
Journal Menu
► ▼ Journal Menu-
- Viruses Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserNeed Help?
Announcements
11 September 2024
Behind the Paper: “Cellular N-Myristoyl Transferases Are Required for Mammarenavirus Multiplication”—a New Weapon against Human Pathogenic Mammarenaviruses: Targeting Host Cell N-myristoyltransferases
Authors: Haydar Witwit, Carlos Alberto Betancourt, Beatrice Cubitt, Roaa Khafaji, Heinrich Kowalski, Nathaniel Jackson, Chengjin Ye, Luis Martinez-Sobrido and Juan C. de la Torre
The Challenge
Several mammarenaviruses, chiefly Lassa virus (LASV) in Western Africa and Junin (JUNV) in the Argentine Pampas cause hemorrhagic fever (HF) diseases associated with high morbidity and mortality, posing important public health problems in their endemic regions. In addition, mounting evidence indicates that the worldwide-distributed mammarenavirus lymphocytic choriomeningitis virus (LCMV) is a neglected human pathogen of clinical significance in paediatric and transplantation medicine [1–3]. Current anti-mammarenavirus therapy is limited to an off-label use of ribavirin for which efficacy remains controversial [4]. Hence, the importance of developing novel therapeutics to combat human pathogenic mammarenaviruses.
Our Discovery: A New Way to Fight Mammarenavirus Infections
Instead of targeting directly the activity of a viral protein, we focused on targeting N-myristoylation directed by the host cell N-myristoyltransferases (NMT1 and NMT2), a process that the virus relies on to complete both its cell entry and budding exiting processes [5,6]. We found that treatment with specific NMT inhibitors, DDD85646 or IMP-1088, effectively stop propagation of LCMV, as well as the HF causing mammarenaviruses LASV and JUNV [7].
Why This Matters
Our findings support the use of NMT inhibitors as a novel antiviral strategy to combat LASV and other human pathogenic mammarenaviruses. Targeting a host cell process can pose a much higher genetic barrier for the selection of drug-resistant variants, a common problem with direct antiviral drugs. NMT inhibitors can be also incorporated into combination therapy protocols.
A Deep Dive into NMT Inhibition in Mammarenavirus Life Cycle
Mammarenaviruses are enveloped viruses with a bi-segmented negative-stranded (NS) RNA genome [8]. Each genome RNA segment, L (ca 7.3 kb) and S (ca 3.5 kb), uses an ambisense coding strategy to direct the synthesis of two polypeptides in opposite orientation, separated by a non-coding intergenic region (IGR). The L genome segment encodes the viral RNA dependent RNA polymerase (L), and Z matrix protein, whereas the S genome segment encodes the viral glycoprotein precursor (GPC) and the viral nucleoprotein (NP). GPC is co-and post-translationally processed to produce a stable signal peptide (SSP), and the mature GP1 and GP2 subunits that together with the SSP form the spikes that decorate the virus surface and mediate cell entry via receptor-mediated endocytosis [9–11]. GP1 mediates binding to the cellular receptor and GP2 the pH-dependent fusion event in the late endosome required for release of the virus ribonucleoprotein (vRNP) complexes into the cell cytoplasm where they direct the replication and transcription of the viral genome [12,13].
Early studies have shown that N-myristoylation is required for the role of the mammarenavirus matrix Z protein in assembly and budding [5,6], and for the role of the SSP in the GP2-mediate fusion event [12]. These findings were based on the use of 2-hydroxy-myristic acid (2-HMA) and 2-HMA analogs as inhibitors of N-myristoyltransferases (NMT1 and NMT2) responsible for catalyzing N-myristoylation in mammalian cells [5,14]. However, recent studies have demonstrated that 2-HMA acts off-target and does not inhibit N-myristoylation in a concentration range consistent with activity on NMT [15]. Therefore, we have revisited the contribution of N-myristoylation in mammarenavirus infection using the validated on-target specific NMT1/2 inhibitor DDD85646 [16]. We found that DDD85646 exhibits a very potent antiviral activity against LCMV in cultured cells. Cell-based assays probing different steps of the LCMV life cycle revealed that DDD85646 exerted its anti-LCMV activity by interfering with Z budding activity and GP2 mediated fusion between viral and cellular membranes, a process that requires the participation of myristoylated SSP [7] (Fig. 1).
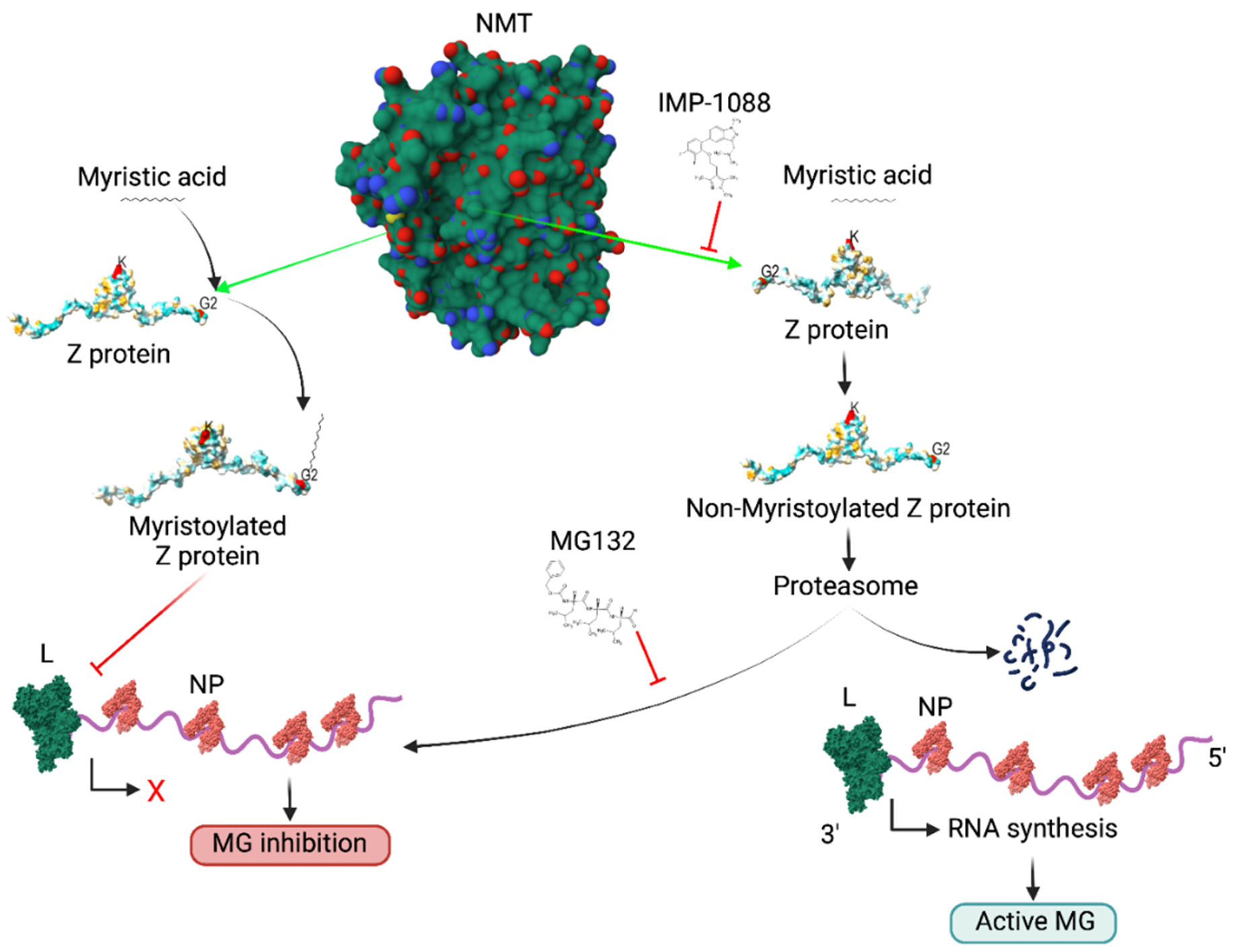
Figure 1: Proposed model of the effect of the NMT inhibitor DDD85646 on mammarenavirus cell entry and budding
NMT isozymes facilitate the addition of myristic acid to glycine (2) of SSP and Z protein, which protect them from proteasome mediated degradation. Myristoylated SSP interacts with GP2 to facilitate the fusion event in the late endosome required to complete the virus cell entry process, whereas myristoylated Z directs the virus assembly and budding process. Inhibition of SSP and Z myristoylation by DDD85646 results in proteasome mediated degradation of SSP and Z which results in inhibition of virus multiplication. Z myr and SSP myr indicate myristoylated Z and SSP respectively.
During our studies, we observed only a minimal increase in Z protein levels in the presence of an NMT inhibitor, an unexpected finding as inhibition of Z myristoylation would interfere with the Z mediated budding process, thus resulting in accumulation of intracellular Z protein. This unexpected result led us to consider that non-myristoylated Z might be targeted for degradation. We found that treatment with the proteasome inhibitor MG132 rescued Z protein expression in the presence of an NMT inhibitor.
Experimental Validation
The Z proteins have been shown to inhibit in a dose-dependent manner the activity of the mammarenavirus vRNP, responsible for directing replication and transcription of the viral genome, in cell-based minigenome (MG) assays. We therefore predicted that Z inhibitory effect on the MG activity would be diminished in the presence of the pan-NMT inhibitor IMP-1088, and that treatment with MG132 would restore, in the presence of IMP-1088, the Z inhibitory effect on the MG activity (Fig. 2).
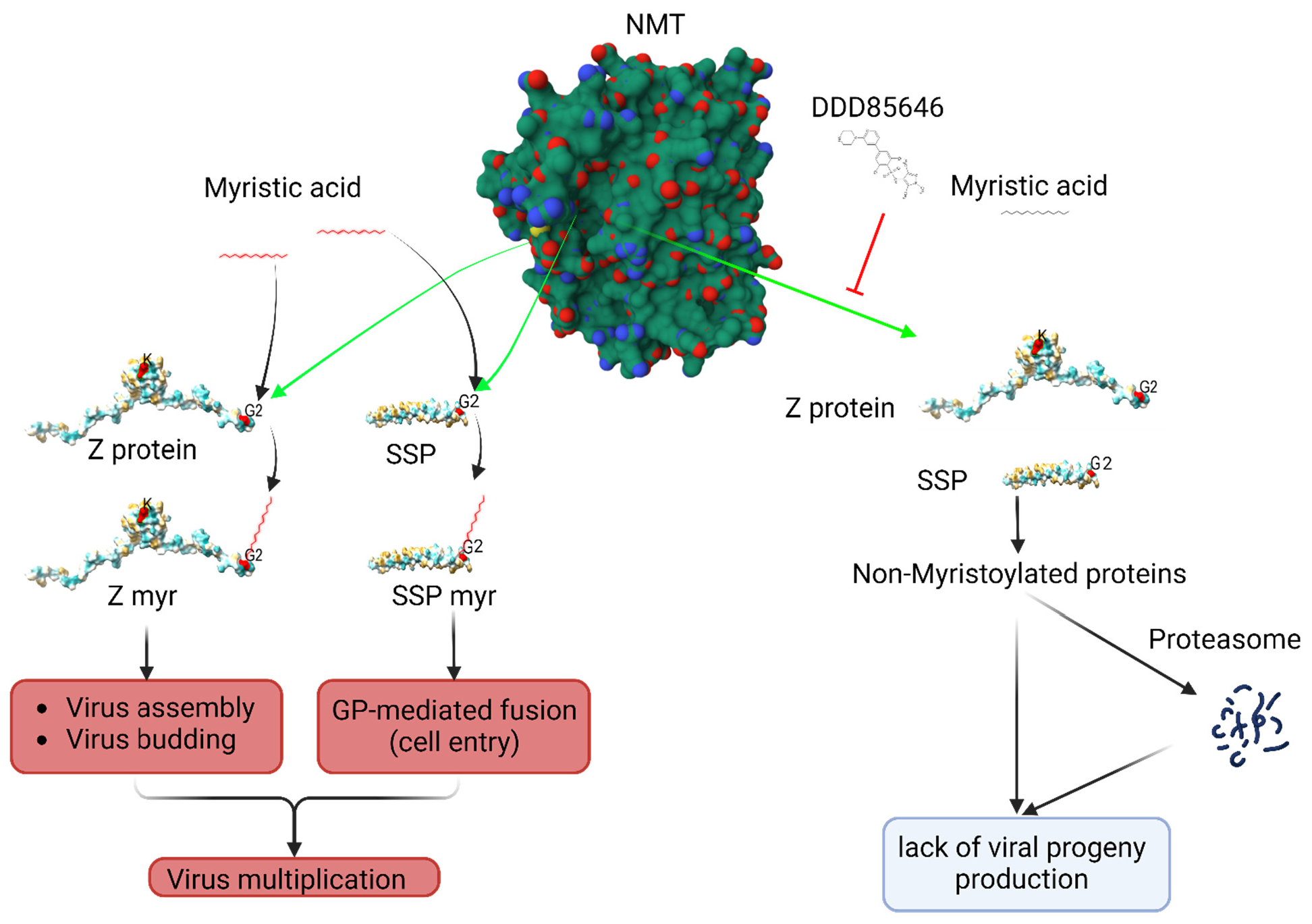
Figure 2: Proposed model of the effect of the NMT (IMP-1088) and proteosome (MG132) inhibitors on the activity of the LCMV MG
NMT isozymes facilitate the addition of myristic acid to glycine (2) of Z protein, which protects the latter from degradation, thus allowing for the Z dose-dependent inhibitory effect on viral RNA synthesis mediated by the vRNP and the consequent inhibition of the activity of the LCMV MG. Inhibition of Z myristoylation by IMP-1088 results in proteasome mediated degradation of Z, which will relief the Z inhibitory effect on the activity of the LCMV MG (right side of the graph). Treatment with the proteasome inhibitor MG132 prevents IMP-1088 induced Z protein degradation, thus resulting in LCMV MG inhibition. L polymerase (green), NP (red) and RNA (purple) are indicated. Red X indicates cessation activity, black angled arrow indicates RNA synthesis.
Treatment with IMP-1088 resulted in the expected reduced levels of Z expression, which correlated with increased levels of MG activity (Fig. 3). Treatment with MG132 restored expression levels of Z to those observed in the absence of IMP-1088, which resulted in the corresponding inhibitory effect on the activity of the LCMV MG (Fig. 3).
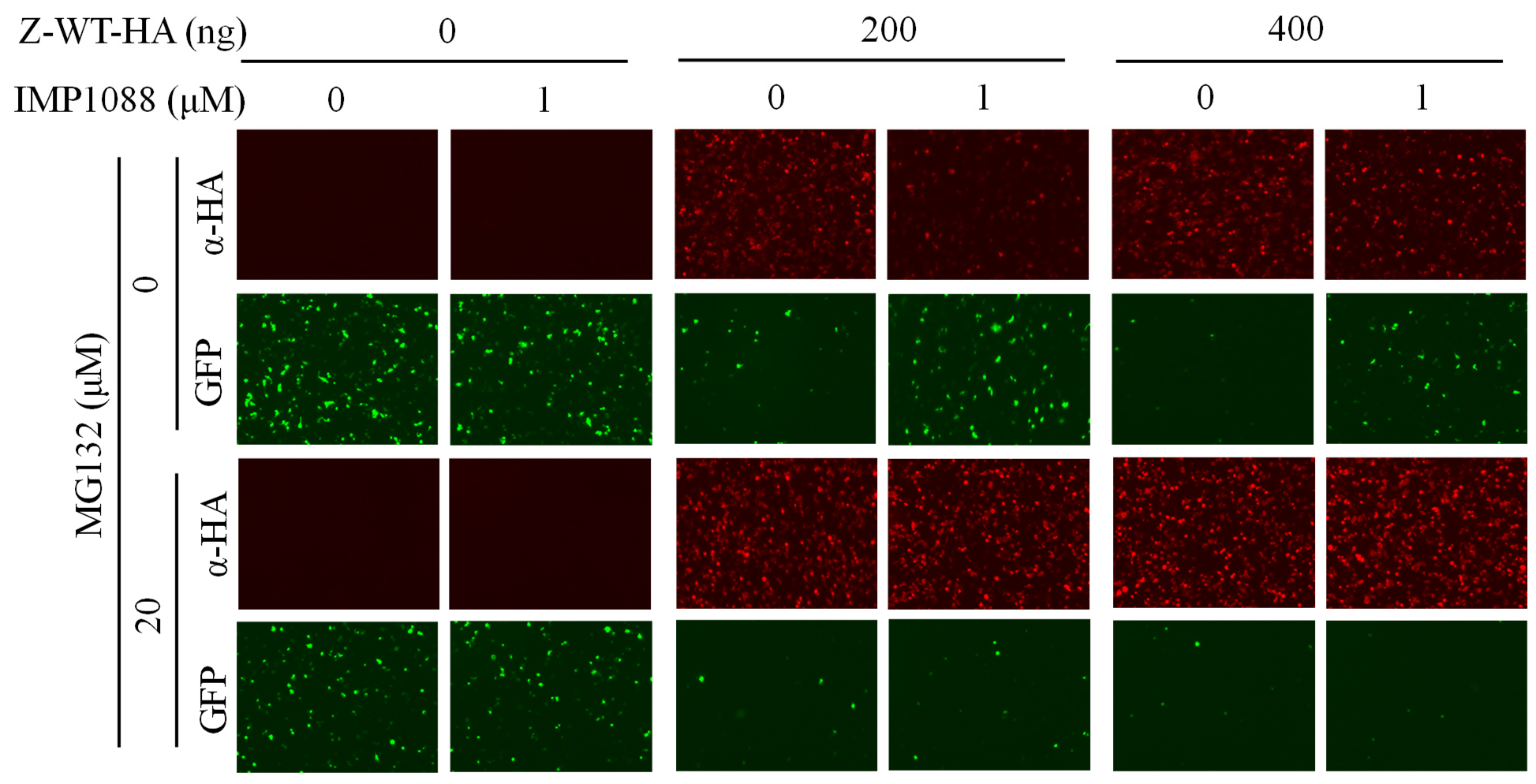
Figure 3: Effect of treatments with the NMT (IMP-1088) and proteasome (MG132) inhibitors on the activity of the LCMV MG
HEK293T cells were seeded at 2x105 per well in ploy-L-lysine treated M12 plate. Next day, cells were transfected with plasmids expressing LCMV L and NP proteins, required for the formation of a functional vRNP, and a plasmid directing intracellular synthesis of an LCMV MG directing expression of GFP (MG-GFP), together with incremental amounts of LCMV-Z tagged with HA (Z-WT-HA). At 18 h post-transfection, cells were treated with the indicated compounds. At 24 h post-treatment, cells were fixed with 4% PFA, and stained with anti-HA, followed by secondary fluorescence antibody to identify cells expressing the Z protein. The activity of the LCMV MG-GFP was assessed based on expression levels of GFP. Images were acquired at 4X magnification (BZ-X710 Keyence).
Future Directions
Our findings open the door to a new class of broad-spectrum anti-mammarenavirus therapy targeting host-cell lipidation processes. NMT inhibitors could be also incorporated into combination therapy strategies with direct acting antivirals, an approach expected to pose a high genetic barrier to the emergence of drug-resistant viruses, and to facilitate drug formulations with reduced toxicity. Notably, the small molecule NMT inhibitor PCLX-001 has been shown to be safe and well tolerated in humans [17,18], supporting the interest of exploring the repurposing of NMT inhibitors to treat infections by human pathogenic mammarenaviruses. NMT inhibitors have been shown to inhibit other viruses with myristoylated proteins, including picornaviruses [16] and vaccinia virus [19]. The use of specific pan-NMT inhibitors as a broad-spectrum antiviral strategy against viruses with myristoylated proteins warrants further investigation.
For more details on the experiments and results, please refer to our paper, which can be found at https://doi.org/10.3390/v16091362.
References
- Yadav, K.; Mathur, G.; Ford, B.; Miller, R.; Group, C.W. A Case Cluster of Lymphocytic Choriomeningitis Virus Transmitted Via Organ Transplantation.: Abstract# D2381. Transplantation 2014, 98, 768.
- MacNeil, A.; Ströher, U.; Farnon, E.; Campbell, S.; Cannon, D.; Paddock, C.D.; Drew, C.P.; Kuehnert, M.; Knust, B.; Gruenenfelder, R.; et al. Solid Organ Transplant–Associated Lymphocytic Choriomeningitis, United States, 2011. Emerg Infect Dis 2012, 18, 1256–1262. https://doi.org/10.3201/eid1808.120212.
- Schafer, I.J.; Miller, R.; Ströher, U.; Knust, B.; Nichol, S.T.; Rollin, P.E. A Cluster of Lymphocytic Choriomeningitis Virus Infections Transmitted Through Organ Transplantation — Iowa, 2013. MMWR Morb Mortal Wkly Rep 2014, 63, 249.
- Carrillo-Bustamante, P.; Nguyen, T.H.T.; Oestereich, L.; Günther, S.; Guedj, J.; Graw, F. Determining Ribavirin’s Mechanism of Action against Lassa Virus Infection. Sci Rep 2017, 7, 11693. https://doi.org/10.1038/s41598-017-10198-0.
- Perez, M.; Greenwald, D.L.; de La Torre, J.C. Myristoylation of the RING Finger Z Protein Is Essential for Arenavirus Budding. J Virol 2004, 78, 11443–11448. https://doi.org/10.1128/JVI.78.20.11443-11448.2004.
- Capul, A.A.; Perez, M.; Burke, E.; Kunz, S.; Buchmeier, M.J.; de la Torre, J.C. Arenavirus Z-Glycoprotein Association Requires Z Myristoylation but Not Functional RING or Late Domains. J Virol 2007, 81, 9451–9460. https://doi.org/10.1128/JVI.00499-07.
- Witwit, H.; Betancourt, C.A.; Cubitt, B.; Khafaji, R.; Kowalski, H.; Jackson, N.; Ye, C.; Martinez-Sobrido, L.; de la Torre, J.C. Cellular N-Myristoyl Transferases Are Required for Mammarenavirus Multiplication. Viruses 2024, 16, 1362. https://doi.org/10.3390/v16091362.
- Radoshitzky, S.R., Buchmeier, M.J., de la Torre, J.C. 2022 Fields Virology: Emerging Viruses: Arenaviridae. Fields Virology, 7th Ed, Vol I: Emerging Viruses. Knipe, D.M., Howley, P.M., Whelan, S. (Eds). In.
- Kunz, S.; Edelmann, K.H.; de la Torre, J.-C.; Gorney, R.; Oldstone, M.B.A. Mechanisms for Lymphocytic Choriomeningitis Virus Glycoprotein Cleavage, Transport, and Incorporation into Virions. Virology 2003, 314, 168–178. https://doi.org/10.1016/s0042-6822(03)00421-5.
- Rojek, J.M.; Lee, A.M.; Nguyen, N.; Spiropoulou, C.F.; Kunz, S. Site 1 Protease Is Required for Proteolytic Processing of the Glycoproteins of the South American Hemorrhagic Fever Viruses Junin, Machupo, and Guanarito. J Virol 2008, 82, 6045–6051. https://doi.org/10.1128/JVI.02392-07.
- Fedeli, C.; Moreno, H.; Kunz, S. Novel Insights into Cell Entry of Emerging Human Pathogenic Arenaviruses. J Mol Biol 2018, 430, 1839–1852. https://doi.org/10.1016/j.jmb.2018.04.026.
- York, J.; Nunberg, J.H. Role of the Stable Signal Peptide of Junín Arenavirus Envelope Glycoprotein in pH-Dependent Membrane Fusion. J Virol 2006, 80, 7775–7780. https://doi.org/10.1128/JVI.00642-06.
- York, J.; Nunberg, J.H. Myristoylation of the Arenavirus Envelope Glycoprotein Stable Signal Peptide Is Critical for Membrane Fusion but Dispensable for Virion Morphogenesis. J Virol 2016, 90, 8341–8350. https://doi.org/10.1128/JVI.01124-16.
- Cordo, S.M.; Candurra, N.A.; Damonte, E.B. Myristic Acid Analogs Are Inhibitors of Junin Virus Replication. Microbes and Infection 1999, 1, 609–614. https://doi.org/10.1016/S1286-4579(99)80060-4.
- Kallemeijn, W.W.; Lueg, G.A.; Faronato, M.; Hadavizadeh, K.; Goya Grocin, A.; Song, O.-R.; Howell, M.; Calado, D.P.; Tate, E.W. Validation and Invalidation of Chemical Probes for the Human N-Myristoyltransferases. Cell Chem Biol 2019, 26, 892-900.e4. https://doi.org/10.1016/j.chembiol.2019.03.006.
- Ramljak, I.C.; Stanger, J.; Real-Hohn, A.; Dreier, D.; Wimmer, L.; Redlberger-Fritz, M.; Fischl, W.; Klingel, K.; Mihovilovic, M.D.; Blaas, D.; et al. Cellular N-Myristoyltransferases Play a Crucial Picornavirus Genus-Specific Role in Viral Assembly, Virion Maturation, and Infectivity. PLOS Pathogens 2018, 14, e1007203. https://doi.org/10.1371/journal.ppat.1007203.
- Sangha, R.S.; Jamal, R.; Spratlin, J.L.; Kuruvilla, J.; Sehn, L.H.; Weickert, M.; Berthiaume, L.G.; Mackey, J.R. A First-in-Human, Open-Label, Phase I Trial of Daily Oral PCLX-001, an NMT Inhibitor, in Patients with Relapsed/Refractory B-Cell Lymphomas and Advanced Solid Tumors. JCO 2023, 41, e15094–e15094. https://doi.org/10.1200/JCO.2023.41.16_suppl.e15094.
- Sangha, R.; Davies, N.M.; Namdar, A.; Chu, M.; Spratlin, J.; Beauchamp, E.; Berthiaume, L.G.; Mackey, J.R. Novel, First-in-Human, Oral PCLX-001 Treatment in a Patient with Relapsed Diffuse Large B-Cell Lymphoma. Curr Oncol 2022, 29, 1939–1946. https://doi.org/10.3390/curroncol29030158.
- Priyamvada, L.; Kallemeijn, W.W.; Faronato, M.; Wilkins, K.; Goldsmith, C.S.; Cotter, C.A.; Ojeda, S.; Solari, R.; Moss, B.; Tate, E.W.; et al. Inhibition of Vaccinia Virus L1 N-Myristoylation by the Host N-Myristoyltransferase Inhibitor IMP-1088 Generates Non-Infectious Virions Defective in Cell Entry. PLoS Pathog 2022, 18, e1010662. https://doi.org/10.1371/journal.ppat.1010662.




