Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Gran Canaria
2.2. Samples
2.3. Study Measurements
2.4. Statistical Analyses
3. Results
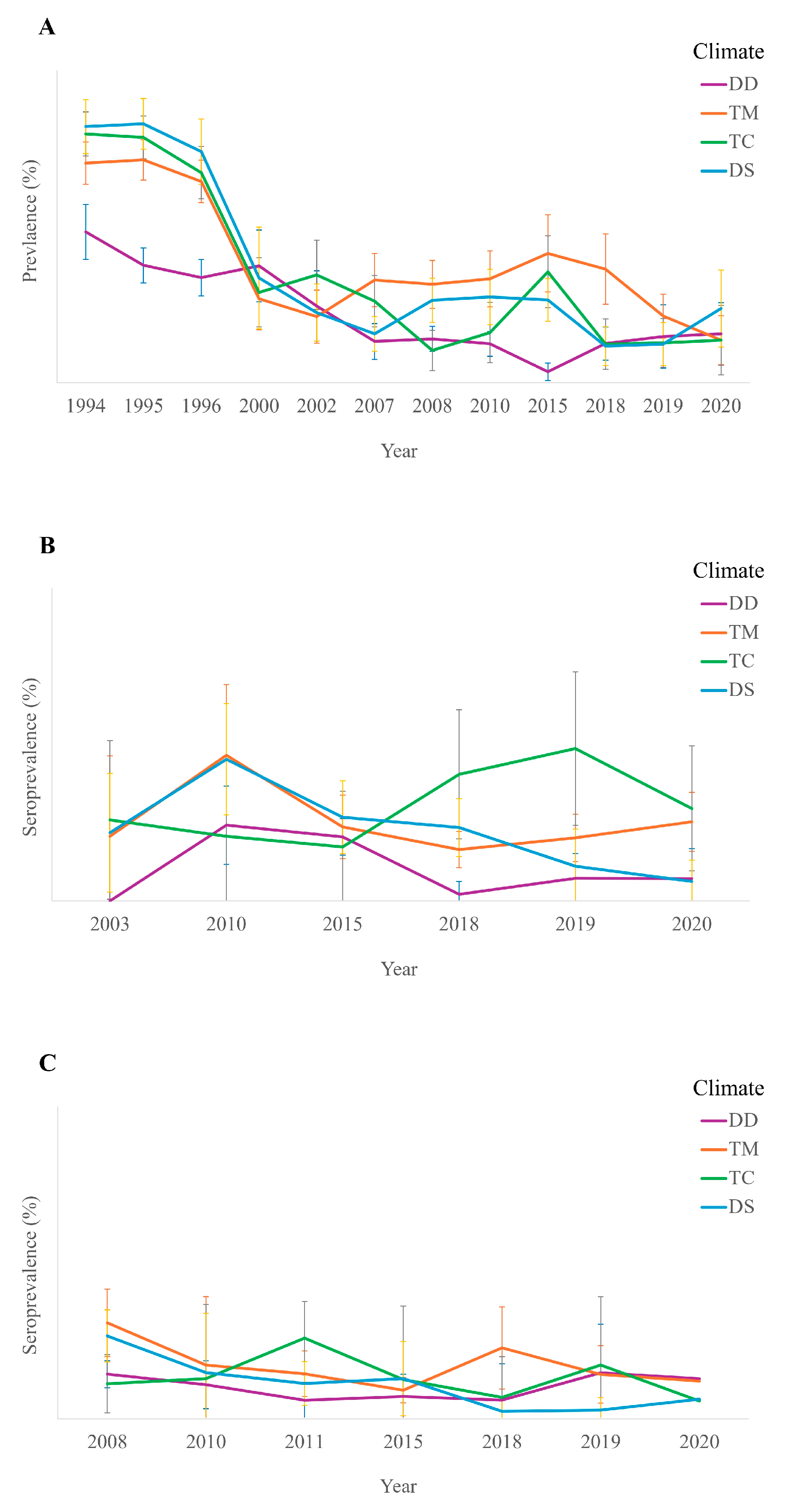
3.1. Total Prevalence According to the Year in Dogs and Cats
3.2. Total Prevalence According to the Year in Humans
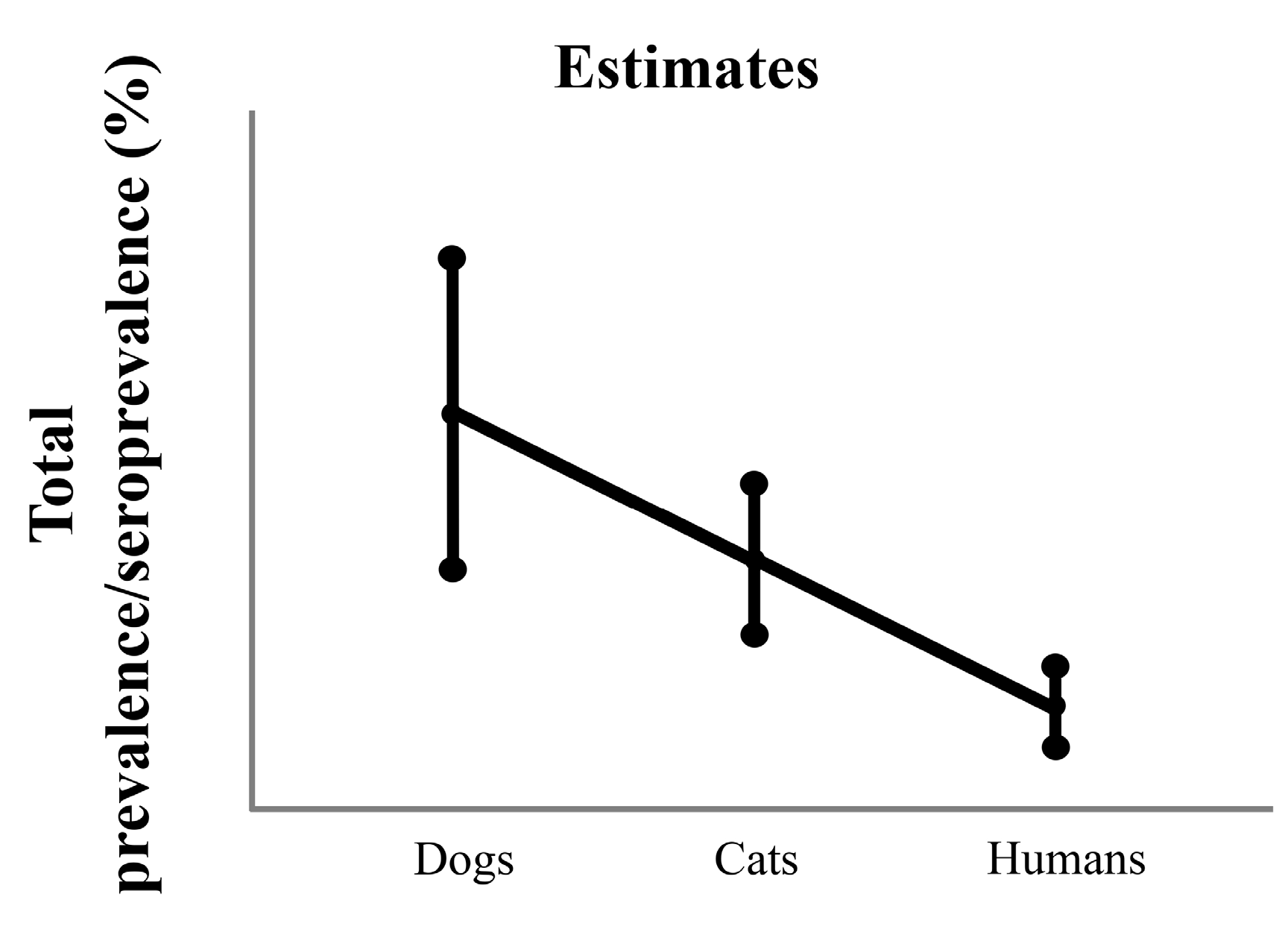
3.3. Difference in Prevalence between 3 Study Groups in General
3.3.1. Total Prevalence
3.3.2. According to the Climate Conditions
3.4. Between 2018 and 2020
3.5. Influence of Habitat on Dogs and Cats between 2018 and 2020
Positives’ Evolution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Hays, K.M.; Rodriguez, J.Y.; Little, S.E.; Litster, A.L.; Mwacalimba, K.K.; Sundstrom, K.D.; Amodie, D.M.; Serrano, M.A.; Guerios, S.D.; Lane, J.N.; et al. Heartworm prevalence in dogs versus cats: Multiple diagnostic modalities provide new insights. Vet. Parasitol. 2020, 277, 100027. [Google Scholar] [CrossRef] [PubMed]
- Albegiri, B.; Ribeiro Campos, D.; Serricella Branco, A.; Bendas, A.; Pereira Brum, R.; Calixto, R.; Câmara Alves, L.; Pinheiro Júnior, J.W.; Batalha Knackfuss, F.; Labarthe, N.; et al. Feline Heartworm in Clinical Settings in a High Canine Prevalence Area. Front. Vet. Sci. 2022, 9, 819082. [Google Scholar]
- Silva, M.J.; Costa, A.R.; Calvinho, P. Human pulmonary dirofilariasis: A pitfall in solitary pulmonary nodule. Pulmonology 2022, 28, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Perles, L.; Dantas-Torres, F.; Krücken, J.; Morchón, R.; Walochnik, J.; Otranto, D. Zoonotic dirofilariases: One, no one, or more than one parasite. Trends Parasitol. 2024, 40, 257–270. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chang, Y.C.; Chang, I.W. Human pulmonary dirofilariasis mimicking a metastatic disease in a cancer patient. Asian J. Surg. 2024, 47, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Cancrini, G.; Gabrielli, S. Vectors of Dirofilaria nematodes: Biology, behaviour and host/parasite relationships. In Dirofilaria immitis and Dirofilaria repens; Genchi, C., Rinaldi, L., Cringoli, G., Eds.; Rolando Editore: Napoli, Italy, 2007; pp. 49–58. [Google Scholar]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm disease (Dirofilaria immitis) and their bectors in Europe—New distribution trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef]
- Morchón, R.; Montoya-Alonso, J.A.; Rodríguez-Escolar, I.; Carretón, E. What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens 2022, 11, 1042. [Google Scholar] [CrossRef]
- Pantchev, N.; Etzold, M.; Daugschies, A.; Dyachenko, V. Diagnosis of imported canine filarial infections in Germany 2008–2010. Parasitol. Res. 2011, 109, 61–76. [Google Scholar] [CrossRef]
- Sun, M.; Zhuo, W.; Guo, S.; Liao, S.; Shi, D.; Liu, J.; Cheng, Z.; Liu, Y.; Niu, X.; Wang, S.; et al. Serological survey of canine dirofilariosis in Chongqing, Kunming, Nanchang, Fuzhou, Guangzhou, Shenzhen, and Nanning in Southern China. Vet. Parasitol. 2012, 185, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Bendas, A.J.R.; Mendes-de-Almeida, F.; Vom Simson, C.; Labarthe, N. Heat pretreatment of canine samples to evaluate efficacy of imidacloprid + moxidectin and doxycycline in heartworm treatment. Parasit. Vectors 2017, 10, 246. [Google Scholar] [CrossRef]
- Sonnberger, K.; Duscher, G.G.; Fuehrer, H.P.; Leschnik, M. Current trends in canine dirofilariosis in Austria—Do we face a pre-endemic status? Parasitol. Res. 2020, 119, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Capelli, G.; Genchi, C. Changing distribution patterns of canine vector borne diseases in Italy: Leishmaniosis vs. dirofilariosis. Parasit. Vectors 2009, 2, S2. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Auer, H.; Leschnik, M.; Silbermayr, K.; Duscher, G.; Joachim, A. Dirofilaria in humans, dogs, and vectors in Austria (1978–2014)—From imported pathogens to the endemicity of Dirofilaria repens. PLoS Negl. Trop. Dis. 2016, 10, e0004547. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranco, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasit. Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; García-Rodríguez, S.N.; Carretón, E.; Rodríguez Escolar, I.; Costa-Rodríguez, N.; Matos, J.I.; Morchón, R. Seroprevalence of feline heartworm in Spain: Completing the epidemiological puzzle of a neglected disease in the cat. Front. Vet. Sci. 2022, 9, 900371. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; García-Rodríguez, S.N.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Matos, J.I.; Rodríguez Escolar, I.; Carretón, E. Expansion of canine heartworm in Spain. Animals 2022, 12, 1268. [Google Scholar] [CrossRef]
- Montoya, J.A.; Morales, M.; Ferrer, O.; Molina, J.M.; Corbera, J.A. The prevalence of Dirofilaria immitis in Gran Canaria, Canary Islands, Spain (1994–1996). Vet. Parasitol. 1998, 75, 221–226. [Google Scholar] [CrossRef]
- Morchón, R.; Ferreira, A.C.; Martín-Pacho, J.R.; Montoya, A.; Mortarino, M.; Genchi, C.; Simón, F. Specific IgG antibody response against antigens of Dirofilaria immitis and its Wolbachia endosymbiont bacterium in cats with natural and experimental infections. Vet. Parasitol. 2004, 125, 313–321. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Juste, M.C.; Mellado, I.; Morchón, R.; Simón, F. Epidemiological survey of canine heartworm disease on the island of Gran Canaria (Canary Islands—Spain) between 2000 and 2008. Vet. Parasitol. 2010, 173, 165–168. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Mellado, I.; Carretón, E.; Cabrera-Pedrero, E.D.; Morchón, R.; Simón, F. Canine dirofilariosis caused by Dirofilaria immitis is a risk factor for the human population on the island of Gran Canaria, Canary Islands, Spain. Parasitol. Res. 2010, 107, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Cabrera-Pedrero, E.D.; Carretón, E.; Méndez, J.C.; Mellado, I.; Morchón, R.; Simón, F. Seroprevalence of human dirofilariosis on the island of Gran Canaria, Canary Islands-Spain. Trop. Med. Int. Health 2011, 16, 229. [Google Scholar]
- Montoya-Alonso, J.A.; Carretón, E.; Corbera, J.A.; Juste, M.C.; Mellado, I.; Morchón, R.; Simón, F. Current prevalence of Dirofilaria immitis in dogs, cats and humans from the island of Gran Canaria, Spain. Vet. Parasitol. 2011, 176, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Carretón, E.; Morchón, R.; Silveira-Viera, L.; Falcón, Y.; Simón, F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet. Parasitol. 2016, 216, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.D.; Carretón, E.; Morchón, R.; Falcón-Cordón, Y.; Falcón-Cordón, S.; Simón, F.; Montoya-Alonso, J.A. The Canary Islands as a model of risk of pulmonary dirofilariasis in a hyperendemic area. Parasitol. Res. 2018, 117, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; Matos, J.I.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Carretón, E. Dirofilaria immitis Could be a risk factor for the development of allergic diseases in humans. Animals 2020, 10, 1847. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, S.N.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A. Feline heartworm disease and environmental allergens hypersensitivity: Is there a link? Parasit. Vectors 2023, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Muro, A.; Cordero, M.; Martin, J. A seroepidemiologic survey of human dirofilariosis in Western Spain. Trop. Med. Parasitol. 1991, 42, 106–108. [Google Scholar]
- Levy, J.K.; Burling, A.N.; Crandall, M.M.; Tucker, S.J.; Wood, E.G.; Foster, J.D. Seroprevalence of heartworm infection, risk factors for seropositivity, and frequency of prescribing heartworm preventives for cats in the United States and Canada. J. Am. Vet. Med. Assoc. 2017, 250, 873–880. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 28, 108995. [Google Scholar] [CrossRef] [PubMed]
- Panarese, R.; Iatta, R.; Beugnet, F.; Otranto, D. Incidence of Dirofilaria immitis and Leishmania infantum infections in sheltered dogs from Southern Italy. Transbound. Emerg. Dis. 2020, 69, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Murillo, D.F.B.; Chenoweth, K.; Barua, S.; Kelly, P.J.; Starkey, L.; Blagburn, B.; Wood, T.; Wang, C. Nationwide molecular survey of Dirofilaria immitis and Dirofilaria repens in companion dogs and cats, United States of America. Parasit. Vectors 2022, 15, 367. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Tian, B.; Liu, Y.; Rehman, M.U.; Ranucci, D.; Veronesi, F.; Varcasia, A.; Jia, W.; Liu, J. The seroprevalence of canine dirofilariosis in dogs in the eastern coastal areas of China. Heliyon 2023, 9, e17009. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, C.; Croton, C.; Paterson, M.B.A.; Knott, L.; Henning, J.; Mallyon, J.; Coleman, G.T. Prevalence of canine heartworm infection in Queensland, Australia: Comparison of diagnostic methods and investigation of factors associated with reduction in antigen detection. Parasit. Vectors 2023, 16, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L.H.; Rivasi, R. Dirofilarial infections in Europe. Vector Borne Zoonotic Dis. 2011, 11, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Mortarino, M.; Rinaldi, L.; Cringoli, G.; Traldi, G.; Genchi, M. Changing climate and changing vector-borne disease distribution: The example of Dirofilaria in Europe. Vet. Parasitol. 2011, 176, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Simón, L.; Afonin, A.; López-Díez, L.I.; González-Miguel, J.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A.; Kartashev, V.; Simón, F. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet. Parasitol. 2014, 200, 257–264. [Google Scholar] [CrossRef]
- Morchón, R.; Rodríguez-Escolar, I.; Hernández Lambraño, R.E.; Sánchez Agudo, J.A.; Montoya-Alonso, J.A.; Serafín-Pérez, I.; Fernández-Serafín, C.; Carretón, E. Assessment Heartworm Disease in the Canary Islands (Spain): Risk of Transmission in a Hyperendemic Area by Ecological Niche Modeling and Its Future Projection. Animals 2023, 13, 3251. [Google Scholar] [CrossRef]

| Year | Dogs | Cats | Humans | Diagnostic Method |
|---|---|---|---|---|
| 1994 | 567 [20] | Dogs: Haemagglutination test * | ||
| 1995 | 779 [20] | Dogs: Haemagglutination test * | ||
| 1996 | 688 [20] | Dogs: Haemagglutination test * | ||
| 2000 | 255 [22] | Dogs: Commercial ELISA test kit ** | ||
| 2002 | 310 [22] | Dogs: Commercial ELISA test kit ** | ||
| 2003 | 49 [21] | Cats: Non-commercial ELISA technique | ||
| 2007 | 551 [22] | Dogs: Commercial ELISA test kit ** | ||
| 2008 | 697 [23] | 493 [23] | Dogs: Commercial ELISA test kit ** Humans: Non-commercial ELISA (antibodies) technique | |
| 2010 | 547 [25] | 109 [25] | 100 [25] | Dogs: Commercial ELISA test kit ** Cats: Non-commercial ELISA (antibodies) technique Humans: Non-commercial ELISA (antibodies) technique |
| 2011 | 300 [24] | Humans: Non-commercial ELISA (antibodies) technique | ||
| 2015 | 478 [26] | 338 [26] | 300 [27] | Dogs: Commercial immunochromatographic test kit *** Cats: Non-commercial ELISA (antibodies) technique Humans: Non-commercial ELISA (antibodies) technique |
| 2018 | 404 | 320 | 115 [28] | Dogs: Commercial immunochromatographic test kit *** Cats: Non-commercial ELISA (antibodies) technique Humans: Non-commercial ELISA (antibodies) technique |
| 2019 | 350 | 201 | 163 | Dogs: Commercial immunochromatographic test kit *** Cats: Non-commercial ELISA (antibodies) technique Humans: Non-commercial ELISA (antibodies) technique |
| 2020 | 215 | 186 | 133 | Dogs: Commercial immunochromatographic test kit *** Cats: Non-commercial ELISA (antibodies) technique Humans: Non-commercial ELISA (antibodies) technique |
| Year | Dogs’ Prevalence | Cats’ Seroprevalence | Humans’ Seroprevalence |
|---|---|---|---|
| 1994 | 67.02% [20] | ||
| 1995 | 58.92% [20] | ||
| 1996 | 52.18% [20] | ||
| 2000 | 30.19% [22] | ||
| 2002 | 24.50% [22] | ||
| 2003 | 18.37% [21] | ||
| 2007 | 20.40% [22] | ||
| 2008 | 19.36% [23] | 18.66% [23] | |
| 2010 | 19.20% [25] | 33.03% [25] | 12% [25] |
| 2011 | 13.3% [24] | ||
| 2015 | 20.77% [26] | 21.30% [26] | 9% [27] |
| 2018 | 16.09% | 17.19% | 10.43% [28] |
| 2019 | 15.71% | 17.91% | 9.2% |
| 2020 | 15.81% | 17.20% | 8.27% |
| Dogs | Cats | Humans | ||
|---|---|---|---|---|
| DD Climate positives + | 36.00 ± 33.92 (21.53 ± 13.23) | 9.17 ± 14.66 (9.85 ± 9.56) | 8.14 ± 12.43 (9.99 ± 3.73) | p = 0.023 |
| TM Climate positives + | 54.92 ± 50.09 (37.36 ± 18.94) | 16.00 ± 9.01 (24.60 ± 10.42) | 10.86 ± 7.27 (16.69 ± 7.05) | p = 0.005 |
| TC Climate positives + | 38.25 ± 43.06 (33.44 ± 25.15) | 5.17 ± 2.93 (29.41 ± 11.64) | 3.57 ± 4.83 (12.78 ± 6.56) | p = 0.002 |
| DS Climate positives + | 33.83 ± 24.42 (35.29 ± 25.88) | 9.67 ± 7.15 (21.67 ± 13.21) | 7.29 ± 9.93 (10.68 ± 8.09) | p = 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Alonso, J.A.; García-Rodríguez, S.N.; Matos, J.I.; Costa-Rodríguez, N.; Falcón-Cordón, Y.; Carretón, E.; Morchón, R. Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020. Animals 2024, 14, 2037. https://doi.org/10.3390/ani14142037
Montoya-Alonso JA, García-Rodríguez SN, Matos JI, Costa-Rodríguez N, Falcón-Cordón Y, Carretón E, Morchón R. Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020. Animals. 2024; 14(14):2037. https://doi.org/10.3390/ani14142037
Chicago/Turabian StyleMontoya-Alonso, José Alberto, Sara Nieves García-Rodríguez, Jorge Isidoro Matos, Noelia Costa-Rodríguez, Yaiza Falcón-Cordón, Elena Carretón, and Rodrigo Morchón. 2024. "Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020" Animals 14, no. 14: 2037. https://doi.org/10.3390/ani14142037
APA StyleMontoya-Alonso, J. A., García-Rodríguez, S. N., Matos, J. I., Costa-Rodríguez, N., Falcón-Cordón, Y., Carretón, E., & Morchón, R. (2024). Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020. Animals, 14(14), 2037. https://doi.org/10.3390/ani14142037









