Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
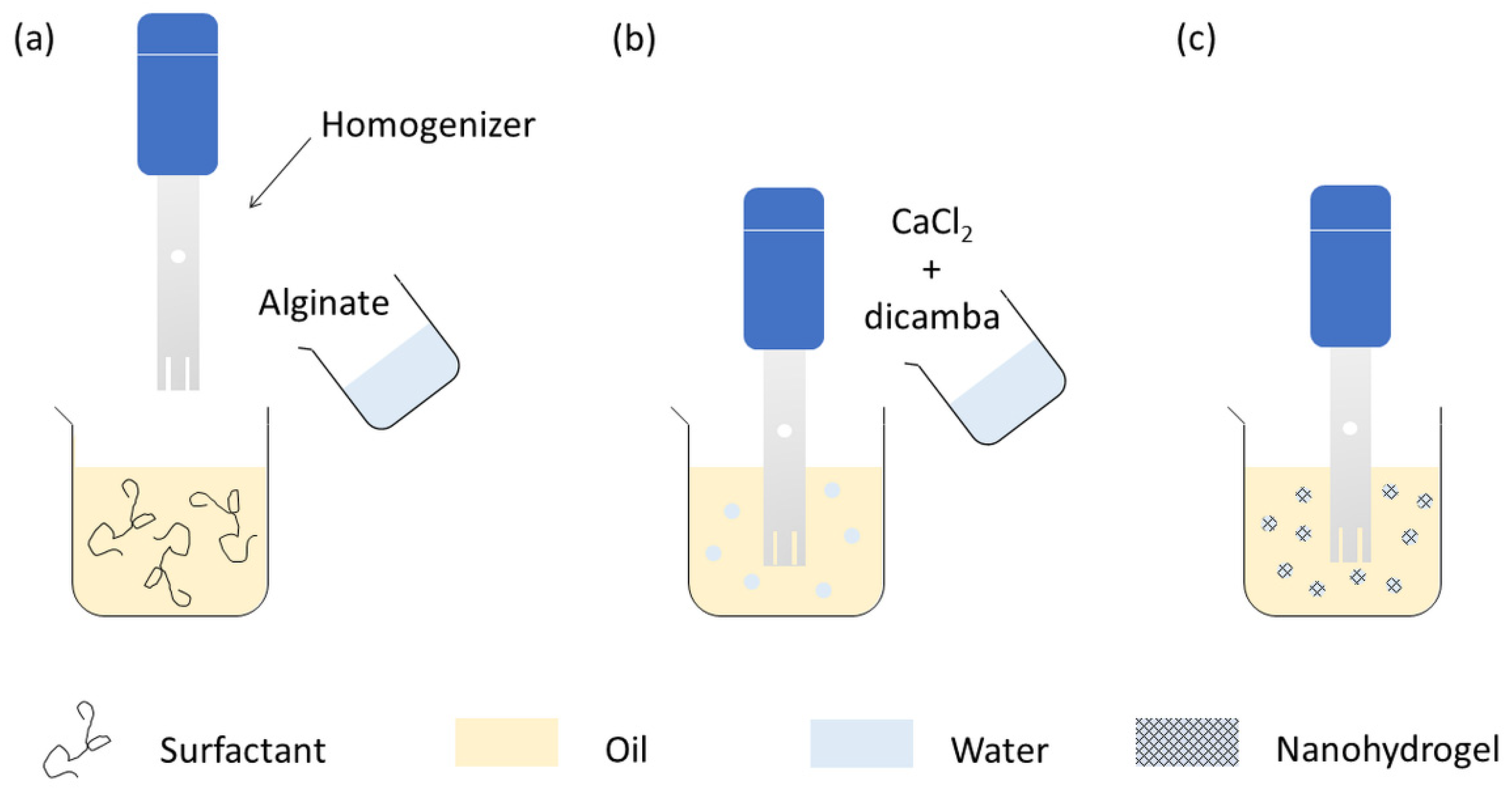
2.2. Synthesis of Unloaded and Loaded Carriers
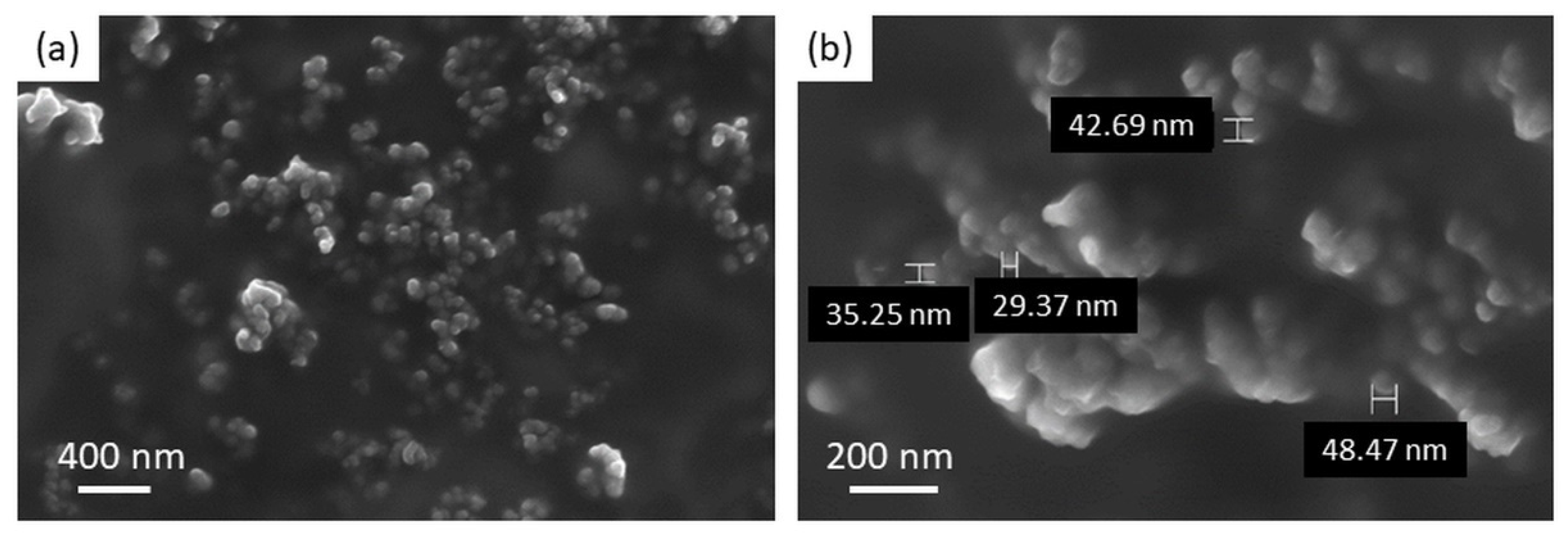
2.3. Dimensional and Morphological Characterization
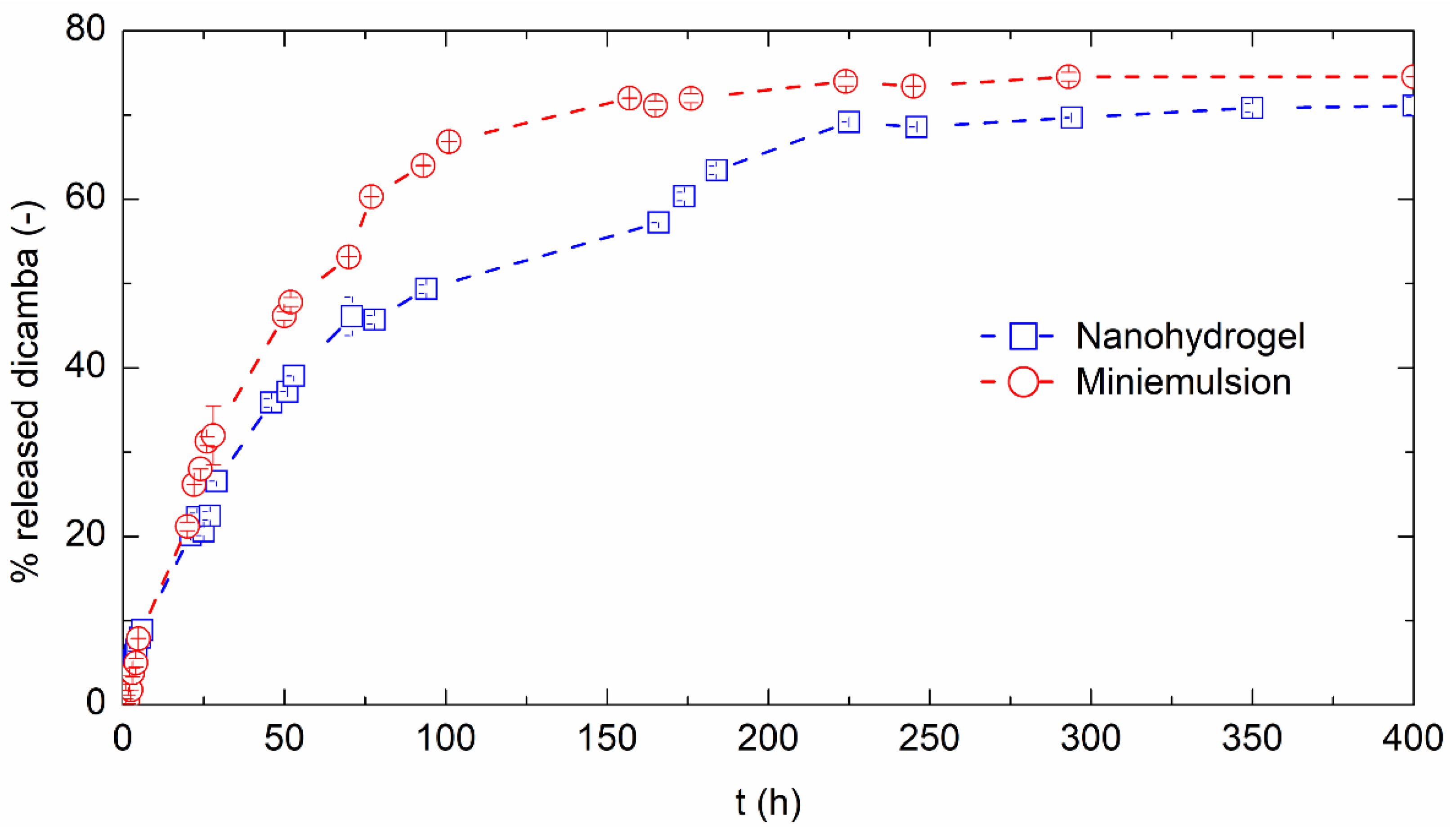
2.4. Release Tests
3. Results and Discussion
3.1. Design of Miniemulsion Formulation and Operative Conditions
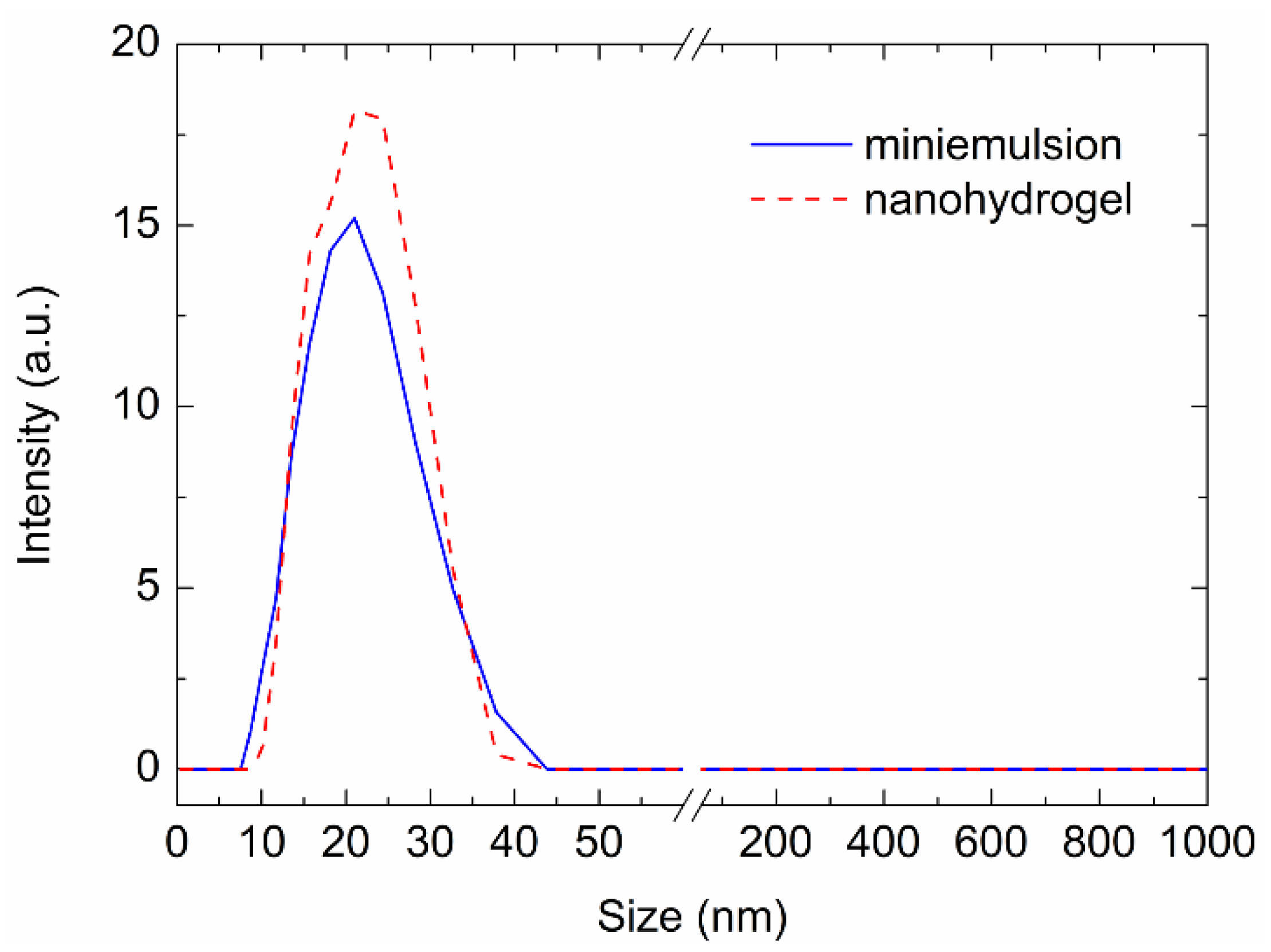
3.2. Nanohydrogel Synthesis
3.3. Loading of the Nanohydrogels with Dicamba
3.4. Controlled Release of Dicamba
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alavanja, M.C.R. Introduction: Pesticides use and exposure extensive worldwide. Rev. Environ. Health 2009, 24, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R. Emerging investigator series: Nanotechnology to develop novel agrochemicals: Critical issues to consider in the global agricultural context. Environ. Sci. Nano 2020, 7, 1867–1873. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects. 2019. Available online: https://population.un.org/wpp/Publications/ (accessed on 28 July 2021).
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Small amounts of pesticides reaching target insects. Environ. Dev. Sustain. 2012, 14, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating Pesticide Degradation in the Environment: Blind Spots and Emerging Opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Krieger, R.I. Preface. In Handbook of Pesticide Toxicology, 2nd ed.; Krieger, R.I., Krieger, W.C., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. xxv–xxvi. [Google Scholar]
- Kah, M.; Brown, C.D. Prediction of the adsorption of ionizable pesticides in soils. J. Agric. Food Chem. 2007, 55, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.; Kah, M.; Brown, C.D. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag. Sci. 2008, 64, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaliene, O.; Papiernik, S.K.; Koskinen, W.C.; Spokas, K.A. Sorption and predicted mobility of herbicides in Baltic soils. J. Environ. Sci. Health B 2007, 42, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.W.; Hall, J.C.; Parkin, G.W.; Wagner-Riddle, C.; Clegg, B.S. Seasonal leaching and biodegradation of dicamba in turfgrass. J. Environ. Qual. 2001, 30, 1360–1370. [Google Scholar] [CrossRef]
- Spadotto, C.A.; Hornsby, A.G.; Gomes, M.A. Sorption and leaching potential of acidic herbicides in Brazilian soils. J. Environ. Sci. Health B 2005, 40, 29–37. [Google Scholar] [CrossRef]
- Tindall, J.A.; Vencill, W.K. Transport of Atrazine, 2,4-D, and Dicamba through Preferential Flowpaths in an Unsaturated Claypan Soil near Centralia, Missouri. J. Hydrol. 1995, 166, 37–59. [Google Scholar] [CrossRef]
- Riter, L.S.; Sall, E.D.; Pai, N.; Beachum, C.E.; Orr, T.B. Quantifying Dicamba Volatility under Field Conditions: Part I, Methodology. J. Agric. Food Chem. 2020, 68, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Bish, M.D.; Farrell, S.T.; Lerch, R.N.; Bradley, K.W. Dicamba Losses to Air after Applications to Soybean under Stable and Nonstable Atmospheric Conditions. J. Environ. Qual. 2019, 48, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Gavlick, W.; Wright, D.; MacInnes, A.; Hemminghaus, J.; Webb, J.; Yermolenka, V.; Su, W. A Method to Determine the Relative Volatility of Auxin Herbicide Formulations. In Pesticide Formulation and Delivery Systems: 35th Volume, Pesticide Formulations, Adjuvants, and Spray Characterization in 2014; Goss, G., Ed.; ASTM International: West Conshohocken, PA, USA, 2016; pp. 24–32. [Google Scholar]
- Sciumbato, A.S.; Chandler, J.M.; Senseman, S.A.; Bovey, R.W.; Smith, K.L. Determining exposure to auxin-like herbicides. II. Practical application to quantify volatility. Weed Technol. 2004, 18, 1135–1142. [Google Scholar] [CrossRef]
- Mueller, T.C. Methods To Measure Herbicide Volatility. Weed Sci. 2015, 63, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Moulick, R.G.; Das, S.; Debnath, N.; Bandyopadhyay, K. Potential use of nanotechnology in sustainable and ‘smart’ agriculture: Advancements made in the last decade. Plant Biotechnol. Rep. 2020, 14, 505–513. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Vurro, M.; Miguel-Rojas, C.; Perez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Kundu, R.; Mukherjee, A. Nanocomposites for Delivering Agrochemicals: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 3691–3702. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Strachan, S.D.; Casini, M.S.; Heldreth, K.M.; Scocas, J.A.; Nissen, S.J.; Bukun, B.; Lindenmayer, R.B.; Shaner, D.L.; Westra, P.; Brunk, G. Vapor Movement of Synthetic Auxin Herbicides: Aminocyclopyrachlor, Aminocyclopyrachlor-Methyl Ester, Dicamba, and Aminopyralid. Weed Sci. 2010, 58, 103–108. [Google Scholar] [CrossRef]
- Marei, A.S.; Soltan, H.R.; Mousa, A.; Khamis, A. Leaching and mobility of carbofuran from granular and alginate controlled release formulations. J. Agric. Sci. 2000, 134, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Pepperman, A.B. Soil Column Mobility of Metribuzin from Alginate-Encapsulated Controlled Release Formulations. J. Agric. Food Chem. 1995, 43, 241–246. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Artusio, F.; Ferri, A.; Gigante, V.; Massella, D.; Mazzarino, I.; Sangermano, M.; Barresi, A.; Pisano, R. Synthesis of high payload nanohydrogels for the ecapsulation of hydrophilic molecules via inverse miniemulsion polymerization: Caffeine as a case study. Drug Dev. Ind. Pharm. 2019, 45, 1862–1870. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef] [Green Version]
- Innocente, N.; Biasutti, M.; Venir, E.; Spaziani, M.; Marchesini, G. Effect of high-pressure homogenization on droplet size distribution and rheological properties of ice cream mixes. J. Dairy Sci. 2009, 92, 1864–1875. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef] [Green Version]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.H.; Ziener, U. Synthesis of nanostructured materials in inverse miniemulsions and their applications. Nanoscale 2013, 5, 10093–10107. [Google Scholar] [CrossRef] [PubMed]
- Landfester, K.; Willert, M.; Antonietti, M. Preparation of polymer particles in nonaqueous direct and inverse miniemulsions. Macromolecules 2000, 33, 2370–2376. [Google Scholar] [CrossRef]
- Capek, I. On inverse miniemulsion polymerization of conventional water-soluble monomers. Adv. Colloid Interface Sci. 2010, 156, 35–61. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Artusio, F.; Bazzano, M.; Pisano, R.; Coulon, P.-E.; Rizza, G.; Schiller, T.; Sangermano, M. Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer 2018, 139, 155–162. [Google Scholar] [CrossRef]
- Schork, F.J.; Luo, Y.; Smulders, W.; Russum, J.P.; Butté, A.; Fontenot, K. Miniemulsion Polymerization. In Polymer Particles; Okubo, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 129–255. [Google Scholar]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Faucheu, J.; Gauthier, C.; Chazeau, L.; Cavaillé, J.-Y.; Mellon, V.; Lami, E.B. Miniemulsion polymerization for synthesis of structured clay/polymer nanocomposites: Short review and recent advances. Polymer 2010, 51, 6–17. [Google Scholar] [CrossRef]
- Weiss, C.K.; Landfester, K. Miniemulsion Polymerization as a Means to Encapsulate Organic and Inorganic Materials. In Hybrid Latex Particles: Preparation with (Mini)emulsion Polymerization; van Herk, A.M., Landfester, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 185–236. [Google Scholar]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Nörnberg, A.B.; Gehrke, V.R.; Mota, H.P.; Camargo, E.R.; Fajardo, A.R. Alginate-cellulose biopolymeric beads as efficient vehicles for encapsulation and slow-release of herbicide. Colloids Surf. Physicochem. Eng. Aspects 2019, 583, 123970. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R.; Anil, P.R.; Raj, D.K. Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde–gelatin nanogels. Mater. Sci. Eng. C 2016, 68, 251–257. [Google Scholar] [CrossRef]
- Artusio, F.; Castellví, A.; Sacristán, A.; Pisano, R.; Gavira, J.A. Agarose Gel as a Medium for Growing and Tailoring Protein Crystals. Cryst. Growth Des. 2020, 20, 5564–5571. [Google Scholar] [CrossRef]
- Artusio, F.; Castellví, A.; Pisano, R.; Gavira, J.A. Tuning Transport Phenomena in Agarose Gels for the Control of Protein Nucleation Density and Crystal Form. Crystals 2021, 11, 466. [Google Scholar] [CrossRef]
- Dalmoro, A.; Barba, A.A.; Lamberti, G.; Grassi, M.; d’Amore, M. Pharmaceutical applications of biocompatible polymer blends containing sodium alginate. Adv. Polym. Tech. 2012, 31, 219–230. [Google Scholar] [CrossRef]
- Nuyttens, D.; Baetens, K.; De Schampheleire, M.; Sonck, B. Effect of nozzle type, size and pressure on spray droplet characteristics. Biosys. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- Antuniassi, U.; Mota, A.; Chechetto, R.; Carvalho, F.; Ovejero, R.; Barbosa, H.; Morris, M.; Araujo, V. Droplet Spectrum Generated by Air Induction Nozzles Spraying Solutions Containing Adjuvants and a Tank Mixture of Glyphosate and Dicamba. In Pesticide Formulation and Delivery Systems: 40th Volume, Formulation, Application and Adjuvant Innovation; Elsik, C., Ed.; ASTM International: West Conshohocken, PA, USA, 2020; p. 36. [Google Scholar]
- Putorti, A. Simultaneous Measurements of Drop Size and Velocity in Large-Scale Sprinkler Flows Using Particle Tracking and Laser-Induced Fluorescence; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2004. Available online: https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=101399 (accessed on 28 July 2021).
- Maldonado-Reina, A.J.; López-Ruiz, R.; Garrido Frenich, A.; Arrebola, F.J.; Romero-González, R. Co-formulants in plant protection products: An analytical approach to their determination by gas chromatography–high resolution mass accuracy spectrometry. Talanta 2021, 234, 122641. [Google Scholar] [CrossRef]
- ElShafei, G.M.S.; El-Said, M.M.; Attia, H.A.E.; Mohammed, T.G.M. Environmentally friendly pesticides: Essential oil-based w/o/w multiple emulsions for anti-fungal formulations. Ind. Crop. Prod. 2010, 31, 99–106. [Google Scholar] [CrossRef]
- Brito, E.S.; de Paula, A.R.; Vieira, L.P.; Dolinski, C.; Samuels, R.I. Combining vegetable oil and sub-lethal concentrations of Imidacloprid with Beauveria bassiana and Metarhizium anisopliae against adult guava weevil Conotrachelus psidii (Coleoptera: Curculionidae). Biocontrol Sci. Technol. 2008, 18, 665–673. [Google Scholar] [CrossRef]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C.; James, T.K. A comparison of dicamba absorption, translocation and metabolism in Chenopodium album populations resistant and susceptible to dicamba. Crop Protect. 2018, 110, 112–116. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, K. Nanoparticle-Based Plant Disease Management: Tools for Sustainable Agriculture. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 29–61. [Google Scholar]

| Sample Code | Pluronic, g | UT Speed, rpm | Alginate, wt % | Dicamba, mg/mL |
|---|---|---|---|---|
| M1 | 0.25 | 14,000 | - | - |
| M2 | 0.30 | 14,000 | - | - |
| M3 | 0.35 | 14,000 | - | - |
| M4 | 0.40 | 14,000 | - | - |
| M5 | 0.30 | 8000 | - | - |
| M6 | 0.30 | 10,000 | - | - |
| H1 | 0.30 | 8000 | 1 | - |
| H2 | 0.30 | 10,000 | 1 | - |
| H3 | 0.30 | 14,000 | 1 | - |
| H4 | 0.30 | 10,000 | 2 | - |
| HD1 | 0.30 | 10,000 | 1 | 1 |
| HD2 | 0.30 | 10,000 | 1 | 3 |
| HD3 | 0.30 | 10,000 | 1 | 30 (+NaOH) |
| HD4 | 0.30 | 10,000 | 1 | 125 (+NaOH) |
| Sample Code | Pluronic, g | UT Speed, rpm | Z-Average, nm | Std Dev, nm | PDI, - |
|---|---|---|---|---|---|
| M1 | 0.25 | 14,000 | 52.2 | 8.0 | 0.19 |
| M2 | 0.30 | 14,000 | 26.7 | 6.2 | 0.23 |
| M3 | 0.35 | 14,000 | 73.6 | 19.8 | 0.46 |
| M4 | 0.40 | 14,000 | 82.7 | 13.6 | 0.58 |
| M5 | 0.30 | 8000 | 131.6 | 77.1 | 0.92 |
| M6 | 0.30 | 10,000 | 21.4 | 3.0 | 0.29 |
| Sample Code | UT Speed, rpm | Alginate, wt % | Z-Average, nm | Std Dev, nm | PDI, - |
|---|---|---|---|---|---|
| H1 | 8000 | 1 | 112.3 | 90.5 | 0.75 |
| H2 | 10,000 | 1 | 20.6 | 4.4 | 0.14 |
| H3 | 14,000 | 1 | 19.3 | 0.3 | 0.35 |
| H4 | 10,000 | 2 | 35.2 | 13.5 | 0.36 |
| Sample Code | Dicamba, mg/mL | Z-Average, nm | Std Dev, nm | PDI, - |
|---|---|---|---|---|
| HD1 | 1 | 15.0 | 1.9 | 0.03 |
| HD2 | 3 | 21.3 | 3.2 | 0.07 |
| HD3 | 30 (+NaOH) | 23.3 | 0.23 | 0.23 |
| HD4 | 125 (+NaOH) | 27.8 | 4.6 | 0.17 |
| Kinetic Release Model | Model Equation | R2 |
|---|---|---|
| Zero Order | F = ko t | 0.78 |
| First Order | ln(1−F) = − k1 t | 0.90 |
| Higuchi | F = kH t1/2 | 0.98 |
| Hixson–Crowell | 1−(1−F)1/3 = kHC t | 0.87 |
| Baker–Lonsdale | 3/2[1−(1−F)2/3]–F = kBL t | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes 2021, 9, 1641. https://doi.org/10.3390/pr9091641
Artusio F, Casà D, Granetto M, Tosco T, Pisano R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes. 2021; 9(9):1641. https://doi.org/10.3390/pr9091641
Chicago/Turabian StyleArtusio, Fiora, Dario Casà, Monica Granetto, Tiziana Tosco, and Roberto Pisano. 2021. "Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide" Processes 9, no. 9: 1641. https://doi.org/10.3390/pr9091641
APA StyleArtusio, F., Casà, D., Granetto, M., Tosco, T., & Pisano, R. (2021). Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes, 9(9), 1641. https://doi.org/10.3390/pr9091641









