Structural Characterization of Exopolysaccharide Produced by Leuconostoccitreum B-2 Cultured in Molasses Medium and Its Application in Set Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Bacterium and Culture Conditions
2.2. Methods for Determination of the Biomass and Crude M-EPS Yield
2.3. Purification of Crude M-EPS and UV-Vis Spectroscopy Analysis
2.4. Structural Analysis of M-EPS
2.4.1. Determination of Monosaccharide Composition
2.4.2. Determination of Molecular Weight
2.4.3. FT-IR Spectrum
2.4.4. NMR Spectroscopy
2.4.5. Scanning Electron Microscope (SEM)
2.5. Statistical Optimization of M-EPS Production
2.5.1. Plackett–Burman Design (PB)
2.5.2. Steepest Ascent Experiment
2.5.3. Central Composite Design (CCD)
2.5.4. Validation Test and Determination of Optimal Sugar Production Time
2.6. Manufacture of Set Yogurt
2.7. Characterization of M-EPS-Added Set Yogurt
2.7.1. Measurement of Water Holding Capacity (WHC) and pH
2.7.2. Microstructure of Yogurt
2.8. Statistical Analysis
3. Results and Discussion
3.1. Production and Purification of the M-EPS
3.2. Structural Analysis of M-EPS
3.2.1. Monosaccharide Composition of M-EPS
3.2.2. Molecular Weight
3.2.3. FT-IR Spectrum Analysis
3.2.4. NMR Spectroscopy Analysis
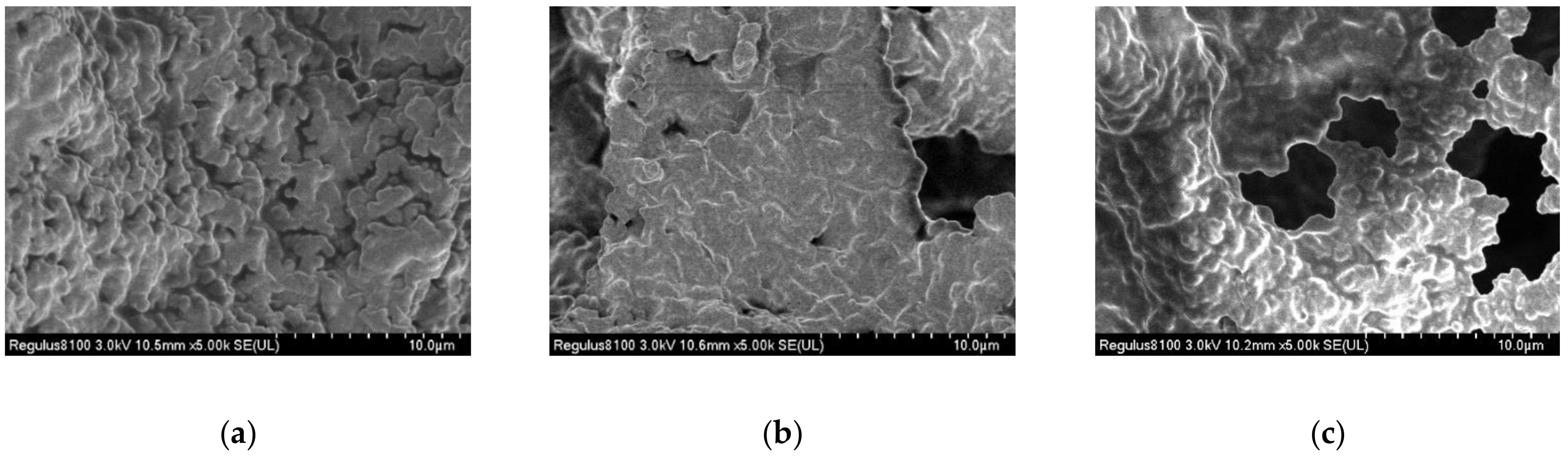
3.2.5. SEM Analysis
3.3. Optimization of M-EPS Production
3.3.1. Plackett–Burman Design
3.3.2. Path of Steepest Ascent
3.3.3. Response Optimization
3.3.4. Validation Test and Determination of Optimal Sugar Production Time
3.4. Characterization of M-EPS-Added Set Yogurt
3.4.1. WHC and pH of Set Yogurt Product
3.4.2. Micromorphology of Set Yogurt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of Bacterial Homopolysaccharide Production and Properties during Food Processing. Biology 2022, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Arriba, M.G.; Hernandez-Alcantara, A.M.; Mohedano, M.L.; Chiva, R.; Celador-Lera, L.; Velazquez, E.; Prieto, A.; Duenas, M.T.; Tamame, M.; Lopez, P. Lactic Acid Bacteria Isolated from Fermented Doughs in Spain Produce Dextrans and Riboflavin. Foods 2021, 10, 2004. [Google Scholar] [CrossRef]
- Kim, G.; Bae, J.H.; Cheon, S.; Lee, D.H.; Kim, D.H.; Lee, D.; Park, S.H.; Shim, S.; Seo, J.H.; Han, N.S. Prebiotic activities of dextran from Leuconostoc mesenteroides SPCL742 analyzed in the aspect of the human gut microbial ecosystem. Food Funct. 2022, 13, 1256–1267. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Esmaeilnejad-Moghadam, B.; Mokarram, R.R.; Hejazi, M.A.; Khiabani, M.S.; Keivaninahr, F. Low molecular weight dextran production by Leuconostoc mesenteroides strains: Optimization of a new culture medium and the rheological assessments. Bioact. Carbohydr. Diet. Fibre. 2019, 18, 100181. [Google Scholar] [CrossRef]
- Diana, C.R.; Humberto, H.S.; Jorge, Y.F. Structural characterization and rheological properties of dextran produced by native strains isolated of Agave salmiana. Food Hydrocoll. 2019, 90, 1–8. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Jiang, J.; Guo, S.; Ping, W.; Ge, J. The response surface optimization of exopolysaccharide produced by Weissella confusa XG-3 and its rheological property. Prep. Biochem. Biotechnol. 2020, 50, 1014–1022. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Patel, S.N.; Lata, K.; Singh, U.; Krishania, M.; Sangwan, R.S.; Singh, S.P. A novel approach of integrated bioprocessing of cane molasses for production of prebiotic and functional bioproducts. Bioresour. Technol. 2016, 219, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Nacher-Vazquez, M.; Ballesteros, N.; Canales, A.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I.; Prieto, A.; Aznar, R.; Lopez, P. Dextrans produced by lactic acid bacteria exhibit antiviral and immunomodulatory activity against salmonid viruses. Carbohydr. Polym. 2015, 124, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Bottari, B.; Morreale, F.; Savo Sardaro, M.L.; Angelino, D.; Raimondi, S.; Rossi, M.; Pellegrini, N. Potential prebiotic effect of a long-chain dextran produced by Weissella cibaria: An in vitro evaluation. Int. J. Food Sci. Nutr. 2020, 71, 563–571. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, D.; Zhang, W.; Stressler, T.; Mu, W. Lactic acid bacteria-derived α-glucans: From enzymatic synthesis to miscellaneous applications. Biotechnol. Adv. 2021, 47, 107708. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Design and application of dextran carrier. J. Drug Delivery Sci. Technol. 2020, 55, 101392. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Valli, V.; Gomez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef]
- Luo, J.; Guo, S.; Wu, Y.; Wan, Y. Separation of Sucrose and Reducing Sugar in Cane Molasses by Nanofiltration. Food Bioprocess Technol. 2018, 11, 913–925. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2020, 346, 128860. [Google Scholar] [CrossRef] [PubMed]
- Muhammadi; Afzal, M. Optimization of water absorbing exopolysaccharide production on local cheap substrates by Bacillus strain CMG1403 using one variable at a time approach. J. Microbiol. 2014, 52, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, J.; Moorthy, I.G.; Sivashankar, R.; Sivasubramanian, V. Optimization of dextran production by Weissella cibaria NITCSK4 using Response Surface Methodology-Genetic Algorithm based technology. Carbohydr. Polym. 2017, 174, 103–110. [Google Scholar] [CrossRef]
- Jiang, J.; Guo, S.; Ping, W.; Zhao, D.; Ge, J. Optimization production of exopolysaccharide from Leuconostoc lactis L2 and its partial characterization. Int. J. Biol. Macromol. 2020, 159, 630–639. [Google Scholar] [CrossRef]
- Du, R.; Xing, H.; Yang, Y.; Jiang, H.; Zhou, Z.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the Texture of Yogurt by Use of Exopolysaccharide Producing Lactic Acid Bacteria. Biomed. Res. Int. 2016, 2016, 7945675. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady Shetty, P. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Doleyres, Y.; Schaub, L.; Lacroix, C. Comparison of the Functionality of Exopolysaccharides Produced In Situ or Added as Bioingredients on Yogurt Properties. J. Dairy Sci. 2005, 88, 4146–4156. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Ke, C.; Bai, Y.; Liu, X.; Li, S. Production of welan gum from cane molasses by Sphingomonas sp. FM01. Carbohydr. Polym. 2020, 244, 116485. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Lee, Y.J.; Cho, D.-H.; Ahn, K.-H.; Hong, J.-E.; Park, Y.-I.; Kim, H.-S. A Novel Galactoglucomannan Exopolysaccharide Produced by Oil Fermentation with Pseudozyma sp. SY16. Biotechnol. Bioprocess Eng. 2020, 25, 742–748. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, Purification, and Characterization of Exopolysaccharide Produced by Leuconostoc citreum N21 from Dried Milk Cake. Trans. Tianjin Univ. 2018, 25, 161–168. [Google Scholar] [CrossRef]
- Du, R.; Pei, F.; Kang, J.; Zhang, W.; Wang, S.; Ping, W.; Ling, H.; Ge, J. Analysis of the structure and properties of dextran produced by Weissella confusa. Int. J. Biol. Macromol. 2022, 204, 677–684. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ping, W.; Ge, J. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Jaros, D.; Kobsch, J.; Rohm, H. Exopolysaccharides from Basidiomycota: Formation, isolation and techno-functional properties. Eng. Life Sci. 2018, 18, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Choudhuri, I.; Khanra, K.; Maity, P.; Patra, A.; Maity, G.N.; Pati, B.R.; Nag, A.; Mondal, S.; Bhattacharyya, N. Structure and biological properties of exopolysaccharide isolated from Citrobacter freundii. Int. J. Biol. Macromol. 2021, 168, 537–549. [Google Scholar] [CrossRef]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Zou, Q.S.; Pu, Y.Y.; Chen, S. Optimization of the Components of Medium Producing Dextran from Immobilized Cells of Leuconostoc mesenteroides. Adv. Mater. Res. 2011, 418–420, 212–216. [Google Scholar] [CrossRef]
- Aramsangtienchai, P.; Kongmon, T.; Pechroj, S.; Srisook, K. Enhanced production and immunomodulatory activity of levan from the acetic acid bacterium, Tanticharoenia sakaeratensis. Int. J. Biol. Macromol. 2020, 163, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, P.; Liu, X.; Wang, J.; Yu, J. Optimization of Cultural Conditions for Antioxidant Exopolysaccharides from Xerocomus badius Grown in Shrimp Byproduct. Biomed Res. Int. 2016, 2016, 2043787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, H.; Du, R.; Zhao, F.; Han, Y.; Xiao, H.; Zhou, Z. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Q.; Qu, L.; Liang, L.; Han, Y.; Wang, X.; Zhou, Z. Pilot-scale production of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its application in set yogurt. J. Dairy Sci. 2022, 105, 1072–1083. [Google Scholar] [CrossRef]
- Ale, E.C.; Perezlindo, M.J.; Pavon, Y.; Peralta, G.H.; Costa, S.; Sabbag, N.; Bergamini, C.; Reinheimer, J.A.; Binetti, A.G. Technological, rheological and sensory characterizations of a yogurt containing an exopolysaccharide extract from Lactobacillus fermentum Lf2, a new food additive. Food Res. Int. 2016, 90, 259–267. [Google Scholar] [CrossRef]
- Fu, R.; Li, J.; Zhang, T.; Zhu, T.; Cheng, R.; Wang, S.; Zhang, J. Salecan stabilizes the microstructure and improves the rheological performance of yogurt. Food Hydrocoll. 2018, 81, 474–480. [Google Scholar] [CrossRef]
- Körzendörfer, A.; Hinrichs, J. Manufacture of high-protein yogurt without generating acid whey—Impact of the final pH and the application of power ultrasound on texture properties. Int. Dairy J. 2019, 99, 104541. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Xiao, H.; Zhou, Z.; Han, Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 87, 187–195. [Google Scholar] [CrossRef]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Characterization and Anti-Inflammatory Potential of an Exopolysaccharide from Submerged Mycelial Culture of Schizophyllum commune. Front. Pharmacol. 2017, 8, 252. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT—Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Vedyashkina, T.A.; Revin, V.V.; Gogotov, I.N. Optimizing the Conditions of Dextran Synthesis by the Bacterium Leuconostoc mesenteroides Grown in a Molasses-Containing Medium. Appl. Biochem. Microbiol. 2005, 41, 361–364. [Google Scholar] [CrossRef]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, J.; Du, R.; Guo, S.; Ping, W.; Ling, H.; Ge, J. Purification and characterization of an exopolysaccharide from Leuconostoc lactis L2. Int. J. Biol. Macromol. 2019, 139, 1224–1231. [Google Scholar] [CrossRef]
- Zehir Senturk, D.; Dertli, E.; Erten, H.; Simsek, O. Structural and technological characterization of ropy exopolysaccharides produced by Lactobacillus plantarum strains isolated from Tarhana. Food Sci. Biotechnol. 2020, 29, 121–129. [Google Scholar] [CrossRef]
- Wangpaiboon, K.; Waiyaseesang, N.; Panpetch, P.; Charoenwongpaiboon, T.; Nepogodiev, S.A.; Ekgasit, S.; Field, R.A.; Pichayangkura, R. Characterisation of insoluble α-1,3-/α-1,6 mixed linkage glucan produced in addition to soluble α-1,6-linked dextran by glucansucrase (DEX-N) from Leuconostoc citreum ABK-1. Int. J. Biol. Macromol. 2020, 152, 473–482. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohydr. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Wang, S.; Zhao, F.; Fang, F.; Guo, J.; Long, M.; Shen, Y. Differences in exopolysaccharides of three microbial aggregates. Environ. Technol. 2021, 1–13. [Google Scholar] [CrossRef]
- Netsopa, S.; Niamsanit, S.; Sakloetsakun, D.; Milintawisamai, N. Characterization and Rheological Behavior of Dextran from Weissella confusa R003. Int. J. Polym. Sci. 2018, 2018, 5790526. [Google Scholar] [CrossRef] [Green Version]
- Tilwani, Y.M.; Lakra, A.K.; Domdi, L.; Yadav, S.; Jha, N.; Arul, V. Optimization and physicochemical characterization of low molecular levan from Enterococcus faecium MC-5 having potential biological activities. Process Biochem. 2021, 110, 282–291. [Google Scholar] [CrossRef]
- Wang, Y.; Du, R.; Qiao, X.; Zhao, B.; Zhou, Z.; Han, Y. Optimization and characterization of exopolysaccharides with a highly branched structure extracted from Leuconostoc citreum B-2. Int. J. Biol. Macromol. 2020, 142, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.A.; Asgher, M.; Bilal, M. Sustainable Production, Optimization, and Partial Characterization of Exopolysaccharides by Macrococcus brunensis. Waste Biomass Valorization 2021, 12, 6847–6859. [Google Scholar] [CrossRef]
- Sathishkumar, R.; Thirumalaikumar, E.; Rajeswari, M.V.; Arun, J.; Vimal, S.; Babu, M.M.; Citarasu, T. Extraction, statistical optimization, and immunomodulatory activity of exopolysaccharide from seaweed-associated Bacillus megaterium DSKPDF CMST3. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Karadayi, Y.I.; Aykutoglu, G.; Arslan, N.P.; Baltaci, M.O.; Adiguzel, A.; Taskin, M. Production of water-soluble sulfated exopolysaccharide with anticancer activity from Anoxybacillus gonensis YK25. J. Chem. Technol. Biotechnol. 2020, 96, 1258–1266. [Google Scholar] [CrossRef]
- Razack, S.A.; Velayutham, V.; Thangavelu, V. Medium optimization and in vitro antioxidant activity of exopolysaccharide produced by Bacillus subtilis. Korean J. Chem. Eng. 2013, 31, 296–303. [Google Scholar] [CrossRef]
- Srikanth, S.; Swathi, M.; Tejaswini, M.; Sharmila, G.; Muthukumaran, C.; Jaganathan, M.K.; Tamilarasan, K. Statistical optimization of molasses based exopolysaccharide and biomass production by Aureobasidium pullulans MTCC 2195. Biocatal. Agric. Biotechnol. 2014, 3, 7–12. [Google Scholar] [CrossRef]
- Hassan, S.W.; Ibrahim, H.A. Production, Characterization and Valuable Applications of Exopolysaccharides from Marine Bacillus subtilis SH1. Pol. J. Microbiol. 2017, 66, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Song, Q.; Zhao, F.; Han, Y.; Zhou, Z. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef]
- Ai, H.; Liu, M.; Yu, P.; Zhang, S.; Suo, Y.; Luo, P.; Li, S.; Wang, J. Improved welan gum production by Alcaligenes sp. ATCC31555 from pretreated cane molasses. Carbohydr. Polym. 2015, 129, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Abarquero, D.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Study of exopolysaccharides from lactic acid bacteria and their industrial applications: A review. Int. J. Food Sci. Technol. 2021, 57, 16–26. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Huang, T.; Guo, S.; Wang, Y.; Wang, J.; Kwok, L.-Y.; Dan, T.; Zhang, H.; Bilige, M. Probiotic Lactobacillus casei Zhang improved the properties of stirred yogurt. Food Biosci. 2020, 37, 100718. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, R.; Li, J. Effects of the β-glucan, curdlan, on the fermentation performance, microstructure, rheological and textural properties of set yogurt. LWT 2020, 128, 109449. [Google Scholar] [CrossRef]







| Run | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | M-EPS Yield (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 45.50 ± 0.08 |
| 2 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 23.38 ± 0.55 |
| 3 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 42.24 ± 0.44 |
| 4 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 22.81 ± 0.17 |
| 5 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 40.98 ± 0.46 |
| 6 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 23.76 ± 0.43 |
| 7 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 10.43 ± 0.49 |
| 8 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 40.19 ± 0.06 |
| 9 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 19.26 ± 0.17 |
| 10 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 36.24 ± 0.35 |
| 11 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 43.31 ± 0.24 |
| 12 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 18.26 ± 0.57 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37.21 ± 0.45 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37.69 ± 0.38 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37.02 ± 0.19 |
| Run | X1 | X4 | X8 | M-EPS Yield (g/L) |
|---|---|---|---|---|
| Origin | 200 | 12 | 9 | 36.24 ± 0.31 |
| 1 | 225 | 11.5 | 8.8 | 39.81 ± 0.39 |
| 2 | 250 | 11 | 8.6 | 40.98 ± 0.33 |
| 3 | 275 | 10.5 | 8.4 | 44.00 ± 0.16 |
| 4 | 300 | 10 | 8.2 | 41.67 ± 0.19 |
| 5 | 325 | 9.5 | 8 | 26.79 ± 0.07 |
| Trial No. | X1 | X4 | X8 | M-EPS Yield (g/L) | |
|---|---|---|---|---|---|
| Estimated Value | Predicted Value | ||||
| 1 | −1 | −1 | −1 | 36.64 ± 0.19 | 35.08 |
| 2 | 1 | −1 | −1 | 44.00 ± 0.24 | 43.30 |
| 3 | −1 | 1 | −1 | 35.55 ± 0.13 | 34.37 |
| 4 | 1 | 1 | −1 | 43.50 ± 0.32 | 42.65 |
| 5 | −1 | −1 | 1 | 40.31 ± 0.48 | 39.81 |
| 6 | 1 | −1 | 1 | 45.79 ± 0.35 | 45.60 |
| 7 | −1 | 1 | 1 | 39.40 ± 0.55 | 38.74 |
| 8 | 1 | 1 | 1 | 44.38 ± 0.17 | 44.59 |
| 9 | −1.682 | 0 | 0 | 32.24 ± 0.38 | 33.90 |
| 10 | 1.682 | 0 | 0 | 45.48 ± 0.21 | 45.73 |
| 11 | 0 | −1.682 | 0 | 43.26 ± 0.06 | 44.36 |
| 12 | 0 | 1.682 | 0 | 42.10 ± 0.26 | 42.92 |
| 13 | 0 | 0 | −1.682 | 34.33 ± 0.13 | 36.23 |
| 14 | 0 | 0 | 1.682 | 41.81 ± 0.17 | 41.84 |
| 15 | 0 | 0 | 0 | 45.98 ± 0.14 | 45.99 |
| 16 | 0 | 0 | 0 | 46.57 ± 0.40 | 45.99 |
| 17 | 0 | 0 | 0 | 45.50 ± 0.12 | 45.99 |
| 18 | 0 | 0 | 0 | 46.33 ± 0.20 | 45.99 |
| 19 | 0 | 0 | 0 | 47.02 ± 0.17 | 45.99 |
| 20 | 0 | 0 | 0 | 44.88 ± 0.16 | 45.99 |
| Factor | Coefficient Estimate | F-Value | p > |F| |
|---|---|---|---|
| Intercept | 30.53 | 47.54 | 0.0045 * |
| X1 | 10.88 | 340.78 | 0.0003 * |
| X2 | −0.33 | 0.3086 | 0.6173 |
| X3 | −1.62 | 7.6 | 0.0703 |
| X4 | −2.05 | 12.14 | 0.0399 * |
| X5 | 0.27 | 0.2128 | 0.676 |
| X6 | 0.18 | 0.098 | 0.7747 |
| X7 | 1.28 | 4.75 | 0.1175 |
| X8 | −2.24 | 14.45 | 0.032 * |
| R2 = 0.9922, Adj R2 = 0.9713, Adeq Precision 18.6162 | |||
| Factor | Coefficient Estimate | F-Value | p > |F| |
|---|---|---|---|
| Intercept | 45.99 | 23.15 | <0.0001 ** |
| X1 | 3.52 | 98.62 | <0.0001 ** |
| X4 | −0.43 | 1.47 | 0.2529 |
| X8 | 1.67 | 22.16 | 0.0008 ** |
| X1X4 | 0.01 | 0.0007 | 0.98 |
| X1X8 | −0.61 | 1.72 | 0.2187 |
| X4X8 | −0.09 | 0.0372 | 0.8508 |
| X12 | −2.18 | 40.11 | <0.0001 ** |
| X42 | −0.83 | 5.82 | 0.0365 * |
| X82 | −2.46 | 50.96 | <0.0001 ** |
| Lack of fit | 4.76 | 0.0561 | |
| R2 = 0.9542, Adj R2 = 0.9130, Adeq Precision 13.0643 | |||
| Addition Dose (%) | WHC (%) | pH |
|---|---|---|
| +0% M-EPS | 72.23 ± 0.01 b | 4.25 ± 0.01 A |
| +0.1% M-EPS | 72.80 ± 0.00 b | 4.23 ± 0.00 AB |
| +0.5% M-EPS | 77.00 ± 0.01 a | 4.20 ± 0.02 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Xu, M.; Pan, L.; Zhou, Z.; Han, Y. Structural Characterization of Exopolysaccharide Produced by Leuconostoccitreum B-2 Cultured in Molasses Medium and Its Application in Set Yogurt. Processes 2022, 10, 891. https://doi.org/10.3390/pr10050891
Liang L, Xu M, Pan L, Zhou Z, Han Y. Structural Characterization of Exopolysaccharide Produced by Leuconostoccitreum B-2 Cultured in Molasses Medium and Its Application in Set Yogurt. Processes. 2022; 10(5):891. https://doi.org/10.3390/pr10050891
Chicago/Turabian StyleLiang, Lu, Min Xu, Lei Pan, Zhijiang Zhou, and Ye Han. 2022. "Structural Characterization of Exopolysaccharide Produced by Leuconostoccitreum B-2 Cultured in Molasses Medium and Its Application in Set Yogurt" Processes 10, no. 5: 891. https://doi.org/10.3390/pr10050891
APA StyleLiang, L., Xu, M., Pan, L., Zhou, Z., & Han, Y. (2022). Structural Characterization of Exopolysaccharide Produced by Leuconostoccitreum B-2 Cultured in Molasses Medium and Its Application in Set Yogurt. Processes, 10(5), 891. https://doi.org/10.3390/pr10050891






