Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DKPs 1–4
2.3. Purification of DKPs 2–3
2.4. Single-Crystal X-ray Diffraction
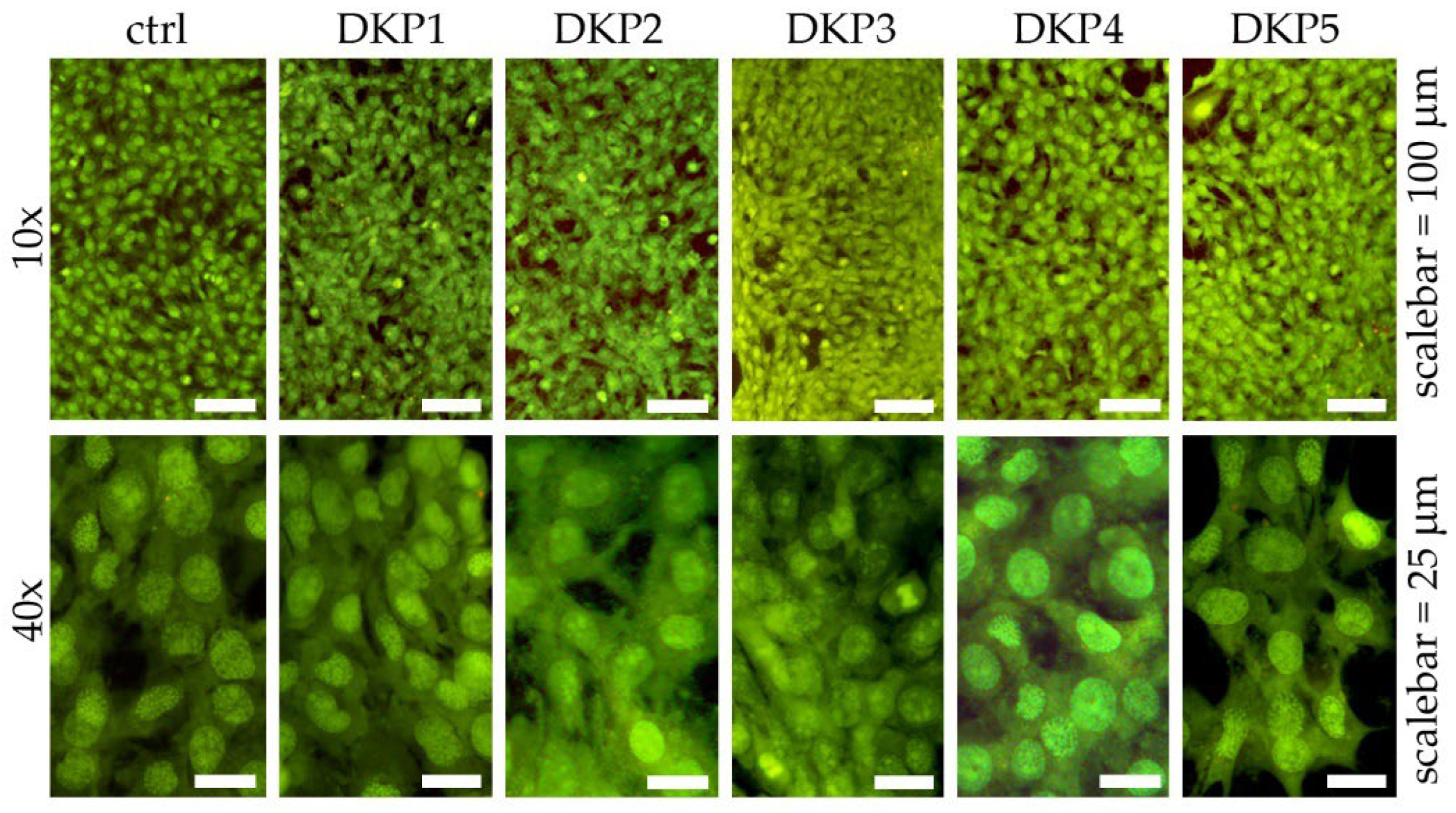
2.5. Live/Dead Cytotoxicity Assay
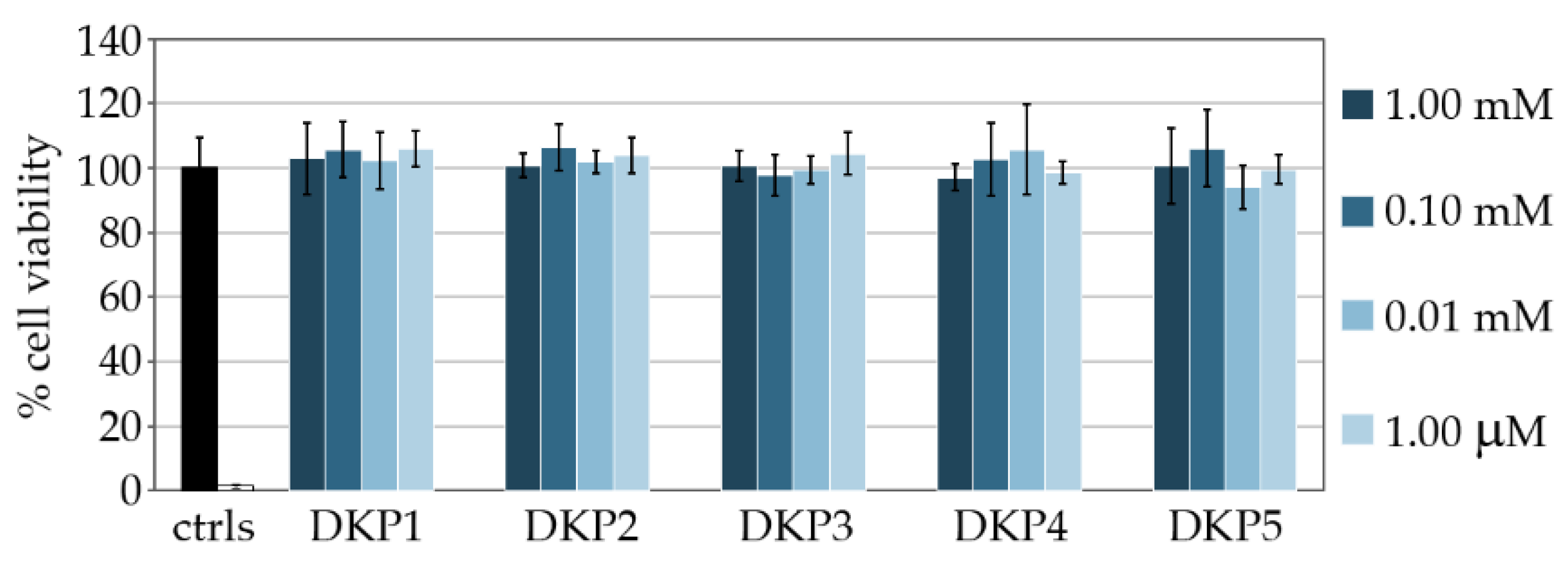
2.6. MTT Cytotoxicity Assay
2.7. Skin Absorption Study
2.7.1. Skin Membrane Preparation
2.7.2. Donor Phase Preparation
2.7.3. In Vitro Permeation and Retention Study
2.7.4. Collection and Treatment of Samples
2.7.5. DKP Quantification by LC-MS
2.7.6. Kp Calculation and Statistical Analysis
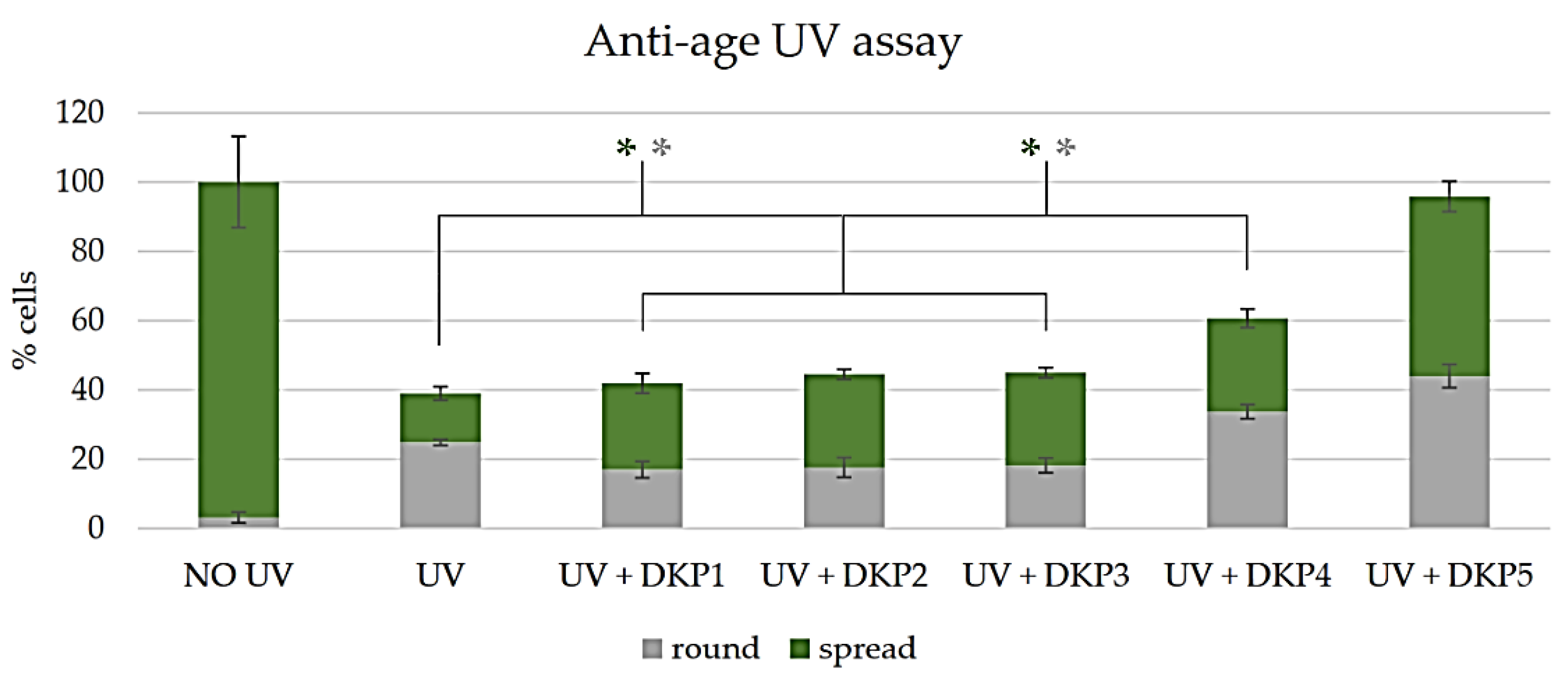
2.8. Anti-Age Assay
3. Results and Discussion
3.1. Synthesis and Characterization of DKPs 1–4
3.2. Single-Crystal X-ray Diffraction Analysis
3.3. Cytocompatibility Assays In Vitro
3.4. Skin Absorption Studies of DKPs
3.5. Anti-Age Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harken, L.; Li, S.M. Modifications of diketopiperazines assembled by cyclodipeptide synthases with cytochrome p(450) enzymes. Appl. Microbiol. Biotechnol. 2021, 105, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Scarel, M.; Marchesan, S. Diketopiperazine gels: New horizons from the self-assembly of cyclic dipeptides. Molecules 2021, 26, 3376. [Google Scholar] [CrossRef]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and biological activities of diketopiperazines from marine organisms: A review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Balachandra, C.; Padhi, D.; Govindaraju, T. Cyclic dipeptide: A privileged molecular scaffold to derive structural diversity and functional utility. ChemMedChem 2021, 16, 2558–2587. [Google Scholar] [CrossRef] [PubMed]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic dipeptides: The biological and structural landscape with special focus on the anti-cancer proline-based scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lee, H.J. Absorption of peptide and peptidomimetic drugs. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 321–341. [Google Scholar] [CrossRef]
- Safiullina, A.S.; Buzyurov, A.V.; Ziganshina, S.A.; Gerasimov, A.V.; Schick, C.; Gorbatchuk, V.V.; Ziganshin, M.A. Using fast scanning calorimetry to study solid-state cyclization of dipeptide l-leucyl-l-leucine. Thermochim. Acta 2020, 692, 178748. [Google Scholar] [CrossRef]
- Bojarska, J.; Wolf, W.M. Ultra-short cyclo-peptides as bio-inspired therapeutics: Proline-based 2,5-diketopiperazines (dkp). Proceedings 2021, 79, 10. [Google Scholar]
- Ressurreição, A.S.M.; Delatouche, R.; Gennari, C.; Piarulli, U. Bifunctional 2,5-diketopiperazines as rigid three-dimensional scaffolds in receptors and peptidomimetics. Eur. J. Org. Chem. 2011, 2011, 217–228. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- André, A.; Touré, A.K.; Stien, D.; Eparvier, V. 2,5-diketopiperazines mitigate the amount of advanced glycation end products accumulated with age in human dermal fibroblasts. Int. J. Cosm. Sci. 2020, 42, 596–604. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Derm. Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Iannuzzi, C. Understanding the role of protein glycation in the amyloid aggregation process. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and progression of non-alcoholic fatty liver disease: The role of advanced glycation end products. Int. J. Mol. Sci. 2019, 20, 5037. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Szydełko, A.; Kotyra, Ł.; Lewandowski, Ł.; Gamian, A.; Kustrzeba-Wójcicka, I. Role of advanced glycation end-products and other ligands for age receptors in thyroid cancer progression. J. Clin. Med. 2021, 10, 4084. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Steenbeke, M.; Speeckaert, R.; Desmedt, S.; Glorieux, G.; Delanghe, J.R.; Speeckaert, M.M. The role of advanced glycation end products and its soluble receptor in kidney diseases. Int. J. Mol. Sci. 2022, 23, 3439. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Romanelli, M.M.C. Sarcopenia in chronic kidney disease: Focus on advanced glycation end products as mediators and markers of oxidative stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- López-Laguna, H.; Voltà-Durán, E.; Parladé, E.; Villaverde, A.; Vázquez, E.; Unzueta, U. Insights on the emerging biotechnology of histidine-rich peptides. Biotechnol. Adv. 2022, 54, 107817. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine meets fenton chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wester, R.C.; Melendres, J.; Sedik, L.; Maibach, H.; Riviere, J.E. Percutaneous absorption of salicylic acid, theophylline, 2, 4-dimethylamine, diethyl hexyl phthalic acid, and p-aminobenzoic acid in the isolated perfused porcine skin flap compared to man in vivo. Toxicol. Appl. Pharmacol. 1998, 151, 159–165. [Google Scholar] [CrossRef]
- Simon, G.A.; Maibach, H.I. The pig as an experimental animal model of percutaneous permeation in man: Qualitative and quantitative observations--an overview. Ski. Pharmacol. Appl. Ski. Physiol. 2000, 13, 229–234. [Google Scholar] [CrossRef]
- Magnano, G.C.; Marussi, G.; Pavoni, E.; Adami, G.; Filon, F.L.; Crosera, M. Percutaneous metals absorption following exposure to road dust powder. Environ. Pollut. 2022, 292, 118353. [Google Scholar] [CrossRef]
- Guth, K.; Schäfer-Korting, M.; Fabian, E.; Landsiedel, R.; van Ravenzwaay, B. Suitability of skin integrity tests for dermal absorption studies in vitro. Toxicol. Vitr. 2015, 29, 113–123. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies. 2004. Available online: https://doi.org/10.1787/9789264078796-en (accessed on 25 August 2022). [CrossRef]
- Pokorna, A.; Bobal, P.; Oravec, M.; Rarova, L.; Bobalova, J.; Jampilek, J. Investigation of permeation of theophylline through skin using selected piperazine-2,5-diones. Molecules 2019, 24, 566. [Google Scholar] [CrossRef]
- Kurbasic, M.; Semeraro, S.; Garcia, A.M.; Kralj, S.; Parisi, E.; Deganutti, C.; De Zorzi, R.; Marchesan, S. Microwave-assisted cyclization of unprotected dipeptides in water to 2,5-piperazinediones and self-assembly study of products and reagents. Synthesis 2019, 51, 2829–2838. [Google Scholar]
- Pérez-Picaso, L.; Escalante, J.; Olivo, H.F.; Rios, M.Y. Efficient microwave assisted syntheses of 2,5-diketopiperazines in aqueous media. Molecules 2009, 14, 2836–2849. [Google Scholar] [CrossRef] [Green Version]
- Naraoka, H.; Harada, K. Stereochemistry of piperazine-2,5-dione formation by self-condensation of dl-amino acid esters. J. Chem. Soc. 1986, 1557–1560. [Google Scholar] [CrossRef]
- López-Cobeñas, A.; Cledera, P.; Sánchez, J.D.; López-Alvarado, P.; Ramos, M.T.; Avendaño, C.; Menéndez, J.C. Microwave-assisted synthesis of 2,5-piperazinediones under solvent-free conditions. Synthesis 2005, 2005, 3412–3422. [Google Scholar] [CrossRef]
- Mendham, A.P.; Potter, B.S.; Palmer, R.A.; Dines, T.J.; Mitchell, J.C.; Withnall, R.; Chowdhry, B.Z. Vibrational spectra and crystal structure of the di-amino acid peptide cyclo(l-met-l-met): Comparison of experimental data and dft calculations. J. Raman Spectrosc. 2010, 41, 148–159. [Google Scholar] [CrossRef]
- Benedetti, E.; Goodman, M.; Marsh, R.E.; Rapoport, H.; Musich, J.A. Cyclo-l-prolyl-l-prolyl, c10h14n2o2. Cryst. Struct. Commun. 1975, 4, 641–645. [Google Scholar]
- Friedrich, A.; Jainta, M.; Nieger, M.; Bräse, S. One-pot synthesis of symmetrical and unsymmetrical diketopiperazines from unprotected amino acids. Synlett 2007, 2007, 2127–2129. [Google Scholar]
- Bettens, F.L.; Bettens, R.P.A.; Brown, R.D.; Godfrey, P.D. The microwave spectrum, structure, and ring-puckering of the cyclic dipeptide diketopiperazine. J. Am. Chem. Soc. 2000, 122, 5856–5860. [Google Scholar] [CrossRef]
- Hirst, J.D.; Persson, B.J. Ab initio calculations of the vibrational and electronic spectra of diketopiperazine. J. Phys. Chem. A 1998, 102, 7519–7524. [Google Scholar] [CrossRef]
- Valle, G.; Guantieri, V.; Tamburro, A.M. On the molecular and crystal structure of cyclo(l-methionyl-l-methionyl). J. Mol. Struct. 1990, 220, 19–24. [Google Scholar] [CrossRef]
- Mishra, A.K.; Choi, J.; Choi, S.J.; Baek, K.H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.; Rompolas, P. Advances in resolving the heterogeneity and dynamics of keratinocyte differentiation. Curr. Opin. Cell Biol. 2020, 67, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of dermis: Scarring and cells involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, H.K.; Eckersley, A.; Ozols, M.; Mellody, K.T.; Sherratt, M.J. Human skin: Composition, structure and visualisation methods. In Skin Biophysics: From Experimental Characterisation to Advanced Modelling; Limbert, G., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar]
- Hu, X.; He, H. A review of cosmetic skin delivery. J. Cosm. Dermatol. 2021, 20, 2020–2030. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Benfeldt, E.; Holmgaard, R. Penetration through the skin barrier. Curr. Probl. Dermatol. 2016, 49, 103–111. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Hopf, N.B.; Champmartin, C.; Schenk, L.; Berthet, A.; Chedik, L.; Du Plessis, J.L.; Franken, A.; Frasch, F.; Gaskin, S.; Johanson, G.; et al. Reflections on the OECD Guidelines for in Vitro Skin Absorption Studies. Regul. Toxicol. Pharmacol. 2020, 117, 104752. [Google Scholar] [CrossRef]
- Li, D.; Kerns, E.H. Permeability. In Drug-like Properties; Chapter 8; Academic Press: London, UK, 2016; pp. 95–111. [Google Scholar]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, a Complex Rate-Controlling Membrane. Curr. Opin. Coll. Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Franklin, L.M.; Walker, S.M.; Hill, G. A DFT study of isolated histidine interactions with metal ions (Ni2+, Cu2+, Zn2+) in a six-coordinated octahedral complex. J. Molec. Model. 2020, 26, 116. [Google Scholar] [CrossRef]
- Ito, Y.; Fukuyama, K.; Horie, N.; Epstein, W.L. Enzymatic activity of metal-binding proteins in epidermal cells. Mol. Cell Biochem. 1984, 60, 183–188. [Google Scholar] [CrossRef]
- Itahana, K.; Campisi, J.; Dimri, G.P. Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods Mol. Biol. 2007, 371, 21–31. [Google Scholar]
- Prasad, C. Cyclo(his-pro): Its distribution, origin and function in the human. Neurosci. Biobehav. Rev. 1988, 12, 19–22. [Google Scholar] [CrossRef]
- Prasad, C.; Jayaraman, A.; Robertson, H.J.; Rao, J.K. Is all cyclo(his-pro) derived from thyrotropin-releasing hormone? Neurochem. Res. 1987, 12, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Peptides affect the intake of specific nutrients and the sympathetic nervous system. Am. J. Clin. Nutrit. 1992, 55, 265s–271s. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Limited proteolysis and physiological regulation: An example from thyrotropin-releasing hormone metabolism. Thyroid 1998, 8, 969–975. [Google Scholar] [CrossRef]
- Kastin, A.J.; Pan, W. Involvement of the blood-brain barrier in metabolic regulation. CNS Neurol. Disord. Drug Targets 2016, 15, 1118–1128. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Grottelli, S.; Galli, F. Focus on cyclo(his-pro): History and perspectives as antioxidant peptide. Amino Acids 2008, 35, 283–289. [Google Scholar] [CrossRef]
- Grottelli, S.; Ferrari, I.; Pietrini, G.; Peirce, M.J.; Minelli, A.; Bellezza, I. The role of cyclo(his-pro) in neurodegeneration. Int. J. Mol. Sci. 2016, 17, 1332. [Google Scholar] [CrossRef]
- Song, M.K.; Bischoff, D.S.; Song, A.M.; Uyemura, K.; Yamaguchi, D.T. Metabolic relationship between diabetes and alzheimer’s disease affected by cyclo(his-pro) plus zinc treatment. BBA Clin. 2017, 7, 41–54. [Google Scholar] [CrossRef]
- Grottelli, S.; Costanzi, E.; Peirce, M.J.; Minelli, A.; Cellini, B.; Bellezza, I. Potential influence of cyclo(his-pro) on proteostasis: Impact on neurodegenerative diseases. Curr. Prot. Pept. Sci. 2018, 19, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Gervasoni, S.; Gilardoni, E.; Mazzolari, A.; Aldini, G.; Vistoli, G. Cyclo(his-pro) exerts protective carbonyl quenching effects through its open histidine containing dipeptides. Nutrients 2022, 14, 1775. [Google Scholar] [CrossRef]
- Marin, D.; Marchesan, S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials 2022, 12, 2147. [Google Scholar] [CrossRef]
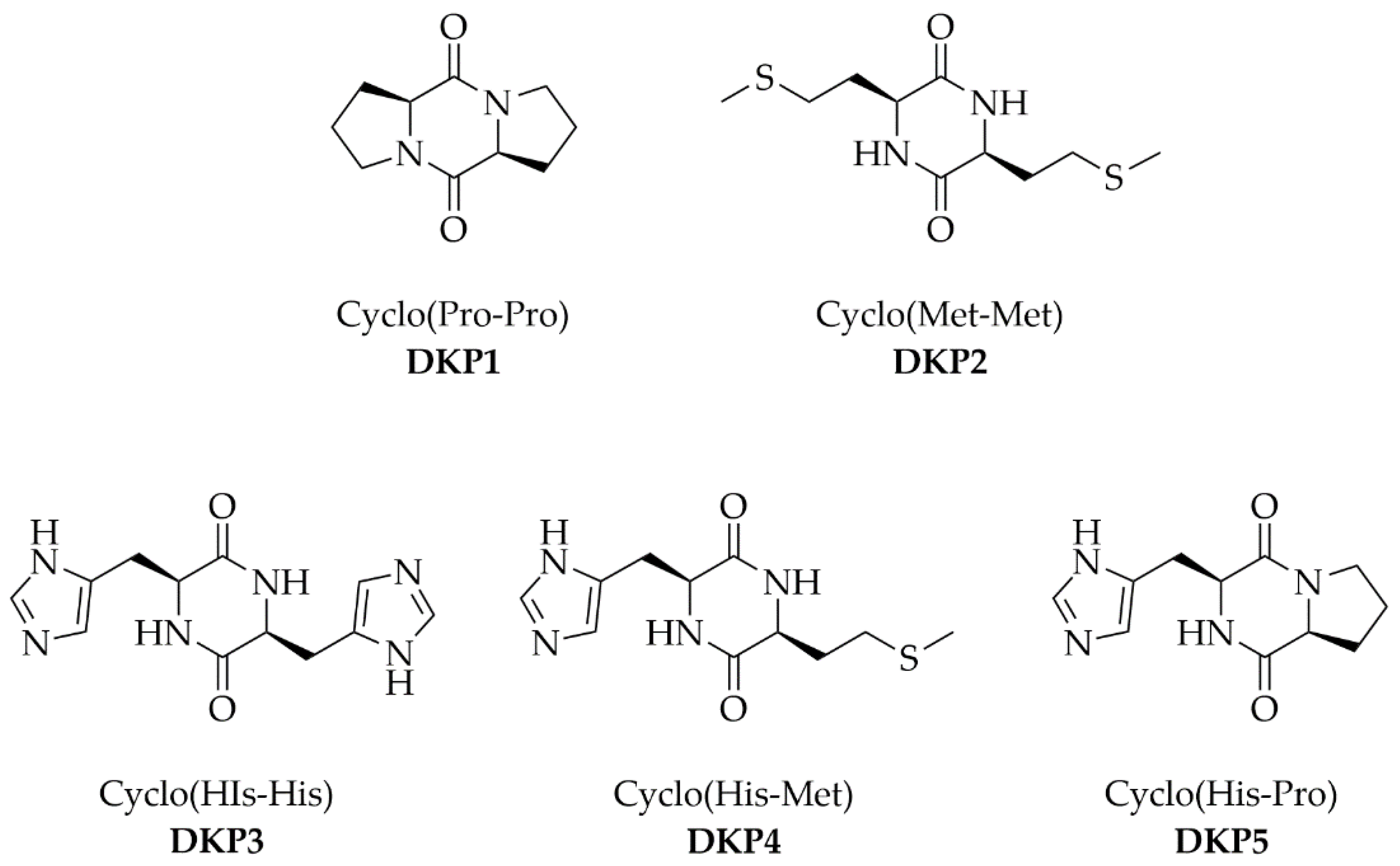




| DKP | Cyclodipeptide | logP 1 | MW (Da) | Ionizable Groups 2 |
|---|---|---|---|---|
| DKP1 | Cyclo (Pro-Pro) | −0.89 | 194 | 0 |
| DKP2 | Cyclo (Met-Met) | −0.64 | 262 | 0 |
| DKP3 | Cyclo (His-His) | −3.60 | 274 | 2 |
| DKP4 | Cyclo (His-Met) | −2.12 | 268 | 1 |
| DKP5 | Cyclo (His-Pro) | −2.24 | 234 | 1 |
| DKP | DC (mg/cm2) | Skin (E + D, mg/cm2) | Skin (SC, mg/cm2) | Total Skin (mg/cm2) | RF (mg/cm2) | Kp (cm/h) |
|---|---|---|---|---|---|---|
| DKP1 | 0.31 ± 0.07 | 0.010 ± 0.003 | 0.000 ± 0.000 | 0.010 ± 0.003 | 0.16 ± 0.05 | 4.59 10−1 |
| DKP2 | 0.38 ± 0.03 | 0.020 ± 0.007 | 0.000 ± 0.000 | 0.020 ± 0.007 | 0.13 ± 0.02 | 3.61 10−1 |
| DKP3 | 0.11 ± 0.01 | 0.005 ± 0.001 | 0.000 ± 0.000 | 0.005 ± 0.001 | 0.04 ± 0.02 | 4.00 10−1 |
| DKP4 | 0.20 ± 0.02 | 0.004 ± 0.000 | 0.000 ± 0.000 | 0.004 ± 0.000 | 0.12 ± 0.03 | 1.82 10−1 |
| DKP5 | 0.38 ± 0.04 | 0.005 ± 0.001 | 0.009 ± 0.000 | 0.014 ± 0.001 | 0.07 ± 0.03 | 0.85 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosetti, V.; Rosetti, B.; Pierri, G.; Bellotto, O.; Adorinni, S.; Bandiera, A.; Adami, G.; Tedesco, C.; Crosera, M.; Magnano, G.C.; et al. Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity. Biomedicines 2022, 10, 2342. https://doi.org/10.3390/biomedicines10102342
Mosetti V, Rosetti B, Pierri G, Bellotto O, Adorinni S, Bandiera A, Adami G, Tedesco C, Crosera M, Magnano GC, et al. Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity. Biomedicines. 2022; 10(10):2342. https://doi.org/10.3390/biomedicines10102342
Chicago/Turabian StyleMosetti, Veronica, Beatrice Rosetti, Giovanni Pierri, Ottavia Bellotto, Simone Adorinni, Antonella Bandiera, Gianpiero Adami, Consiglia Tedesco, Matteo Crosera, Greta Camilla Magnano, and et al. 2022. "Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity" Biomedicines 10, no. 10: 2342. https://doi.org/10.3390/biomedicines10102342
APA StyleMosetti, V., Rosetti, B., Pierri, G., Bellotto, O., Adorinni, S., Bandiera, A., Adami, G., Tedesco, C., Crosera, M., Magnano, G. C., & Marchesan, S. (2022). Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity. Biomedicines, 10(10), 2342. https://doi.org/10.3390/biomedicines10102342









