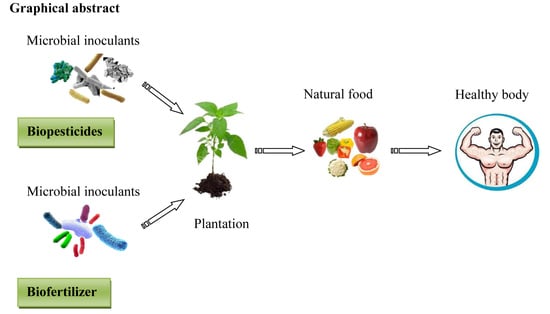
Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product
Abstract
:Simple Summary
Abstract
1. Introduction
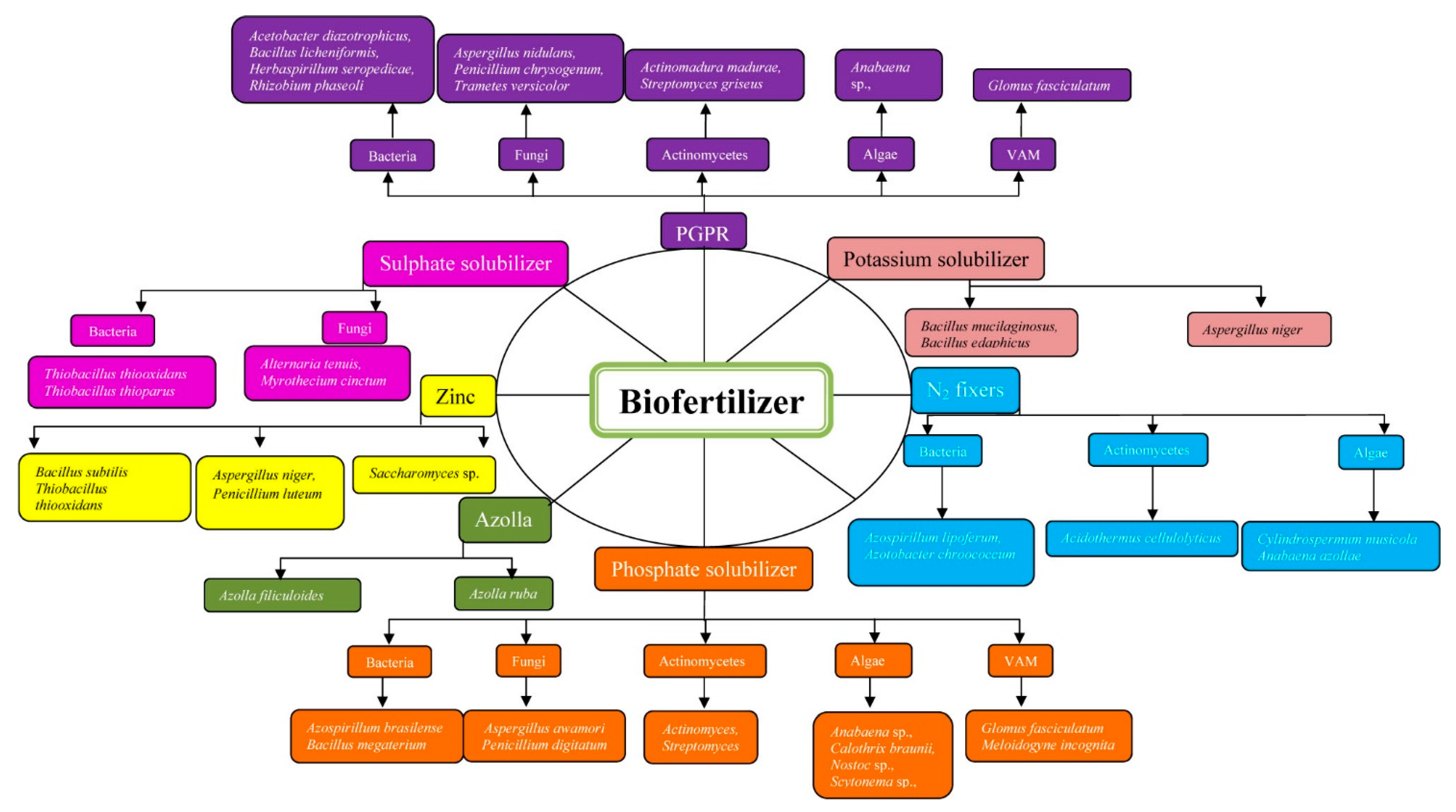
2. Biofertilizer
2.1. Nitrogen Fixers
2.2. Phosphate Solubilizing Microorganisms
2.3. Potassium Solubilizing Microorganisms
2.4. Sulfur Dissolving Microorganisms
2.5. Zinc Solubilizers
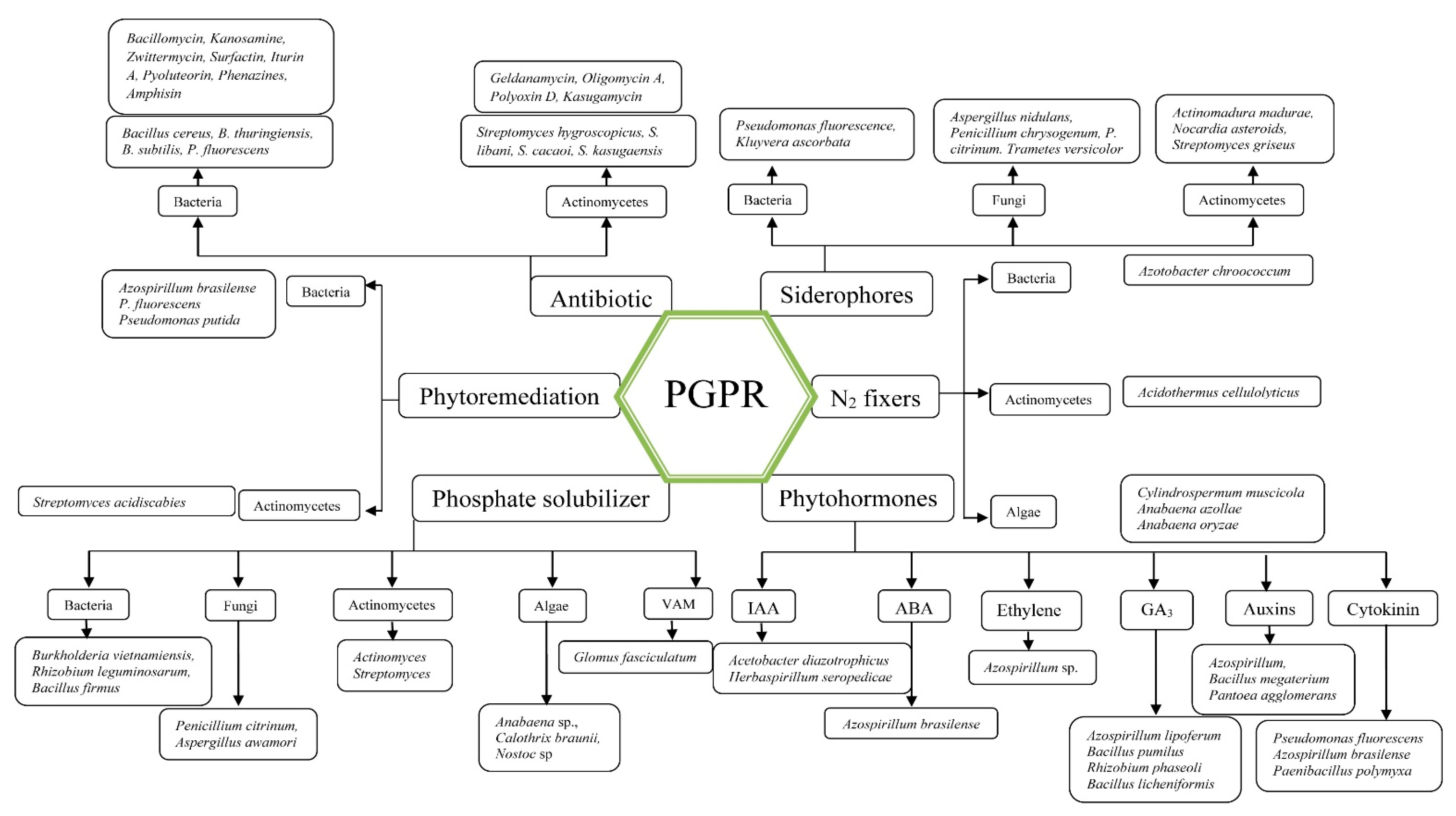
3. Plant Growth Promoting Rhizobacteria (PGPR)
3.1. Phytohormones
3.2. Siderophore
3.3. Phytoremediation of Heavy Metals by PGPR
3.4. Antibiotic
4. Biofertilizer Carrier
5. Biopesticides
5.1. Microbial Pesticides
5.1.1. Bacteria
5.1.2. Fungi
5.1.3. Nematodes
5.1.4. Protozoa
5.1.5. Viruses
5.2. Biochemical Pesticides
5.3. Plant Incorporated Protectants (PIPS)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Santos, E.A.; Ferreira, L.R.; Costa, M.D.; Santos, J.B.; Silva, M.C.S.; Aspiazu, I. The effects of soil fumigation on the growth and mineral nutrition of weeds and crops. Acta Sci. Agron. 2012, 34, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period, A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Swapna, A.L. Development of biofertilizers and its future perspective. J. Pharm. 2013, 2, 327–332. [Google Scholar]
- Rahman, K.M.A.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.P.; Babalola, O.O. Evaluation of plant growth-promoting potential of four rhizobacterial species for indigenous system. J. Cent. South Univ. 2013, 20, 164–171. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. Sust. Agric. Rev. 2017, 22, 281–307. [Google Scholar]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants, potential tool for sustainability of agricultural production systems. Arch. Microb. 2020, 202, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Macik, M.; Gryta, A.; Frac, M. Biofertilizers in agriculture, An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Deepak, B.; Mohammad, W.A.; Ranjan, K.S.; Narendra, T. Biofertilizers function as a key player in sustainable agriculture by improving soil fertility, plant tolerance, and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar]
- Labuschagne, N.; Pretorius, T.; Idris, A.H. Plant growth-promoting Rhizobacteria as Biocontrol Agents against soil-borne Plant diseases. Microbiol. Monogr. 2010, 18, 211–230. [Google Scholar]
- Sharma, K.R.; Raju, S.V.S.; Jaiswal, D.K.; Thakur, S. Biopesticides, an effective tool for insect pest management and current scenario in India. Ind. J. Agric. Allied Sci. 2018, 4, 59–62. [Google Scholar]
- Valicente, F.H.; Tuelher, E.S.; Leite, M.I.S.; Freire, F.L.; Vieira, C.M. Production of Bacillus thuringiensis biopesticide using commercial Lab medium and agricultural by-products as nutrient sources. Braz. J. Maize Sorghum 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Yosefi, K.; Galavi, M.; Ramrodi, M.; Mousavi, S.R. Effect of bio-phosphate and chemical phosphorus fertilizer accompanied with micronutrient foliar application on growth, yield and yield components of maize (Single Cross 704). Aust. J. Crop Sci. 2011, 5, 175–180. [Google Scholar]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Umesha, S.K.; Singh, P.P.; Singh, R. Microbial Biotechnology and Sustainable Agriculture. Biotechnol. Sustain. Agric. 2018, 185–205. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y. Bacterial biofertilizers for sustainable crop production, A review. ARPN J. Agric. Biol. Sci. 2012, 7, 307–316. [Google Scholar]
- Saharan, B.S.; Nehra, V. Plant growth-promoting rhizobacteria, a critical review. Life Sci. Med. Res. 2011, 21, 30. [Google Scholar]
- Tak, H.I.; Ahmad, F.; Babalola, O.O. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev. Environ. Contam. Toxicol. 2013, 223, 33–52. [Google Scholar] [PubMed]
- Youssef, M.M.A.; Eissa, M.F.M. Biofertilizers and their role in management of plant parasitic nematodes, A review. J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar]
- Barman, M.; Paul, S.; Choudhury, A.G.; Roy, P.; Sen, J. Biofertilizers as prospective input for sustainable agriculture in India. Int. J. Curr. Microb. Appl. Sci. 2017, 6, 1177–1186. [Google Scholar] [CrossRef]
- Ramasamy, M.; Geetha, T.; Yuvaraj, M. Role of Biofertilizers in Plant Growth and Soil Health. In Nitrogen Fixation; Everlon Cid Rigobelo and Ademar Pereira Serra; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.J.; Rodelas, B.; Pozo, C.; Salmeron, V.; Martnez, M.V.; Salmeron, V. Liberation of amino acids by heterotrophic nitrogen-fixing bacteria. Amino Acids 2005, 28, 363–367. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria, mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, S.; Ranga Rao, G.V.; Humayun, P.; Rameshwar Rao, V.; Alekhya, G.; Simi, J.; Deepthi, K.; Sree Vidya, M.; Srinivas, V.; Mamtha, L.; et al. Efficacy of botanical extracts and entomopathogens on control of Helicoverpa armigera and Spodoptera litura. Afr. J. Biotechnol. 2011, 10, 16667–16673. [Google Scholar]
- Lacey, L.A.; Neven, L.G. The potential of the fungus, Muscodor albus, as a microbial control agent of potato tuber moth (Lepidoptera, Gelechiidae) in stored potatoes. J. Invert. Pathol. 2006, 91, 195–198. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeno-Orrillo, E.; Lopez-Lopez, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martinez-Romero, J.C.; Reddy, P.M.; Martinez-Romero, E. Nitrogen Fixation in Cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakulin, M.K.; Grudtsyna, A.S.; Pletneva, A. Biological fixation of nitrogen and growth of bacteria of the genus Azotobacter in liquid media in the presence of Perfluorocarbons. Appl. Biochem. Microbiol. 2007, 4, 399–402. [Google Scholar] [CrossRef]
- Dubey, R.C. A Textbook of Biotechnology, 4th ed.; S. Chand & Co. Ltd.: New Delhi, India, 2006; p. 732. ISBN 81-219-2608-4. [Google Scholar]
- Sahoo, R.K.; Ansari, M.W.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterization of efficient nitrogen-fixing Azotobacter strains of the rice fields. Protoplasma 2013, 251, 511–523. [Google Scholar] [CrossRef]
- Gauri, S.S.; Mandal, S.M.; Pati, B.R. Impact of Azotobacter exopolysaccharides on sustainable agriculture. Appl. Microbiol. Biotechnol. 2012, 95, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, D.A.; Ewedab, W.E.; Zayed, M.S.; Hassaneina, M.K. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculants. Ann. Agric. Sci. 2013, 58, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Germination seedling Growth and Yield of Maize. Int. J. Biol. Life Sci. 2009, 5, 1. [Google Scholar]
- Mali, G.V.; Bodhankar, M.G. Antifungal and phytohormone production potential of Azotobacter chroococcum isolates from groundnut (Arachis hypogea L.) rhizosphere. Asian J. Exp. Sci. 2009, 23, 293–297. [Google Scholar]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and molecular characterization of efficient native Azospirillum strains from rice fields for crop improvement. Protoplasma 2014, 251, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR), emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Sharan, A.; Darmwal, N.S.; Gaur, R. Xanthomonas campestris, a novel stress-tolerant, phosphate-solubilizing bacterial strain from saline-alkali soils. World J. Microbiol. Biotechnol. 2008, 24, 753–759. [Google Scholar] [CrossRef]
- Farajzadeh, D.; Yakhchali, B.; Aliasgharzad, N.; Bashir, N.S.; Farajzadeh, M. Plant growth-promoting characterization of indigenous Azotobacteria isolated from soils in Iran. Curr. Microbiol. 2012, 64, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Kundu, S.; Joshi, P.; Nazim, S.; Gupta, A.D.; Mishra, P.K.; Gupta, H.S. Characterization of a cold-tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the North-Western Indian Himalayas. World J. Microbiol. Biotechnol. 2008, 24, 955–960. [Google Scholar] [CrossRef]
- Shahid, M.; Hameed, S.; Imran, A.; Ali, S.; Elsas, J.D. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J. Microbiol. Biotechnol. 2012, 28, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, N.; Vassileva, M.; Bravo, V.; Fernandez-Serrano, M.; Nikolaeva, I. Simultaneous phytase production and rock phosphate solubilization by Aspergillus niger grown on dry olive wastes. Ind. Crops Prod. 2007, 26, 332–336. [Google Scholar] [CrossRef]
- Vazquez, P.; Holguin, G.; Puente, M.; Lopez-cortes, A.; Bashan, Y. Phosphate solubilizing microorganisms associated with the rhizosphere of mangroves in a semi-arid coastal lagoon. Biol. Fertil. Soils 2000, 30, 460–468. [Google Scholar] [CrossRef]
- Koulman, A.; Lee, T.V.; Fraser, K.; Johnson, L.; Arcus, V.; Lott, J.S.; Rasmussen, S.; Lane, G. Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloe festucae in culture and endophyte-infected Lolium perenne. Phytochemistry 2012, 75, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, J.M.; Parker, D.L.; Bargar, J.R.; Jarzecki, A.A.; Tebo, B.M.; Sposito, G.; Duckworth, O.W. Structural dependence of Mn complexation by siderophores, Donor group dependence on complex stability and reactivity. Geochim. Cosmochim. Acta 2012, 88, 106–119. [Google Scholar] [CrossRef]
- Rodrigo, V.; Novelo, E. Seasonal changes in periphyton nitrogen fixation in a protected tropical wetland. Biol. Fertil. Soils 2007, 43, 367–372. [Google Scholar]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Srivastava, R.C. Assembling BNF system in rice plant, frontier areas of research. Curr. Sci. 2013, 104, 3–10. [Google Scholar]
- Dey, H.S.; Tayung, K.; Bastia, A.K. Occurrence of nitrogen-fixing cyanobacteria in local rice fields of Orissa, India. Ecoprint 2010, 17, 77–85. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Siddiqui, Z.A. Use of plant growth-promoting rhizobacteria for the biocontrol of root-rot disease complex of chickpea. Australas. Plant Pathol. 2009, 38, 44–50. [Google Scholar] [CrossRef]
- Widawati, S.; Suliasih, S. Augmentation of potential phosphate solubilizing bacteria (PSB) stimulates the growth of green mustard (Brasica caventis Oed.) in marginal soil. Biodiversitas 2006, 7, 10–14. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Amit, S.; Priyanka, K.; Anju, N.; Ashwani, K. Isolation and Characterization of Phosphate Solublizing Bacteria from Anand Agriculture Soil. Int. J. Life Sci. Pharm. Res. 2012, 23, 256–266. [Google Scholar]
- Kannapiran, E.; Ramkumar, V. Isolation of phosphate Solubilizing bacteria from sediments of Thondi coast, Palk Strait, Southeast coast of India. Ann. Biol. Res. 2011, 25, 157–163. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun-qiao, X.; Ru-an, C.H.I.; Huan, H.E.; Wen-xue, Z.J. Characterization of tricalcium phosphate solubilization by Stenotrophomonas maltophilia YC isolated from pipe mines. J Cent. South Univ. Technol. 2009, 16, 581–587. [Google Scholar]
- Babalola, O.O.; Glick, B.R. The use of microbial inoculants in African agriculture, current practice and prospects. J. Food Agric. Environ. 2012, 10, 540–549. [Google Scholar]
- Alfa, M.I.; Adie, D.B.; Igboro, S.B.; Oranusi, U.S.; Dahunsi, S.O.; Akali, D.M. Assessment of biofertilizer quality and health implications of anaerobic effluent of cow dung and chicken droppings. Renew. Energy 2014, 63, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes, sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.K.; Zhu, Y.G.; Chittleborough, D. Phosphorus release from phosphate rock and an iron phosphate by low-molecular-weight organic acids. J. Environ. Sci. 2004, 16, 5–8. [Google Scholar]
- Park, J.; Bolan, N.; Megharaj, M.; Naidu, R. Isolation of phosphate-solubilizing bacteria and characterization of their effects on lead immobilization. Pedologist 2010, 53, 67–75. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture, exploring Arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.S.; Pathan, M.A.; Gupta, K.P.; Khandkar, U.R. Effect of phosphate solubilizing bacteria at different levels of phosphate on black gram (Phaseolus mungo). Ind. J. Agron. 1993, 38, 131–133. [Google Scholar]
- Chabot, R.; Antoun, H.; Cescas, M.P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant Soil 1996, 184, 311–321. [Google Scholar] [CrossRef]
- Mittal, V.; Singh, O.; Nayyar, H.; Kaur, J.; Tewari, R. Stimulatory effect of phosphate solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol. Biochem. 2008, 40, 718–727. [Google Scholar] [CrossRef]
- Hajra, N.; Shahina, F.; Firoza, K. Biocontrol of root-knot nematode by Arbuscular mycorrhizal fungi in Luffa cylindrical. Pak. J. Nematol. 2013, 31, 77–84. [Google Scholar]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients, Plant Cell Monographs; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 17, pp. 199–224. [Google Scholar]
- Vijay, S.M.; Maurya, B.R.; Jay, P.V. Does a rhizospheric microorganism enhance K+ availability in agricultural soils. Microb. Res. 2014, 169, 337–347. [Google Scholar]
- Troufflard, S.; Mullen, W.; Larson, T.R.; Graham, I.A.; Crozier, A.; Amtmann, A.; Armengaud, P. Potassium deficiency induced the biosynthesis of oxylipins and glucosinolates in Arabiodopsis thaliana. BMC Plant Biol. 2010, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Han, H.S.; Lee, K.D. Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Liu, D.; Lian, B.; Dong, H. Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol. J. 2012, 29, 413–421. [Google Scholar] [CrossRef]
- Sakr, W.R.; Elbagoury, H.M.; Sidky, M.A.; Ali, S.A. Production of organic roselle by natural minerals and biofertilizers. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 985–995. [Google Scholar]
- Sheng, X.F. Growth promotion and increased potassium uptake of cotton and rape by apotassium releasing strain of Bacillus edaphicus. Soil Biol. Biochem. 2005, 37, 1918–1922. [Google Scholar] [CrossRef]
- Amrita, S.; Sunil, K.G. Microbial intervention in agriculture, An overview. Afr. J. Microbiol. Res. 2015, 9, 1215–1226. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y. Solubilization of potassium bearing minerals by a wild type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.B.; Biswas, D.R. Co-inoculation of potassium solubilizing and nitrogen-fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol. Fertil. Soils 2010, 46, 641–648. [Google Scholar] [CrossRef]
- Singh, G.; Biswas, D.R.; Marwah, T.S. Mobilization of potassium from waste mica by plant growth-promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.). J. Plant Nutr. 2010, 33, 1236–1251. [Google Scholar] [CrossRef]
- Hayes, J.E.; Richardson, A.E.; Simpson, R.J. Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biol. Fertil. Soils 2000, 32, 279–286. [Google Scholar] [CrossRef]
- Stamford, N.P.; Santos, P.R.; Moura, A.M.; Santos, C.E.S.; Freitas, A.D.S. Biofertilizer with natural phosphate.; sulfur.; and Acidithio bacillus in a soil with low available-P. Sci. Agric. 2003, 60, 767–773. [Google Scholar] [CrossRef]
- Yang, Z.H.; Stoven, K.; Haneklaus, S.; Singh, B.R.; Schnug, E. Elemental sulfur oxidation by Thiobacillus spp. and aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere 2010, 20, 71–77. [Google Scholar] [CrossRef]
- Costa, A.C.A.; Medronhe, R.A.; Pecanha, R.P. Phosphate rock bioleaching. Biotechnol. Lett. 1992, 14, 233–238. [Google Scholar] [CrossRef]
- Shinde, D.B.; Patil, P.L.; Patil, B.R. Potential use of sulphur oxidizing microorganism as soil inoculant. Crop Res. 1996, 11, 291–295. [Google Scholar]
- Bapiri, A.; Asgharzadeh, A.; Mujallali, H.; Khavazi, K.; Pazira, E. Evaluation of zinc solubilization potential by different strains of Fluorescent Pseudomonads. J. Appl. Sci. Environ. Manag. 2012, 16, 295–298. [Google Scholar]
- Saravanan, V.S.; Kalaiarasan, P.; Madhaiyan, M.; Thangaraju, M. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett. Appl. Microb. 2007, 44, 235–241. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching, metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Bullen, P.; Kemila, A.P.F. Influence of pH on the toxic effect of zinc.; cadmium and pentachlorophenol on pure cultures of soil microorganisms. Environ. Toxicol. Chem. 1997, 16, 146–153. [Google Scholar]
- Zayed, M.S. Improvement of growth and nutritional quality of Moringa oleifera using different biofertilizers. Ann. Agric. Sci. 2012, 57, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress, a survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Podile, A.R. Plant growth-promoting rhizobacteria (PGPR), Bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O. Does nature make provision for backups in the modification of bacterial community structures? Biotechnol. Genet. Eng. Rev. 2014, 30, 31–48. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, Y.F.; Ma, H.Y.; Su, L.N.; Chen, Z.J.; Wang, Q.Y.; Meng, Q.; Fang, S.X. Characterization of copper resistant bacteria and assessment of bacterial communities in rhizosphere soils of copper-tolerant plants. Appl. Soil Ecol. 2010, 44, 49–55. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Evaluation of plant growth-promoting activities of rhizobacterium Pseudomonas putida under herbicide-stress. Ann. Microbiol. 2012, 62, 1531–1540. [Google Scholar] [CrossRef]
- Frebort, I.; Kowalska, M.; Hluska, T.; Frebortova, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth-promoting activities. Microb. Res. 2006, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Implications of bacterial resistance against heavy metals in bioremediation, a review. IIOABJ 2012, 3, 39–46. [Google Scholar]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Manero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Perrig, D.; Boiero, M.L.; Masciarelli, O.A.; Penna, C.; Ruiz, O.A.; Cassan, F.D.; Luna, M.V. Plant growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense and implications for inoculant formulation. Appl. Microbiol. Biotechnol. 2007, 75, 1143–1150. [Google Scholar] [CrossRef]
- Cassan, F.; Perrig, D.; Sgroy, V.; Masciarelli, O.; Penna, C.; Luna, V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination.; promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur. J. Soil Biol. 2009, 45, 28–35. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Ding, Z.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef] [Green Version]
- Khare, E.; Arora, N.A. Effect of indole-3-acetic acid (IAA) produced by Pseudomonas aeruginosa in the suppression of charcoal rot disease of chickpea. Curr. Microbiol. 2010, 61, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Ribaudo, C.M.; Krumpholz, E.M.; Cassan, F.D.; Bottini, R.; Cantore, M.L.; Cura, J.A. Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J. Plant Growth Reg. 2006, 25, 175–185. [Google Scholar] [CrossRef]
- Yaxley, J.R.; Ross, J.J.; Sherriff, L.J.; Reid, J.B. Gibberellin biosynthesis mutations and root development in pea. Plant Physiol. 2001, 125, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, N.K.; Tewari, S.; Singh, R. Multifaceted Plant-Associated Microbes and Their Mechanisms Diminish the Concept of Direct and Indirect PGPRs. In Plant-Microbe Symbiosis, Fundamentals and Advances; Arora, N.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 411–449. [Google Scholar]
- Cornelis, P. Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol. 2010, 86, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Esuola, C.O.; Babalola, O.O.; Heine, T.; Schwabe, R.; Schlomann, M.; Tischler, D. Identification and characterization of a FAD-dependent putrescine N-hydroxylase (GorA) from Gordonia rubripertincta CWB2. J. Mol. Catal. B Enzym. 2016, 134, 378–389. [Google Scholar] [CrossRef]
- Dimkpa, C.; Svatos, A.; Merten, D.; Buchel, G.; Kothe, E. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can. J. Microbiol. 2008, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Gouveia, C.; Dias, T.; Varma, A.; Babalola, O.O. How to disentangle changes in microbial function from changes in the microbial community. In Modern Tools and Techniques to Understand Microbes; Varma, A., Sharma, A.K., Eds.; Springer International Publishing AG: Basel, Switzerland, 2017; pp. 149–158. [Google Scholar]
- Choudhary, R.; Shrivastava, S. Mechanism of zinc resistance in Pseudomonas putida strain S4. World J. Microbiol. Biotechnol. 2001, 17, 149–153. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants, Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallik, E.; Sarig, S.; Okon, Y. Morphology and physiology of plant roots associated with Azospirillum. In Azospirillum Plant Associations; Okon, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 77–86. [Google Scholar]
- Abdul, M.; Elisabeth, G.; Madalena, A. Management of microbial resources in the Environment: A Broad Perspective. In Management of Microbial Resources in the Environment; Springer: Dordrecht, The Netherlands, 2013; Volume 530. [Google Scholar]
- Denton, B.P. Advances in phytoremediation of heavy metals using plant growth-promoting bacteria and fungi. MMG 445 Basic Biotechnol. 2007, 3, 1–5. [Google Scholar]
- Loper, J.E.; Gross, H. Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. Eur. J. Plant Pathol. 2007, 119, 265–278. [Google Scholar] [CrossRef]
- Notz, R.; Maurhofer, M.; Schnider-Keel, U.; Duffy, B.; Haas, D.; Defago, G. Biotic factors affecting the expression of the 2, 4-diacetylphloroglucinol biosynthesis gene phlA in Pseudomonas fluorescens biocontrol strain CHA0 in the rhizosphere. Phytopathology 2001, 91, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Haas, D.; Defago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Abizgildina, R.R.; Pusenkova, L.I. Plant growth-promoting rhizobacteria as an alternative to chemical crop protectors from pathogens (Review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S.; Sheikh, I.H.; Khan, A. Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World J. Microbiol. Biotechnol. 2006, 22, 641–650. [Google Scholar] [CrossRef]
- Lanteigne, C.; Gadkar, V.J.; Wallon, T.; Novinscak, A.; Filion, M. Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology 2012, 102, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Koch, B.; Nielsen, T.H.; Sorensen, D.; Andersen, J.B.; Christophersen, C.; Molin, S.; Givskov, M.; Sorensen, J.; Nybroe, O. Lipopeptide production in Pseudomonas sp. strain DSS73 is regulated by components of sugar beet seed exudate via the Gac two-component regulatory system. Appl. Environ. Microbiol. 2002, 68, 4509–4516. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar]
- Ben Rebah, F.B.; Tyagi, R.D.; Prevost, D. Wastewater sludge as a substrate for growth and carrier for rhizobia, the effect of storage conditions on survival of Sinorhizobium meliloti. Bioresour. Technol. 2002, 83, 145–151. [Google Scholar] [CrossRef]
- Stephens, J.H.; Rask, H.M. Inoculant production and formulation. Field Crops Res. 2000, 65, 249–258. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Castro, I.V. Residues of the cork industry as carriers for the production of legumes inoculants. Silva Lusit. 2005, 13, 159–167. [Google Scholar]
- Bashan, Y.; de-Bashan, L.E. Bacteria. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 103–115. [Google Scholar]
- Tilak, K.V.B.R. Survival of Azospirillum brasilense in different carriers. Curr. Sci. 1979, 48, 412. [Google Scholar]
- Tilak, K.V.B.R.; Subba Rao, N.S. Carries for legume inoculants. Fert. News 1978, 23, 25–28. [Google Scholar]
- Sparrow, S.D.; Han, G.E. Survival of Rhizobium phaseoli in six carrier materials. Agron. J. 1981, 75, 181–184. [Google Scholar] [CrossRef]
- Singaravadivel, K.; Anthoni Raj, S. Rice mill by products as carrier for Rhizobium. Legume Res. 1988, 11, 143–145. [Google Scholar]
- Saxena, S.; Pandey, A.K. Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl. Microbiol. Biotechnol. 2001, 55, 395–403. [Google Scholar] [CrossRef]
- EPA. Ingredients Used in Pesticide Products: Pesticides. What Are Biopesticides? Available online: https://www.epa.gov/ingredients-used-pesticide-products/what-are-biopesticides (accessed on 10 May 2021).
- Cheng, X.L.; Liu, C.J.; Yao, J.W. The current status, development trend, and strategy of the bio-pesticide industry in China. Hubei Agric. Sci. 2010, 49, 2287–2290. [Google Scholar]
- Leahy, J.; Mendelsohn, M.; Kough, J.; Jones, R.; Berckes, N. Biopesticide oversight and registration at the U.S. Environmental Protection Agency. In Biopesticides, State of the Art and Future Opportunities; Seiber, J.N., Coats, J., Duke, S.O., Gross, A.D., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Bailey, K.L.; Mupondwa, E.K. Developing microbial weed control products, commercialization, biological and technological considerations. In Handbook of Sustainable Weed Management; Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; The Haworth Press Inc.: Binghamton, NY, USA, 2006; pp. 431–473. [Google Scholar]
- Lacey, L.A.; Liu, T.X.; Buchman, J.L.; Munyaneza, J.E.; Goolsby, J.A.; Horton, D.R. Entomopathogenic fungi (Hypocreales) for control of potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera, Triozidae) in an area endemic for zebra chip disease of potato. Biol. Control 2011, 56, 271–278. [Google Scholar] [CrossRef]
- Jisha, V.N.; Smitha, R.B.; Benjamin, S.; Al, E.T. An overview on the crystal toxins from Bacillus thuringiensis. Adv. Microbiol. 2013, 3, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007, 17, 547–549. [Google Scholar] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Schunemann, R.; Knaak, N.; Fiuza, L.D. Mode of action and specificity of Bacillus thuringiensis toxins in the control of caterpillars and stink bugs in soybean culture. ISRN Microbiol. 2014, 2014, 135675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro-Ilan, D.I.; Gouge, D.H.; Piggott, S.J.; Fife, P.J. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol. Control 2006, 38, 124–133. [Google Scholar] [CrossRef]
- Szewczyk, B.; Lobo de Souza, M.; Batista de Castro, M.L.; Moscardi, M.L.; Moscardi, F. Baculovirus biopesticides. In Pesticides—Formulations, Effects, Fate; Stoytcheva, M., Ed.; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Moscardi, F.; Morales, L.; Santos, B. The successful use of AgMNPV for the control of velvetbean caterpillar, Anticarsia gemmatalis, in soybean in Brazil. In Proceedings of the VIII International Colloquium on Invertebrate Pathology and Microbial Control Embrapa Soja, Londrina, Brazil, 28 August 2002; pp. 86–91. [Google Scholar]
- Bellinger, R.G. Organic Pesticides and Biopesticides, Clemson Extension, Home and Garden Information Center (HGIC). 2007. Available online: http://www.clemson.edu/extension/hgic (accessed on 15 May 2012).
- Quarles, W. New biopesticides for IPM and organic production. IPM Pract. 2011, 13, 7–8. [Google Scholar]
- Thakore, Y. The biopesticide market for global agricultural use. Ind. Biotechnol. 2006, 2, 194–208. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant-microbe interactions promoting plant growth and health, perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 14, 11–18. [Google Scholar] [CrossRef]
- Afify, A.M.R.; Aboul-Soud, M.A.M.; Foda, M.S.; Sadik, M.W.A.; Kahil, T.; Asar, A.R.; Al-Khedhairy, A.A. Production of alkaline protease and larvicidal biopesticides by an Egyptian Bacillus sphaericus isolate. Afr. J. Biotechnol. 2009, 8, 3864–3873. [Google Scholar]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Kohl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Biopesticides acquire mainstream status. Agrow World Crop. Prot. News 2013, 662, 1–5. [Google Scholar]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.; Soberon, M. Bacillus thuringiensis, a story of a successful bio-insecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect Pathogens as Biological Control Agents, Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Sunitha, V.; Lakshmi, K.V.; Rao, G.V.R. Laboratory evaluation of certain insecticides against pigeonpea pod borer, Maruca vitrata. J. Food Legumes 2008, 21, 137–139. [Google Scholar]
- Langewald, J.; Ouambama, Z.; Mamadou, A.; Peveling, R.; Stolz, I.; Bateman, R. Comparison of an organophosphate insecticide with a mycoinsecticide for the control of Oedaleus senegalensis Krauss (Orthoptera, Acrididae) and other Sahelian grasshoppers in the field at the operational scale. Biocontrol Sci. Technol. 1999, 9, 199–214. [Google Scholar] [CrossRef]
- Uribe, D.; Khachatourians, G.G. Restriction fragment length polymorphism of mitochondrial genome of the entomopathogenic fungus Beauveria bassiana reveals high intraspecific variation. Mycol. Res. 2004, 108, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.J.; Murray, S.L.; Duncan, A.; Khew, G.S.; Davis, M.A. Regulatory genes controlling fatty acid catabolism and peroxisomal functions in the filamentous fungus Aspergillus nidulans. Eukaryot. Cell 2006, 5, 794–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatsuka, J. Specific PCR assays for the detection of DNA from Beauveria bassiana F-263, a highly virulent strain affecting Japanese pine sawyer, Monochamus alternates (Coleoptera, Cerambycidae), by a sequence characterized amplified region (SCAR) marker. Appl. Entomol. Zool. 2007, 42, 619–628. [Google Scholar] [CrossRef]
- Faria, M.R.; Wright, S.P. Mycoinsecticides and mycoacaricides, a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Butt, T.M.; Jackson, C.W.; Magan, N. Fungi as Biocontrol Agents, Progress, Problems, and Potential; CABI: Swansea, UK, 2001; ISBN 9780851993560. [Google Scholar] [CrossRef]
- Leemon, D.M.; Jonsson, N.N. Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J Invertebr. Pathol. 2008, 97, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Koppenhofer, A.M.; Kaya, H.K. Entomopathogenic nematodes and insect pest management. In Microbial Biopesticides; Koul, O., Dhaliwal, G.S., Eds.; Taylor & Francis: London, UK, 2002; pp. 277–305. [Google Scholar]
- Lewis, L.C. Protozoan Control of Pests. In Encyclopedia of Pest Management; Pimental, D., Ed.; Taylor & Francis: London, UK, 2002; pp. 673–676. [Google Scholar]
- Cranshaw, W.; Hammon, R. Grasshopper control in gardens and small acreages. Colo. State Univ. Ext. Bull. 2013, 5, 536. Available online: http://www.ext.colostate.edu/pubs/insect/05536.html/ (accessed on 15 October 2021).
- Szewczyk, B.; Rabalski, L.; Krol, E.; Sihler, W.; de Souza, M.L. Baculovirus biopesticides-a safe alternative to chemical protection of plants. J. Biopestic. 2009, 2, 209–216. [Google Scholar]
- Van Regenmortel, M.H.V.; Fauquet, C.M.; Bishop, D.H.L.; Cartens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: San Diego, CA, USA, 2000; p. 1162. ISBN 0123702003. [Google Scholar]
- Mazid, S.; Kalita, J.C.; Rajkhowa, R.C. A review on the use of biopesticides in insect pest management. Int. J. Sci. Adv. Technol. 2011, 1, 169–178. [Google Scholar]

| Biofertilizer | PGPR | Biopesticide | References |
|---|---|---|---|
| Rhizobium, Azotobacter, Azospirillum brasilense, Azospirillum lipoferum, Azotobacter chroococcum, Acetobacter diazotrophicus, Bacillus licheniformis, B. megaterium, B. mucilagenosus, B. edaphicus, B. subtilis, Actinomyces, Streptomyces, Herbaspirillum seropedicae, Rhizobium phaseoli, Thiobacillus thioxidans, Glomus fasciculatum, Blue Green Algae (BGA), and Azolla. | Acetobacter, Aeromonas hydrophila, Azotobacter, Achromobacter, Alcaligenes, Anabaena, Arthrobacter, Azoarcus, Azospirillum brasilense, A. irakense, A. lipoferum, Azotobacter, Acinetobacter calcoaceticus, A. baumannii, Bacillus polymyxa, Beijerinckia, Burkholderia gladioli, Burkholderia cepacia, Clostridium, Derxia, Enterobacter, Erwinia spp., Ewingella americana, Escherichia vulneris, Flavobacterium, Frankia, Gluconacetobacter, Klebsiella, Mycobacterium phlei, Proteus penneri, Pseudomonas fluorescens, P. luteola, P. alcaligenes, P. putida, Rhizobium leguminosarum, Rahnella aquatilis, Serratia plymuthica, S. ficaria, Sinorhizobium, Shigella spp., Vibrio fluvialis, and Zoogloea | Bacillus thuringiensis, B. thuringiensis var. kurstaki (Bt), B. thuringiensis var. israelensis (Bt), B. thuringiensis var. tenebrionis, B. thuringiensis var. aizawai, B. thuringiensis japonensis, B. popilliae, B. lentimorbus, B. sphaericus, B. pumilus, B. subtilis, B. firmus, Burkholderia cepacia, B. amyloliquefaciens, B. licheniformis, Erwinia amylovora, Pasteuria penetrans, Pasteuria usage, Pseudomonas spp., Streptomyces griseoviridis, and Xanthomonas campestris pv. poannua, | [22,23,24,25] |
| Types | Bacteria | Fungi/VAM | Actinomycetes | Cyanobacteria/Yeast | References |
|---|---|---|---|---|---|
| PSM * | Alcaligenes sp., Aerobacter aerogenes, Achromobacter sp., Actinomadura oligospora, Agrobacterium sp., Azospirillum brasilense, Bacillus circulans, B.cereus, B.fusiformis, B. pumilus, B. megaterium, B. mycoides, B. polymyxa, B. coagulans, B.chitinolyticus, B. subtilis, Bradyrhizobium sp., Brevibacterium sp., Citrobacter sp., Pseudomonas putida, P. striata, P. fluorescens, P. calcis, P. corrugate, Flavobacterium sp., Nitrosomonas sp., Erwinia sp., Micrococcus sp., Escherichia intermedia, Enterobacter asburiae, Serratia phosphoticum, Nitrobacter sp., Thiobacillus ferroxidans, T. thioxidans, Rhizobium meliloti, and Xanthomonas sp. | Aspergillus awamori, A. niger, A. terreus, A. flavus, A. nidulans, A. foetidus, A. wentii, Fusarium oxysporum, Alternaria teneius, Achrothcium sp., Penicillium digitatum, P. lilacinium, P. balaji, P. funicolosum, Cephalosporium sp., Cladosprium sp., Curvularia lunata, Cunnighamella, Candida sp., Chaetomium globosum, Humicolainslens, H. lanuginosa, Helminthosporium sp., Paecilomycesfusisporous, Pythium sp., Phoma sp., Populosporamytilina, Myrotheciumroridum, Morteirella sp., Micromonospora sp., Oideodendron sp., Rhizoctonia solani, Rhizopus sp., Mucor sp., Trichoderma viridae, Torula thermophila, Schwanniomyces occidentalis, and Sclerotium rolfsii. Glomus fasciculatum (VAM) | Actinomyces sp. and Streptomyces sp. | Anabaena sp., Calothrix braunii, Nostoc sp., and Scytonema sp., | [22,34,35,37,38,39,40,41,42,43,44,45] |
| SSM * | Acidothiobacillus, Thiomicrospira, Thiosphaera, Paracoccus, Xanthobacter, Alcaligenes, Pseudomonas, Thiobacillus thiooxidans, T. ferrooxidans, T. thioparus, T. denitrificans, and T. novellus | Aureobasidium, Epicoccum, Penicillium, Aspergillus, Alternariatenuis, Aureobasidiumpullulans, Epicoccumnigrum, Scolecobasidiumconstrictum, and Myrotheciumcinctum | |||
| NO3 * | Azospirillum lipoferum, A. brasilense, Azoarcus, Azotobacter chroococcum, A. peroxydans, A. nitrogenifigens, Rhizobium, Bradyrhizobium, Sinorhizobium, Azorhizobium, Mesorhizobium, H. seropedicae, H. rubrisubalbicans Burkholderia sp., Rhizobium leguminosarum bv. trifolii, B. vietnamiensis, Gluconacetobacterkombuchae, G. johannae, G. azotocaptans, G. diazotrophicus, and Swaminathania salitolerans | Acidothermus cellulolyticus | Cylindrospermum musicola and Anabaena azollae | ||
| Siderophore | Bacillus sp., Ochrobactrum, Kluyvera ascorbata, Salmonella, Enterobacter, Yersinia, Mycobacterium, B. megaterium, Ochrobactrum anthropi, Proteus vulgaris, Pseudomonas fluorescence, P. putida, Escherichia coli, Salmonella, Klebsiella pneumoniae, Vibrio cholerae, V. anguillarum, Aeromonas, Aerobacter aerogenes, Yersinia, and Mycobacterium | Aspergillus nidulans, A. versicolor, Penicillium chrysogenum, P. citrinum, Mucor, Rhizopus, Trametes versicolor, Ustilago sphaerogina, Debaromyces sp., and Rhodotorula minuta | Nocardia asteroids, Streptomyces griseus, and Actinomadura madurae | Saccharomyces cerevisiae (Yeast) | |
| ZSB * | Bacillus subtilis, Gluconacetobacter diazotrophicus, Thiobacillus thioxidans, and T. ferroxidans | Aspergillus niger and Penicillium luteum | Saccharomyces sp. (Yeast) |
| Microorganisms | Acids | References |
|---|---|---|
| Bacillus pumils, B. subtilis, B. licheniformis, B. megaterium BHUPSB14, and Paenibacillus polymyxa | Gibberellins, Ethylene, Cytokinin, and ACC deaminase | [44,45,100] |
| Pseudomonas tabaci, P. putida, P. syringae, P. fluorescens, P. fluorescens G20-18, P. fluorescens BHUPSB06, P. aeruginosa, P. cepacia, and P. corrugata | Ethylene, Indole-3-acetic acid, Cytokinin, and ACC deaminase | |
| Rhizobium leguminosarum | Indole-3-acetic acid, Cytokinin, and HCN | |
| Azospirillum brasilense and A. lipoferum, | Indole-3-acetic acid, Zeatin, and ethylene, Gibberellic acid (GA3), and Abscisic acid (ABA) | |
| Rhizobacterial isolates | Auxins | |
| Aeromonas veronii, Agrobacterium sp., Bradyrhizobium sp., Comamonas acidovorans, Azotobacter chroococcum, Mesorhizobium ciceri, Azospirillum amazonense, Rhizobium sp., Azotobacter sp., Kebsiellaoxytoca, Erwinia herbicola, Bacillus subtilis, Serratia marcescens, and Enterobacter asburiae | Indole-3-acetic acid | |
| Alcaligenes piechaudii and Enterobacter cloacae | Indole-3-acetic acid, ACC deaminase | |
| Variovorax paradoxus | ACC deaminase | |
| Pantoea agglomerans and Pantoea herbicola | IAA and Auxin | |
| Gluconobacter diazotrophicus | GA3, indole-3-acetic acid, and gibberellin GA1 |
| Microorganisms | Enzymes | Acids | References |
|---|---|---|---|
| Bacillus circulans, B.cereus, B. fusiformis, B.pumilus var.2, B. megaterium, B. mycoides, B. polymyxa, B. coagulans B. chitinolyticus, B. subtilis, B. subtilisvar.2, B. licheniformis, B. amyloliquefaciens, B. atrophaeus, Paenibacillus macerans, and B. japonicum | Phytase and D-a-glycerophosphate | Lactic, malic, citric, itaconic, isovaleric, isobutyric, acetic, gluconic, propionic, heptonic, Caproic, Isocaproic, Formic, valeric, succinic, Oxalic, oxalacetic, malonic, and IAA | [95,100,101] |
| Bradyrhizobium sp., | Phytate | IAA | |
| Burkholderia cepacia, Citrobacter sp., and Citrobacter freundii | Acid phosphatase | Gluconic acid | |
| Escherichia intermedia and E. freundii | - | Lactic | |
| Enterobacter asburiae, E. aerogenes, E. cloacae, E. aerogenes, and E. intermedium | Acid phosphatase | Lactic, itaconic, isovaleric, isobutyric, acetic, 2-ketogluconic, gluconic, succinic, acetic, glutamic, oxaloacetic, pyruvic, malic, fumaric, and alpha-ketoglutaric | |
| Pseudomonas putida, P. striata, P. fluorescens, P. calcis, P. mendocina, and P. aeruginosa | Acid phosphatase, Phytase, and Phosphonoacetate hydrolase | Lactic, malic, citric, gluconic, 2-ketogluconic acid, and tartaric | |
| Proteus mirabilis | Acid phosphatase | ||
| Serratia phosphoticum and S. marcescens | Acid phosphatase | Gluconic acid and IAA | |
| Rhizobium meliloti, R. leguminosarum, R. leguminosarum bv.phaseoli, R. leguminosarum bv. Trifolii, and R. leguminosarum bv. Viciae | Phytate | 2-ketogluconic acid, HCN, and IAA | |
| Klebsiella aerogenes | C-P Lyase | ||
| Sinorhizobium meliloti | Phytate | IAA, malic, succinic, and fumaric | |
| Stenotrophomonas maltophilia | Gluconic acid | ||
| Mesorhizobium cireri and M. mediterraneum | Phytate | ||
| Acetobacter sp. | Gluconic acid |
| Materials | Category | Reference |
|---|---|---|
| Preservative and Culture media ( liquid and powder) | Bacterial cultures (lyophilized) | Bashan and de-Bashan, [129] |
| Alginate and xanthan gum | Biopolymer | |
| Black ash, paddy husk, black ash plus husk mixture, husk powder and pressmud, soybean and peanut oils, farmyard manure, plant debris, wheat bran, composts, spent mushroom composts, sugar industry waste, agricultural waste material, soybean meal, coconut shell powder, and teak leaf powder | Waste materials (Plant) | |
| Lignite, pressmud, charcoal, inorganic soil, coal, clays and peat | Soils | |
| Carrageenan, polyacrylamide, calcium sulfate, polysaccharide-like alginate, ground rock phosphate, vermiculite, and perlite | Inert materials |
| Micro Organisms | Pest Control | Weed Control | Plant Disease Control | Nematicides Control | Fungicides | Reference |
|---|---|---|---|---|---|---|
| Bacteria | Bacillus thuringiensis, B. thuringiensis var. kurstaki, B. thuringiensis var. israelensis, B. thuringiensis var. tenebrionis, B. thuringiensis var. aizawai, B. thuringiensis japonensis, B. popilliae, B. lentimorbus, B. sphaericus, Erwinia amylovora, and B. pumilus | Xanthomonas campestris pv. Poannua | Bacillus pumilus, B. subtilis, Pseudomonas spp., Streptomyces griseoviridis, and Burkholderia cepacia | Bacillus firmus, Pasteuria penetrans, and Pasteuria usage | Bacillus amyloliquefaciens, B. licheniformis, B. pumilus, and B. subtilis | [24,25,137,140,141,142,143,144,145,146]. |
| Fungi | Beauveria bassiana, Metarhizium anisopliae, Entomophaga, Zoopthora, Paecilomyces fumosoroseus, Normuraea, Lecanicillium lecanii, L. longisporum, Lagenidium giganteum, and Verticillium lecanii | Colletotrichum gloeosporioides, Chondrostereum purpureum and Cylindrobasidium laeve | Ampelomyces quisqualis, Candida sp., Clonostachys rosea f. catenulate, Coniothyrium minitans, Pseudozyma flocculosa, Trichoderma harzianum, T. koningii, T. viride, and Chaetomium cupreum | Paecilomyces lilacinus, Myrothecium verrucaria, Verticillium chlamydosporium, and Pochonia chlamydosporia | ||
| Protozoa | Nosema locustae, Thelohania, and Vairimorpha | |||||
| Nematodes | Steinernema feltiae, S. carpocapsae, S. glaseri, S. riobravis, and Heterorhabditis heliothidis | |||||
| Virus | Tussock moth NPV, Pine sawfly NPV, Granulosis viruses, Codling moth granulosis virus (GV), Gypsy moth nuclear polyhedrosis (NPV), Nuclear polyhedrosis viruses, non-occluded baculoviruses, Adoxophyes orana granulovirus (GV)+ Homona magnanima GV, Cydia pomonella granulovirus, Nucleopolyhedrovirus Neodiprion abietis, Heliothis zea NPV, Anagrapha falcifera NPV, Spodoptera exigua NPV, Mamestra configurata NPV, Ectropis obliqua hypulina NPV, Laphygma exigua NPV, Prodenia litura NPV, Buzura suppressaria NPV, Gynaephora ruoergensis NPV, Mythimna separata NPV, Periplaneta fuliginosa densovirus virus, Pieris rapae GV, Mythimna separata GV, and Plutella xylostella GV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. https://doi.org/10.3390/biology10111111
Seenivasagan R, Babalola OO. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology. 2021; 10(11):1111. https://doi.org/10.3390/biology10111111
Chicago/Turabian StyleSeenivasagan, Renganathan, and Olubukola Oluranti Babalola. 2021. "Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product" Biology 10, no. 11: 1111. https://doi.org/10.3390/biology10111111
APA StyleSeenivasagan, R., & Babalola, O. O. (2021). Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology, 10(11), 1111. https://doi.org/10.3390/biology10111111