L.; Liu, Q.; Liu, G. In-Situ Preparation of Silver Salts/Collagen Fiber Hybrid Composites and their Photocatalytic and Antibacterial Activities. J. Hazard. Mater. 2018, 359, 274–280. [Google Scholar] [CrossRef]
- Nagaraj, S.; Cheirmadurai, K.; Thanikaivelan, P. Visible-Light Active Collagen-TiO2 Nanobio-Sponge for Water Remediation: A Sustainable Approach. Clean. Mater. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Ghafuri, H.; Esmaili, E.; Talebi, M. Fe3O4@SiO2/collagen: An Efficient Magnetic Nanocatalyst for the Synthesis of Benzimidazole and Benzothiazole Derivatives. C. R. Chim. 2016, 19, 942–950. [Google Scholar] [CrossRef]
- Jia, H.; Liu, S.; Zheng, G.P.; Zheng, X.C.; Wang, X.Y.; Liu, P. Collagen-Graphene Oxide Magnetic Hybrids Anchoring Pd (0) Catalysts for Efficient H2 Generation from Ammonia Borane. Int. J. Hydrog. Energy 2019, 44, 27022–27029. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, Z.H.; Yang, Y.; Chen, Y.; Chen, X.; Jiang, H.L. Facile Synthesis of Graphene-Supported Ni-CeOx Nanocomposites as Highly Efficient Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane. Nano Res. 2018, 11, 4412–4422. [Google Scholar] [CrossRef]
- Xue, Z.; Huang, P.; Li, T.; Qin, P.; Xiao, D.; Liu, M.; Chen, P.; Wu, Y. A Novel “Tunnel-like” Cyclopalladated Arylimine Catalyst Immobilized on Graphene Oxide Nano-Sheet. Nanoscale 2017, 9, 781–791. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Hu, E.; Yuen, C.W.M.; Jiang, S.X. Konjac Glucomannan/Graphene Oxide Hydrogel with Enhanced Dyes Adsorption Capability for Methyl Blue and Methyl Orange. Appl. Surf. Sci. 2015, 357, 866–872. [Google Scholar] [CrossRef]
- Aghdam, B.M.; Bahari, S.; Molaei, R. The Pd (0) Nanoparticles Stabilized by Collagen Fibres as a Recyclable Heterogeneous Catalyst for the Stille Reaction under Aerobic Condition. J. Chem. Sci. 2013, 125, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Tang, R.; He, Q.; Liao, X.; Shi, B. Highly Stable Pt Nanoparticle Catalyst Supported by Polyphenol-Grafted Collagen Fiber and Its Catalytic Application in the Hydrogenation of Olefins. J. Chem. Technol. Biotechnol. 2009, 84, 1702–1711. [Google Scholar] [CrossRef]
- Liao, Z.; Lan, Y.; Wang, K.; Lei, M.; Liao, Y.; Mao, H.; Ma, J.; Zhao, S. In Situ Growing PtCo Bimetallic Catalyst on Plant Tannin-Grafted Collagen Fiber for Catalytic Hydrogenation of Cinnamaldehyde with Desirable Performance. Chem. Res. Chin. Univ. 2018, 34, 285–289. [Google Scholar] [CrossRef]
- Ma, J.; Huang, X.; Liao, X.; Shi, B. Preparation of Highly Active Heterogeneous Au@ Pd Bimetallic Catalyst Using Plant Tannin Grafted Collagen Fiber as the Matrix. J. Mol. Catal. A Chem. 2013, 366, 8–16. [Google Scholar] [CrossRef]
- Akabori, S.; Sakurai, S.; Izumi, Y.; Fujii, Y. An Asymmetric Catalyst. Nature 1956, 178, 323–324. [Google Scholar] [CrossRef]
- Akamatsu, A.; Izumi, Y.; Akabori, S. Studies on the Silk-Platinum Catalyst. I. Its Preparation and Activity. Bull. Chem. Soc. Jpn. 1961, 34, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Sajiki, H.; Ikawa, T.; Yamada, H.; Tsubouchi, K.; Hirota, K. Preparation of Silk Fibroin-Supported Pd (0) catalyst for chemoselective hydrogenation: Reduction of palladium (II) acetate by methanol on the Protein. Tetrahedron Lett. 2003, 44, 171–174. [Google Scholar] [CrossRef]
- Ikawa, T.; Sajiki, H.; Hirota, K. Highly Chemoselective Hydrogenation Method Using Novel Finely Dispersed Palladium Catalyst on Silk-Fibroin: Its Preparation and Activity. Tetrahedron 2005, 61, 2217–2231. [Google Scholar] [CrossRef]
- Sajiki, H.; Ikawa, T.; Hirota, K. Markedly Chemoselective Hydrogenation with Retention of Benzyl Ester and N-Cbz Functions Using a Heterogeneous Pd-Fibroin Catalyst. Tetrahedron Lett. 2003, 44, 8437–8439. [Google Scholar] [CrossRef]
- Xia, Y.; Wan, J.; Gu, Q. Silk Fibroin Fibers Supported with High Density of Gold Nanoparticles: Fabrication and Application as Catalyst. Gold Bull. 2011, 44, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ju, M.; Cho, O.H.; Kim, Y.; Nam, K.T. Tyrosine-Rich Peptides as a Platform for Assembly and Material Synthesis. Adv. Sci. 2019, 6, 1801255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Chen, W.; Itoh, H.; Naka, K.; Ni, Q.; Yamane, H.; Chujo, Y. Preparation of a Novel Core–Shell Nanostructured Gold Colloid–Silk Fibroin Bioconjugate by the Protein in Situ Redox Technique at Room Temperature. Chem. Commun. 2001, 37, 2518–2519. [Google Scholar] [CrossRef]
- Akbarzadeh, P.; Koukabi, N. Fibroin-Functionalized Magnetic Carbon Nanotube as a Green Support for Anchoring Silver Nanoparticles as a Biocatalyst for A3 Coupling Reaction. Appl. Organomet. Chem. 2019, 34, e5395. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.; Yang, W.; Lu, B.; Ke, X.; Zhang, B.; Tang, J. Porous Co3O4 Nanorods-Reduced Graphene Oxide with Intrinsic Peroxidase-like Activity and Catalysis in the Degradation of Methylene Blue. ACS Appl. Mater. Interfaces 2013, 5, 3809–3815. [Google Scholar] [CrossRef]
- Fei, X.; Jia, M.; Du, X.; Yang, Y.; Zhang, R.; Shao, Z.; Zhao, X.; Chen, X. Green Synthesis of Silk Fibroin-Silver Nanoparticle Composites with Effective Antibacterial and Biofilm-Disrupting Properties. Biomacromolecules 2013, 14, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Shao, Z.; Chen, X. Synthesis of Hierarchical Three-Dimensional Copper Oxide Nanostructures through a Biomineralization-Inspired Approach. Nanoscale 2013, 5, 7991–7997. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Y.; Chen, X.; Shao, Z. Templating Effect of Silk Fibers in the Oriented Deposition of Aragonite. Chem. Commun. 2008, 44, 5511–5513. [Google Scholar] [CrossRef]
- Luo, K.Y.; Shao, Z.Z. A Novel Regenerated Silk Fibroin-Based Hydrogels with Magnetic and Catalytic Activities. Chin. J. Polym. Sci. 2017, 35, 515–523. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, H.; Tong, M.; Cao, J.; Wu, W. Synergetic Effects of Graphene-CoPc/Silk Fibroin Three-Dimensional Porous Composites as Catalysts for Acid Red G Degradation. RSC Adv. 2019, 9, 24751–24759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.; Wang, Z.; Zhao, N.; Xu, J. Aerogels Derived from Polymer Nanofibers and Their Applications. Macromol. Rapid Commun. 2018, 39, e1700724. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godiya, C.B.; Cheng, X.; Deng, G.; Li, D.; Lu, X. Silk Fibroin/Polyethylenimine Functional Hydrogel for Metal Ion Adsorption and Upcycling Utilization. J. Environ. Chem. Eng. 2019, 7, 102806. [Google Scholar] [CrossRef]
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-Based Materials for Flexible Supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Imtiaz, S.; Zhang, J.; Zafar, Z.A.; Ji, S.; Huang, T.; Anderson, J.A.; Zhang, Z.; Huang, Y. Biomass-Derived Nanostructured Porous Carbons for Lithium-Sulfur Batteries. Sci. China Mater. 2016, 59, 389–407. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Wu, D.; Li, M.; Zeng, Q.; Xu, F.; Huang, Z.; Fu, R. Template-Free Fabrication of Hierarchical Porous Carbon by Constructing Carbonyl Crosslinking Bridges between Polystyrene Chains. J. Mater. Chem. 2010, 20, 731–735. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically Porous Carbon Derived from Polymers and Biomass: Effect of Interconnected Pores on Energy Applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Deng, D.; Tang, R.; Liao, X.; Shi, B. Using Collagen Fiber as a Template to Synthesize Hierarchical Mesoporous Alumina Fiber. Langmuir 2008, 24, 368–370. [Google Scholar] [CrossRef]
- Deng, D.; Wu, H.; Liao, X.; Shi, B. Synthesis of Unique Mesoporous ZrO2-Carbon Fiber from Collagen Fiber. Microporous Mesoporous Mater. 2008, 116, 705–709. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, X.; Liao, X.; Shi, B. Preparation of Fibrous Sulfated Zirconia (SO42−/ZrO2) Solid Acid Catalyst Using Collagen Fiber as the Template and Its Application in Esterification. J. Mol. Catal. A Chem. 2011, 347, 46–51. [Google Scholar] [CrossRef]
- Xiao, G.; Zhou, J.; Huang, X.; Liao, X.; Shi, B. Facile Synthesis of Mesoporous Sulfated Ce/TiO2 Nanofiber Solid Superacid with Nanocrystalline Frameworks by Using Collagen Fibers as a Biotemplate and Its Application in Esterification. RSC Adv. 2014, 4, 4010–4019. [Google Scholar] [CrossRef]
- Xiao, G.; Huang, X.; Liao, X.; Shi, B. One-Pot Facile Synthesis of Cerium-Doped TiO2 Mesoporous Nanofibers Using Collagen Fiber as the Biotemplate and Its Application in Visible Light Photocatalysis. J. Phys. Chem. C 2013, 117, 9739–9746. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.; Ma, B.; Wang, J.; Song, W.; Fan, X.; Li, Y.; Zhang, F.; Peng, W. Ultra-Small Mo2 C Nanodots Encapsulated in Nitrogen-Doped Porous Carbon for pH-Universal Hydrogen Evolution: Insights into the Synergistic Enhancement of HER Activity by Nitrogen Doping and Structural Defects. J. Mater. Chem. A 2019, 7, 4734–4743. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and β-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chem. 2019, 131, 4977–4982. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-Derived Porous Carbon Materials with Different Dimensions for Supercapacitor Electrodes: A Review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Lee, Y.H.; Li, F.; Chang, K.H.; Hu, C.C.; Ohsaka, T. Novel Synthesis of N-Doped Porous Carbons from Collagen for Electrocatalytic Production of H2O2. Appl. Catal. B 2012, 126, 208–214. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, Y.; Yuan, L.; Zhang, Y.; Liu, C.; Wang, Q.; Wu, H. Synergistic Effect of WN/Mo2C Embedded in Bioderived Carbon Nanofibers: A Rational Design of a Shuttle Inhibitor and an Electrocatalyst for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 14, 18578–18588. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, L.; Tang, Y.; Zhou, J.; Shi, B. Novel g-C3N4/C/Fe2O3 Composite for Efficient Photocatalytic Reduction of Aqueous Cr(VI) under Light Irradiation. Ind. Eng. Chem. Res. 2021, 60, 13594–13603. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ashokkumar, M.; Gullapalli, H.; Gong, Y.; Vajtai, R.; Thanikaivelan, P.; Ajayan, P.M. Nitrogen-Rich Carbon Nano-Onions for Oxygen Reduction Reaction. Carbon 2018, 130, 645–651. [Google Scholar] [CrossRef]
- Telay Mekonnen, B.; Meiyazhagan, A.; Ragothaman, M.; Kalirajan, C.; Palanisamy, T. Bi-Functional Iron Embedded Carbon Nanostructures from Collagen Waste for Photocatalysis and Li-ion Battery Applications: A Waste to Wealth Approach. J. Clean. Prod. 2019, 210, 190–199. [Google Scholar] [CrossRef]
- Kang, Q.; Qin, Y.; Lu, Q.; Gao, F. Waste Leather-Derived (Cr, N)-Co-Doped Carbon Cloth Coupling with Mo2C nanoparticles as a self-supported electrode for highly active hydrogen evolution reaction Performances. J. Power Sources 2020, 476, 228706. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Song, Y.; Wang, F. Photochemical Solid-Phase Synthesis of Platinum Single Atoms on Nitrogen-Doped Carbon with High Loading as Bifunctional Catalysts for Hydrogen Evolution and Oxygen Reduction Reactions. ACS Catal. 2018, 8, 8450–8458. [Google Scholar] [CrossRef]
- He, D.; Niu, J.; Dou, M.; Ji, J.; Huang, Y.; Wang, F. Nitrogen and Oxygen Co-Doped Carbon Networks with a Mesopore-Dominant Hierarchical Porosity for High Energy and Power Density Supercapacitors. Electrochim. Acta 2017, 238, 310–318. [Google Scholar] [CrossRef]
- Dou, M.; He, D.; Shao, W.; Liu, H.; Wang, F.; Dai, L. Pyrolysis of Animal Bones with Vitamin B12: A Facile Route to Efficient Transition Metal-Nitrogen-Carbon (TM-N-C) Electrocatalysts for Oxygen Reduction. Chem. Eur. J. 2016, 22, 2896–2901. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient Oxygen Reduction Reaction (ORR) Catalysts Based on Single Iron Atoms Dispersed on a Hierarchically Structured Porous Carbon Framework. Angew. Chem. Int. Ed. Engl. 2018, 57, 9038–9043. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Dou, M.; Wang, F. Towards High-Performance Electrocatalysts for Oxygen Reduction: Inducing Atomic-Level Reconstruction of Fe-Nx Site for Atomically Dispersed Fe/N-Doped Hierarchically Porous Carbon. Chem. Eur. J. 2018, 24, 8848–8856. [Google Scholar] [CrossRef]
- Ke, L.; Zhao, K.; Yan, X.; Cao, X.; Wu, X.; Zhang, C.; Luo, T.; Ding, T.; Yan, N. Facile Mineralization and Valorization of Cr-Containing Leather Shavings for Electrocatalytic H2O2 Generation and Organic Pollutant Removal. Chem. Eng. J. 2022, 437, 135036. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized Silk Fabric for Ultrastretchable, Highly Sensitive, and Wearable Strain Sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Zhang, M.; Jian, M.; Zhang, Y. An All-Silk-Derived Dual-Mode e-Skin for Simultaneous Temperature–Pressure Detection. ACS Appl. Mater. Interfaces 2017, 9, 39484–39492. [Google Scholar] [CrossRef]
- Lei, Y.; Shi, Q.; Han, C.; Wang, B.; Wu, N.; Wang, H.; Wang, Y. N-doped Graphene Grown on Silk Cocoon-Derived Interconnected Carbon Fibers for Oxygen Reduction Reaction and Photocatalytic Hydrogen Production. Nano Res. 2016, 9, 2498–2509. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Wang, H. Astridia Velutina-like S, N-Codoped Hierarchical Porous Carbon from Silk Cocoon for Superior Oxygen Reduction Reaction. RSC Adv. 2016, 6, 73560–73565. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Zhang, H.; Zhang, P.; Tian, Z.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Intrinsic Defects in Biomass-Derived Carbons Facilitate Electroreduction of CO2. Nano Res. 2020, 13, 729–735. [Google Scholar] [CrossRef]
- Iwazaki, T.; Obinata, R.; Sugimoto, W.; Takasu, Y. High Oxygen-Reduction Activity of Silk-Derived Activated Carbon. Electrochem. Commun. 2009, 11, 376–378. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhang, Y.; Zhang, W.; Li, Y.; Zhu, X.; Wang, P.; Hu, D. Porous Carbon Nanofibers Derived from Silk Fibroin through Electrospinning as N-Doped Metal-Free Catalysts for Hydrogen Evolution Reaction in Acidic and Alkaline Solutions. ACS Appl. Mater. Interfaces 2022, 14, 834–849. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Xia, K.; Xie, N.; Wang, H.; Zhang, Y. Silk-Derived 2D Porous Carbon Nanosheets with Atomically-Dispersed Fe-Nx-C Sites for Highly Efficient Oxygen Reaction Catalysts. Small 2019, 15, e1804966. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Zhang, W.; Li, Y.; Wang, Y.; Wang, P.; Hu, D. Dual Metal-Loaded Porous Carbon Materials Derived from Silk Fibroin as Bifunctional Electrocatalysts for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 30678–30692. [Google Scholar] [CrossRef]
- Gong, S.; Xiao, X.; Wang, W.; Sam, D.K.; Lu, R.; Xu, Y.; Liu, J.; Wu, C.; Lv, X. Silk Fibroin-Derived Carbon Aerogels Embedded with Copper Nanoparticles for Efficient Electrocatalytic CO2-to-CO Conversion. J. Colloid Interface Sci. 2021, 600, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, L.; Cao, C.; Zhang, F.; Lang, F.; Wang, H.; Yang, H.; Shen, J. Salt-Induced Silk Gel-Derived N and Trace Fe Co-Doped 3D Porous Carbon as an Oxygen Reduction Catalyst in Microbial Fuel Cells. Nanoscale 2019, 11, 13431–13439. [Google Scholar] [CrossRef] [PubMed]
- Sam, D.K.; Wang, W.; Gong, S.; Sam, E.K.; Lv, X.; Wang, J.; Liu, J. CO2 Assisted Synthesis of Silk Fibroin Driven Robust N-Doped Carbon Aerogels Coupled with Nickel–Cobalt Particles as Highly Active Electrocatalysts for HER. Int. J. Hydrog. Energy 2021, 46, 21525–21533. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Yu, L.; Ren, P.; Wu, X.; Sun, L.; Jiao, F.; Bao, X. Silicon Carbide-Derived Carbon Nanocomposite as a Substitute for Mercury in the Catalytic Hydrochlorination of Acetylene. Nat. Commun. 2014, 5, 3688. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Dai, L.; Lin, X.; Chen, J.F.; Zhang, J.; Feng, X.; Müllen, K.; Zhu, X.; Dai, S. Chemical Approaches to Carbon-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1804863. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, W.; Luo, J.; Chen, W.; Cao, T.; Zheng, L.; Dong, J.; Zhang, J.; Zhang, M.; Han, Y.; et al. A Cocoon Silk Chemistry Strategy to Ultrathin N-Doped Carbon Nanosheet with Metal Single-Site Catalysts. Nat. Commun. 2018, 9, 3861. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, L.; Lou, Z.; Zheng, Y.; Wang, K.; Zhao, L.; Han, W.; Jiang, K.; Shen, G. Biomimetic, Biocompatible and Robust Silk Fibroin-MXene Film with STable 3D Cross-Link Structure for Flexible Pressure Sensors. Nano Energy 2020, 78, 105252. [Google Scholar] [CrossRef]
- Su, I.; Jung, G.S.; Narayanan, N.; Buehler, M.J. Perspectives on Three-Dimensional Printing of Self-Assembling Materials and Structures. Curr. Opin. Biomed. Eng. 2020, 15, 59–67. [Google Scholar] [CrossRef]
- Kim, T.S.; Song, H.J.; Kim, J.C.; Ju, B.; Kim, D.W. 3D Architectures of Cox P Using Silk Fibroin Scaffolds: An Active and Stable Electrocatalyst for Hydrogen Generation in Acidic and Alkaline Media. Small 2018, 14, e1801284. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, F.; Lin, Y. Refining Cocoon to Prepare (N, S, and Fe) Ternary-Doped Porous Carbon Aerogel as Efficient Catalyst for the Oxygen Reduction Reaction in Alkaline Medium. J. Power Sources 2018, 384, 48–57. [Google Scholar] [CrossRef]




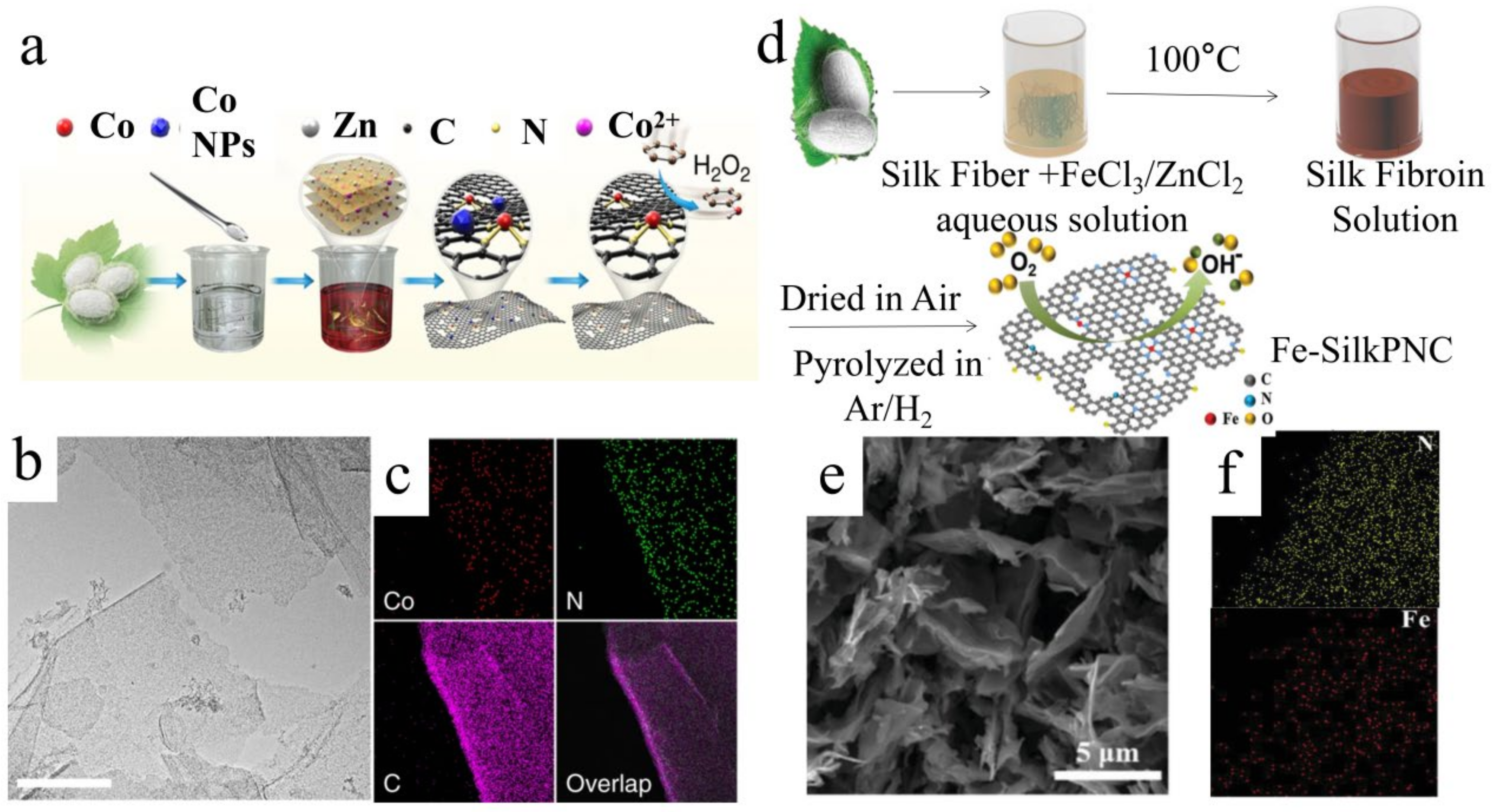
| Metal Nanoparticles | Cross-Linking Agent | Type of Catalytic Reaction | Catalytic Efficiency | References |
|---|---|---|---|---|
| Pd | EGCG | Hydrogenation of nitrobenzene and its derivatives | Nitrobenzene conversion rate 98%, selectivity 99% | [89] |
| Pd | EGCG | Hydrogenation of allyl alcohol | Conversion rate 99.8%, selectivity 89.04% | [19] |
| Pd | EGCG | Stille coupling reactions | The yield of static coupling of aryl iodide with vinylbutane is 90% | [99] |
| Pt | EGCG | Hydrogenation of typical olefins | Conversion rate > 99.5% Selectivity > 99% | [100] |
| Pd | Black wattle tannin | Hydrogenation of quinoline | Conversion rate 99.3%, selectivity 100% | [90] |
| PtCox | BT | Hydrogenation of cinnamaldehyde | The conversion of cinnamaldehyde was 93.56% | [101] |
| Au@Pd | BT | Liquid-phase hydrogenation of cyclohexene | The conversion was 92.70% | [102] |
| Ru | BT | Hydrolyze ammonia borane | TOF was as high as 322 molH2 mol−1min−1 and Ea was as low as 32.41 kJ mol−1 for AB hydrolysis | [91] |
| Raw Materials | Catalysis | Carbonization Temperature and Time | Reaction | Medium | Activity | Ref. |
|---|---|---|---|---|---|---|
| Cattle bone | SA-Fe-HPC | 400 °C; 2 h 900 °C; 1 h | ORR | Acidic electrolyte | E1/2 = 0.81 V, Jd = 5.5 mA cm−2 | [143] |
| Alkaline electrolytes | E1/2 = 0.63 V, Jd = 2.8 mA cm−2 | |||||
| Pig bone | Fe-N-HPC-AH | 400 °C; 3 h, 800 °C; 1 h, 800 °C; 2 h | ORR | Alkaline electrolyte | Eonset was 0.97 V, E1/2 was 0.870 V | [144] |
| Cattle bones | Co-N-HPC | 400 °C; 3 h, 850 °C; 1 h 800 °C; 2 h | ORR | Alkaline electrolyte | E1/2 = 0.835 V Jk = 20.4 mA cm−2 | [142] |
| Cowhide | FeCu/CCol-CNCA | 800 °C; 5 h | ORR | Alkaline electrolyte | The current limit density reached 7.32 mA cm−2 | [55] |
| Cattle bone | Pt1/NPC | 400 °C; 3 h 800 °C; 1 h 1000 °C; 3 h | HER | Acidic electrolyte | At −10 mA cm−2, the potential is −0.025 V. The TOF was 2.93 s−1 | [140] |
| ORR | Alkaline electrolytes | E1/2 = 0.835 V The largest Jk was 3.23 mA cm−2 | ||||
| Cr-containing leather | Cr/CF600 | 600 °C; 3 h | ORR | Alkaline electrolytes | The oORR yields H2O2 with selectivity ~86% at 0.62 V | [145] |
| Rat tail tropocollagen | Ru-CCS | 800 °C; 2 h | HER | Acidic electrolyte | Eonset was 11.0 mV The TOF was 3.70 s−1 at an overpotential of 50 mV, | [72] |
| Goat skin | 750-8 N-CNO | 750 °C; 8 h | ORR | Alkaline electrolytes | 50 mV onset potential at 10 mA cm−2 | [138] |
| Waste leather | Mo2C@CNCC | 900 °C; 3 h | HER | Alkaline electrolytes | 272 mV overpotential at 10 mA cm−2 | [139] |
| Catalysis | Structure | Carbonization Temperature and Time | Reaction | Medium | Activity | Ref. |
|---|---|---|---|---|---|---|
| A-350–1000 | Particles | 350 °C; 1 h 1000 °C; 1 h | CO2 reduction reaction | Acidic electrolyte | The Faradaic efficiency was 89% and maintained good selectivity for about 10 days. | [150] |
| 4%-SPCNF | 1D | 900 °C; 4 h | HER | Acidic electrolyte | The overpotential was 310.86 ± 12.93 mV and Tafel slope was 95.93 mV dec−1 | [152] |
| Alkaline electrolyte | The overpotential was 401.3 ± 7.92 mV and Tafel slope was 138.43 mV dec−1 | |||||
| Fe–Nx–C | 2D | 220 °C 45 min, 320 °C; 2.5 h, 900 °C; 2 h | ORR | Alkaline media | Half-wave potential (E1/2) was 0.853 V, remarkable stability with only 11 mV loss in E1/2 after 30,000 cycles | [153] |
| CoW@ACSF | Particles | 900 °C; -- | HER | Acidic electrolyte | The overpotential was 138.42 ± 10.39 mV at 10 mA cm−2 | [154] |
| ORR | Alkaline electrolytes | The overpotential was 492.05 ± 19.04 mV at 10 mA cm−2 | ||||
| SF-Cu/CA | 3D | 100 °C; 30 min, 225 °C; 2 h, 800 °C; 2 h | CO2 reduction reaction | —— | The current density was 29.4 mA cm−2 and Faraday efficiency was 83.06% | [155] |
| NFe0.5-C | 3D | 900 °C; 2 h | ORR | Acidic electrolyte | The positive initial potential was 0.274 V and half-wave potential 0.095 V. | [156] |
| SFCA-NiCo | 3D | 225 °C; 30 min 800 °C; 2 h | HER | Alkaline electrolytes | Eonset was 52.0 mV 179 mV overpotential at 10 mA cm−2 | [157] |
| CA-NiCoFe-600 | 3D | 600 °C; 2 h | OER | —— | 321 mV overpotential at 10 mA cm−2 and the Tafel slope was 42 mV dec−1 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, J.; Yang, W.; Pei, Y. Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts. Polymers 2023, 15, 375. https://doi.org/10.3390/polym15020375
Chen J, Liu J, Yang W, Pei Y. Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts. Polymers. 2023; 15(2):375. https://doi.org/10.3390/polym15020375
Chicago/Turabian StyleChen, Jiankang, Jie Liu, Wen Yang, and Ying Pei. 2023. "Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts" Polymers 15, no. 2: 375. https://doi.org/10.3390/polym15020375
APA StyleChen, J., Liu, J., Yang, W., & Pei, Y. (2023). Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts. Polymers, 15(2), 375. https://doi.org/10.3390/polym15020375









