Daptomycin versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Outcomes Assessed
2.6. Quality Assessment
2.7. Statistical Analyses
2.8. Ethics
3. Results
3.1. Literature Search
3.2. Study Description
3.3. Quality of Included Studies
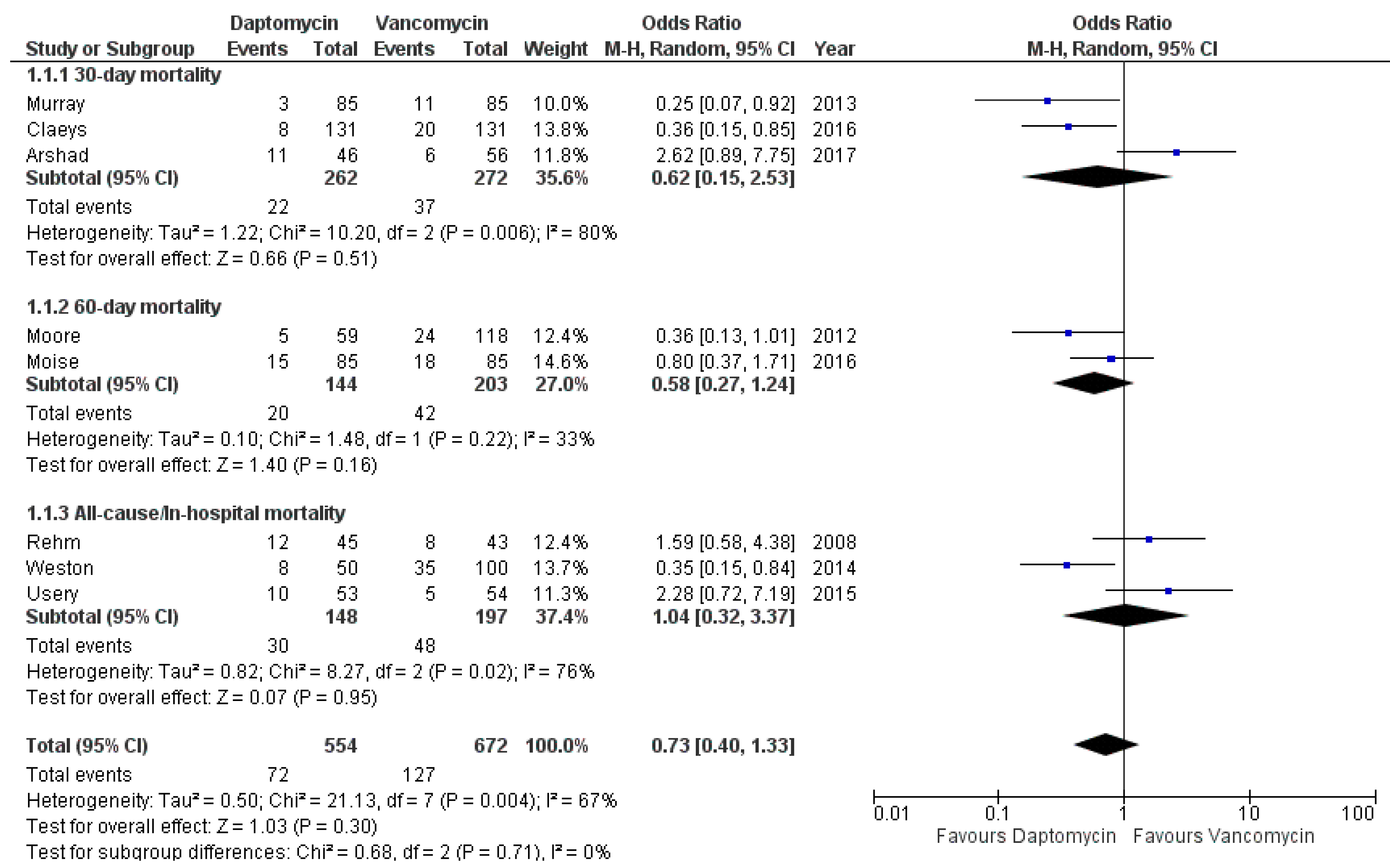
3.4. Mortality
3.5. Clinical Failure
3.6. Infection Recurrence
3.7. Infection Persistence
3.8. Length of Stay
3.9. Safety Profile
3.10. 30-Day Re-Admission
3.11. Sources of Heterogeneity and Sensitivity Analyses
3.12. Publication Bias
3.13. Certainty of Evidence According to the GRADE Framework
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.D.; Chen, Y.Y.; Chen, T.L.; Cheng-Yi, L. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am. J. Infect. Control 2008, 36, 118–122. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [Green Version]
- Kern, W.V. Management of Staphylococcus aureus bacteremia and endocarditis: Progresses and challenges. Curr. Opin. Infect. Dis. 2010, 23, 346–358. [Google Scholar] [CrossRef]
- Ayau, P.; Bardossy, A.C.; Sanchez, G.; Ortiz, R.; Moreno, D.; Hartman, P.; Rizvi, K.; Prentiss, T.C.; Perri, M.B.; Mahan, M.; et al. Risk Factors for 30-Day Mortality in Patients with Methicillin-Resistant Staphylococcus aureus Bloodstream Infections. Int. J. Infect. Dis. 2017, 61, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Cortés, L.E.; Del Toro, M.D.; Gálvez-Acebal, J.; Bereciartua-Bastarrica, E.; Fariñas, M.C.; Sanz-Franco, M.; Natera, C.; Corzo, J.E.; Lomas, J.M.; Pasquau, J.; et al. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2013, 57, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Schweizer, M.L.; Vaughan-Sarrazin, M.S.; Perencevich, E.N.; Livorsi, D.J.; Diekema, D.J.; Richardson, K.K.; Beck, B.F.; Alexander, B.; Ohl, M.E. Association of Evidence-Based Care Processes With Mortality in Staphylococcus aureus Bacteremia at Veterans Health Administration Hospitals, 2003–2014. JAMA Intern. Med. 2017, 177, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Yamamoto, M.; Matsumura, Y.; Yokota, I.; Takakura, S.; Teramukai, S.; Ichiyama, S. Complete adherence to evidence-based quality-of-care indicators for Staphylococcus aureus bacteremia resulted in better prognosis. Infection 2017, 45, 83–91. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.; Bacci, M.R.; Feder, D.; da Luz Gonçalves Pedreira, M.; Sorgini Peterlini, M.A.; Azzalis, L.A.; Campos Junqueira, V.B.; Fonseca, F.L.A. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.J.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 24 February 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Sedgwick, P. Meta-analyses: What is heterogeneity? BMJ 2015, 350, h1435. [Google Scholar] [CrossRef] [Green Version]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Trigkidis, K.K.; Falagas, M.E. Fluoroquinolones or macrolides in combination with β-lactams in adult patients hospitalized with community acquired pneumonia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2017, 23, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Van Aert, R.; Jackson, D. A new justification of the Hartung-Knapp method for random-effects meta-analysis based on weighted least squares regression. Res. Syn. Meth. 2019, 10, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, S.J.; Boucher, H.; Levine, D.; Campion, M.; Eisenstein, B.I.; Vigliani, G.A.; Corey, G.R.; Abrutyn, E. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: Subset analysis of patients infected with methicillin-resistant isolates. J. Antimicrob. Chemother. 2008, 62, 1413–1421. [Google Scholar] [CrossRef]
- Arshad, S.; Huang, V.; Hartman, P.; Perri, M.B.; Moreno, D.; Zervos, M.J. Ceftarolinefosamil monotherapy for methicillin-resistant Staphylococcus aureus bacteremia: A comparative clinical outcomes study. Int. J. Infect. Dis. 2017, 57, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Usery, J.B.; Vo, N.H.; Finch, C.K.; Cleveland, K.O.; Gelfand, M.S.; Self, T.H. Evaluation of the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Am. J. Med. Sci. 2015, 349, 36–41. [Google Scholar] [CrossRef]
- Moore, C.L.; Osaki-Kiyan, P.; Haque, N.Z.; Perri, M.B.; Donabedian, S.; Zervos, M.J. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: A case-control study. Clin. Infect. Dis. 2012, 54, 51–58. [Google Scholar] [CrossRef]
- Murray, K.P.; Zhao, J.J.; Davis, S.L.; Kullar, R.; Kaye, K.S.; Lephart, P.; Rybak, M.J. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: A matched cohort study. Clin. Infect. Dis. 2013, 56, 1562–1569. [Google Scholar] [CrossRef] [Green Version]
- Weston, A.; Golan, Y.; Holcroft, C.; Snydman, D.R. The efficacy of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bloodstream infection in patients with impaired renal function. Clin. Infect. Dis. 2014, 58, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative Effectiveness of Vancomycin Versus Daptomycin for MRSA Bacteremia With Vancomycin MIC >1 mg/L: A Multicenter Evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef]
- Claeys, K.C.; Zasowski, E.J.; Casapao, A.M.; Lagnf, A.M.; Nagel, J.L.; Nguyen, C.T.; Hallesy, J.A.; Compton, M.T.; Kaye, K.S.; Levine, D.P.; et al. Daptomycin Improves Outcomes Regardless of Vancomycin MIC in a Propensity-Matched Analysis of Methicillin-Resistant Staphylococcus aureus Bloodstream Infections. Antimicrob. Agents Chemother. 2016, 60, 5841–5848. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Souli, M.; Ruffin, F.; Choi, S.H.; Park, L.P.; Gao, S.; Lent, N.C.; Sharma-Kuinkel, B.K.; Thaden, J.T.; Maskarinec, S.A.; Wanda, L.; et al. Changing Characteristics of Staphylococcus aureus Bacteremia: Results From a 21-Year, Prospective, Longitudinal Study. Clin. Infect. Dis. 2019, 69, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Diep, B.A.; Villaruz, A.E.; Braughton, K.R.; Jiang, X.; DeLeo, F.R.; Chambers, H.F.; Lu, Y.; Otto, M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 5883–5888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessa, F.C.; Mu, Y.; Ray, S.M.; Dumyati, G.; Bulens, S.; Gorwitz, R.J.; Fosheim, G.; DeVries, A.S.; Schaffner, W.; Nadle, J.; et al. Impact of USA300 methicillin-resistant Staphylococcus aureus on clinical outcomes of patients with pneumonia or central line-associated bloodstream infections. Clin. Infect. Dis. 2012, 55, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Yaw, L.K.; Robinson, J.O.; Ho, K.M. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: An observational cohort study. Lancet Infect. Dis. 2014, 14, 967–975. [Google Scholar] [CrossRef]
- Minejima, E.; Mai, N.; Bui, N.; Mert, M.; Mack, W.J.; She, R.C.; Nieberg, P.; Spellberg, B.; Wong-Beringer, A. Defining the Breakpoint Duration of Staphylococcus aureus Bacteremia Predictive of Poor Outcomes. Clin. Infect. Dis. 2020, 70, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, C.; Liao, L.; Wang, Z.; Hu, Y.; Deng, C.; Liu, L. Adjuvant β-Lactam Therapy Combined with Vancomycin or Daptomycin for Methicillin-Resistant Staphylococcus aureus Bacteremia: A Systematic Review and Meta-analysis. Antimicrob. Agents Chemother. 2020, 64, e01377-20. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Zasowski, E.J.; Trinh, T.D.; Lagnf, A.M.; Bhatia, S.; Sabagha, N.; Abdul-Mutakabbir, J.C.; Alosaimy, S.; Mynatt, R.P.; Davis, S.L.; et al. Daptomycin Plus β-Lactam Combination Therapy for Methicillin-resistant Staphylococcus aureus Bloodstream Infections: A Retrospective, Comparative Cohort Study. Clin. Infect. Dis. 2020, 71, 1–10. [Google Scholar] [CrossRef]
- Gudiol, F.; Aguado, J.M.; Almirante, B.; Bouza, E.; Cercenado, E.; Domínguez, M.A.; Gasch, O.; Lora-Tamayo, J.; Miró, J.M.; Palomar, M.; et al. Executive summary of the diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2015, 33, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Goodman, A.L.; Horner, C.; Jenkins, A.; Brown, E.M. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC Antimicrob. Resist. 2021, 3, dlaa114. [Google Scholar] [CrossRef] [PubMed]
- Gould, F.K.; Denning, D.W.; Elliott, T.S.J.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.T.; Spry, M.J.; Watkin, R.W.; Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: A report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2012, 67, 269–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, H.; Fitzpatick, F.; Harvey, B.J. Gender differences in rates of carriage and bloodstream infection caused by methicillin-resistant Staphylococcus aureus: Are they real, do they matter and why? Clin. Infect. Dis. 2015, 61, 1708–1714. [Google Scholar]
- Smit, J.; López-Cortés, L.E.; Kaasch, A.J.; Søgaard, M.; Thomsen, R.W.; Schønheyder, H.C.; Rodríguez-Baño, J.; Nielsen, H. Gender differences in the outcome of community-acquired Staphylococcus aureus bacteraemia: A historical population-based cohort study. Clin. Microbiol. Infect. 2017, 23, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, H.; Tariq, W.; Talha, K.M.; Palraj, B.R.V.; Sohail, M.R.; Baddour, L.M.; Mahmood, M. Association between high vancomycin minimum inhibitory concentration and clinical outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: A meta-analysis. Infection 2021, 1–9. [Google Scholar] [CrossRef]
- Kalil, A.C.; Van Schooneveld, T.C.; Fey, P.D.; Rupp, M.E. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: A systematic review and meta-analysis. JAMA 2014, 312, 1552–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovici, L.; Scudeller, L.; Kalil, A.; Huttner, A.; Leeflang, M.M.G.; Bielicki, J.; Allerberger, F.; Paul, M.; Rodríguez-Baño, J. Guidance on reporting multivariable regression models in CMI. Clin. Microbiol. Infect. 2020, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Hershberger, E.; Moldovan, T.; Grucz, R.G. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against Staphylococci and Enterococci, including vancomycin- intermediate and -resistant strains. Antimicrob. Agents Chemother. 2000, 44, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Caparas, J.V.; Hu, J.P. Safe administration of vancomycin through a novel midline catheter: A randomized, prospective clinical trial. J. Vasc. Access 2014, 15, 251–256. [Google Scholar] [CrossRef]
- Ye, Z.K.; Li, C.; Zhai, S.D. Guidelines for therapeutic drug monitoring of vancomycin: A systematic review. PLoS ONE 2014, 9, e99044. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar]
- Dalton, B.R.; Rajakumar, I.; Langevin, A.; Langevin, A.; Ondro, C.; Sabuda, D.; Griener, T.P.; Dersch-Mills, D.; Rennert-May, E. Vancomycin area under the curve to minimum inhibitory concentration ratio predicting clinical outcome: A systematic review and meta-analysis with pooled sensitivity and specificity. Clin. Microbiol. Infect. 2020, 26, 436–446. [Google Scholar] [CrossRef]
- Bhavnani, S.M.; Prakhya, A.; Hammel, J.P.; Ambrose, P.G. Cost-Effectiveness of daptomycin versus vancomycin and gentamicin for patients with methicillin-resistant Staphylococcus aureus bacteremia and/or endocarditis. Clin. Infect. Dis. 2009, 49, 691–698. [Google Scholar] [CrossRef]
- Schweizer, M.L.; Richardson, K.; Vaughan Sarrazin, M.S.; Goto, M.; Livorsi, D.J.; Nair, R.; Alexander, B.; Beck, B.F.; Jones, M.P.; Puig-Asensio, M.; et al. Comparative Effectiveness of Switching to Daptomycin Versus Remaining on Vancomycin Among Patients With Methicillin-resistant Staphylococcus aureus (MRSA) Bloodstream Infections. Clin. Infect. Dis. 2021, 72, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Timbrook, T.T.; Caffrey, A.R.; Luther, M.K.; Lopes, V.; LaPlante, K.L. Association of Higher Daptomycin Dose (7 mg/kg or Greater) with Improved Survival in Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia. Pharmacotherapy 2018, 38, 189–196. [Google Scholar] [CrossRef] [PubMed]





| Author, yr [Reference] | Country | Design | No. of Centers | Study Period | Sample Size | MRSA Associated Endocarditis | Group | Primary Endpoints 1 | Mortality | Clinical Failure (Composite Outcome) | Relapse | Persistent BSI | Safety Assessment 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daptomycin (Daily Dose, Treatment Duration, Combination Therapy [%, Drug]) | Vancomycin (Trough Concentration or Daily Dose, Treatment Duration, Combination Therapy [%, Drug]) | |||||||||||||
| Arshad S et al. 2017 [27] | USA | Retrospective matched cohort study | 1 | Nov 2009–Dec 2013 | 102 (46 DAP vs. 56 VAN) | DAP: 19/46 VAN: 13/56 | N/A 4–8 weeks N/A | N/A 4–8 weeks N/A | 1–3, 5 | DAP: 11/46 VAN: 6/56 | DAP: 15/46 10/56 | DAP: 1/46 1/56 | N/A | N/A |
| Claeys C et al. 2016 [33] | USA | Retrospective matched cohort study | 3 | Jan 2010–Mar 2015 | 262 (131 VAN vs. 131 DAP) | DAP: 25/131 VAN: 25/131 | 8.2 mg/kg (IQR, 6.4–10.0) N/A 24.4% (13% ceftaroline, 6.9% rifampin) | 17.7 mg/L (IQR, 13–222.0) N/A 20.6% (10.7% ceftaroline, 4.6% rifampin) | 1–6 | DAP: 8/131 VAN: 20/131 | DAP: 38/131 VAN: 59/131 | DAP: 0/131 VAN: 0/131 | DAP: 20/131 VAN: 37/131 | DAP: 2 (3/131) VAN: 1 (12/131) |
| Moise PA et al. 2016 [32] | USA | Retrospective matched cohort study | 11 | 2005–2012 | 170 (85 DAP vs. 85 VAN) | 31/170 (no group subdivision) | 6 mg/kg (IQR 6–8) 16 days (IQR, 10–35) 74% (13% gentamicin, 15% rifampin, 46% β-lactam) | 17.5 mg/L (IQR,14.0–22.0) 16 days (IQR, 9–31) 98% (16% gentamicin, 11% rifampin, 71% β-lactam) | 1–4, 6 | DAP: 15/85 VAN: 18/85 | DAP: 9/85 VAN: 20/85 | DAP: 0/85 VAN: 0/85 | DAP: 8/72 VAN: 6/66 | DAP: 1 (6/64) VAN: 1 (14/62) |
| Moore CL et al. 2012 [29] | USA | Retrospective case–control study | 1 | 2005–2009 | 177 (59 DAP vs. 118 VAN) | DAP: 17/59 VAN: 34/118 | 6 mg/kg (dose adjustment if GFR ≤30 mL/min) 12 days (IQR, 8–18) 3 days) | 10-20 mg/L 15 days (IQR, 10–24) 3 days) | 1–4, 6 | DAP: 5/59 VAN: 24/118 | DAP: 10/59 VAN: 37/118 | DAP: 2/59 VAN: 6/118 | N/A | DAP: 1 (1/59) VAN: 1 (13/63) |
| Weston A et al. 2014 [31] | USA | Retrospective case–control study | 1 | Jan 2001–Aug 2011 | 267 (50 DAP vs. 100 VAN) | DAP: 13/50 VAN: 11/100 | 6.8 mg/kg (range, 5.1–10.8) (dose adjustment if GFR ≤30 mL/min) Average 28 days N/A | 15.3 μg/mL (range, 8.2–25.6) Average 21 days N/A | 1–4 | DAP: 8/50 VAN: 35/100 | DAP: 17/50 VAN: 51/100 | DAP: 6/50 VAN: 5/100 | DAP: 7/50 VAN: 21/100 | DAP: 2 (2/50) VAN: N/A |
| Murray KP et al. 2013 [30] | USA | Retrospective matched cohort study | 4 | Jan 2005–Mar 2012 | 170 (85 DAP vs. 85 VAN) | DAP: 20/85 VAN: 20/85 | 8.4 mg/kg (IQR, 6.3–9.9) 10 days (IQR, 8–17) 30.6% (14.1% aminoglycoside, 16.5% rifampin) | 17.6 µg/mL (IQR, 14.9–21.2) 9 days (IQR, 6-16) 47.1% (25.9% aminoglycoside, 21.2% rifampin) | 1–4 | DAP: 3/85 VAN: 11/85 | DAP: 17/85 VAN: 41/85 | DAP: 0/85 VAN: 3/85 | DAP: 16/85 VAN: 36/85 | DAP: 2 (1/85) VAN: 1 (22/85) |
| Usery JB et al. 2015 [28] | USA | Retrospective cohort study | 1 | Jun 2008–Nov 2010 | 122 (53 DAP vs. 54 VAN) | DAP: 6/53 VAN: 6/54 | 6.7 mg/kg (range, 4.9–8.5) 16.4 days (range, 6.8–26) N/A | 13.6 mg/kg (range, 9.6–17.6) 13.6 days (range, 6.5–20.7) N/A | 1–3, 7 | DAP: 10/53 VAN: 5/54 | DAP: 11/53 VAN: 9/54 | DAP: 4/42 VAN: 8/49 | DAP: 19/53 VAN: 11/54 | N/A |
| Rehm SJ et al. 2008 [26] | International | Subset analysis of an open-label randomized trial | Multicentre | Aug 2002–Mar 2005 | 88 (45 DAP vs. 43 VAN plus gentamicin) | DAP: 13/45 VAN: 10/43 | 6 mg/kg 10-14 days for uncomplicated bacteraemia; at least 28 days for complicated bacteraemia and endocarditis N/A | 14.9 μg/mL 10-14 days for uncomplicated bacteraemia; at least 28 days for complicated bacteraemia and endocarditis 100% (gentamicin 1 mg/kg every 8 hours for the first 4 days of treatment) | 1–4, 6, 7 | DAP: 12/45 VAN: 8/43 | DAP: 25/45 VAN: 29/43 | DAP: 12/45 VAN: 9/43 | DAP: 3/45 VAN: 7/43 | DAP: 1, 3 (3/45) VAN: 1, 3 (7/43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraolo, A.E.; Giaccone, A.; Gentile, I.; Saracino, A.; Bavaro, D.F. Daptomycin versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 1014. https://doi.org/10.3390/antibiotics10081014
Maraolo AE, Giaccone A, Gentile I, Saracino A, Bavaro DF. Daptomycin versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(8):1014. https://doi.org/10.3390/antibiotics10081014
Chicago/Turabian StyleMaraolo, Alberto Enrico, Agnese Giaccone, Ivan Gentile, Annalisa Saracino, and Davide Fiore Bavaro. 2021. "Daptomycin versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 8: 1014. https://doi.org/10.3390/antibiotics10081014









