Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility
2.2. Search Strategy
2.3. Selection Process
2.4. Data Extraction and Outcomes
2.5. Study Risk of Bias Assessment
2.6. Measures of Treatment Effect
2.7. Statistical Analysis
2.7.1. Analysis Populations
2.7.2. Mean Pain Intensity Reduction
2.7.3. Responder Rate
2.7.4. Adverse Events
3. Results
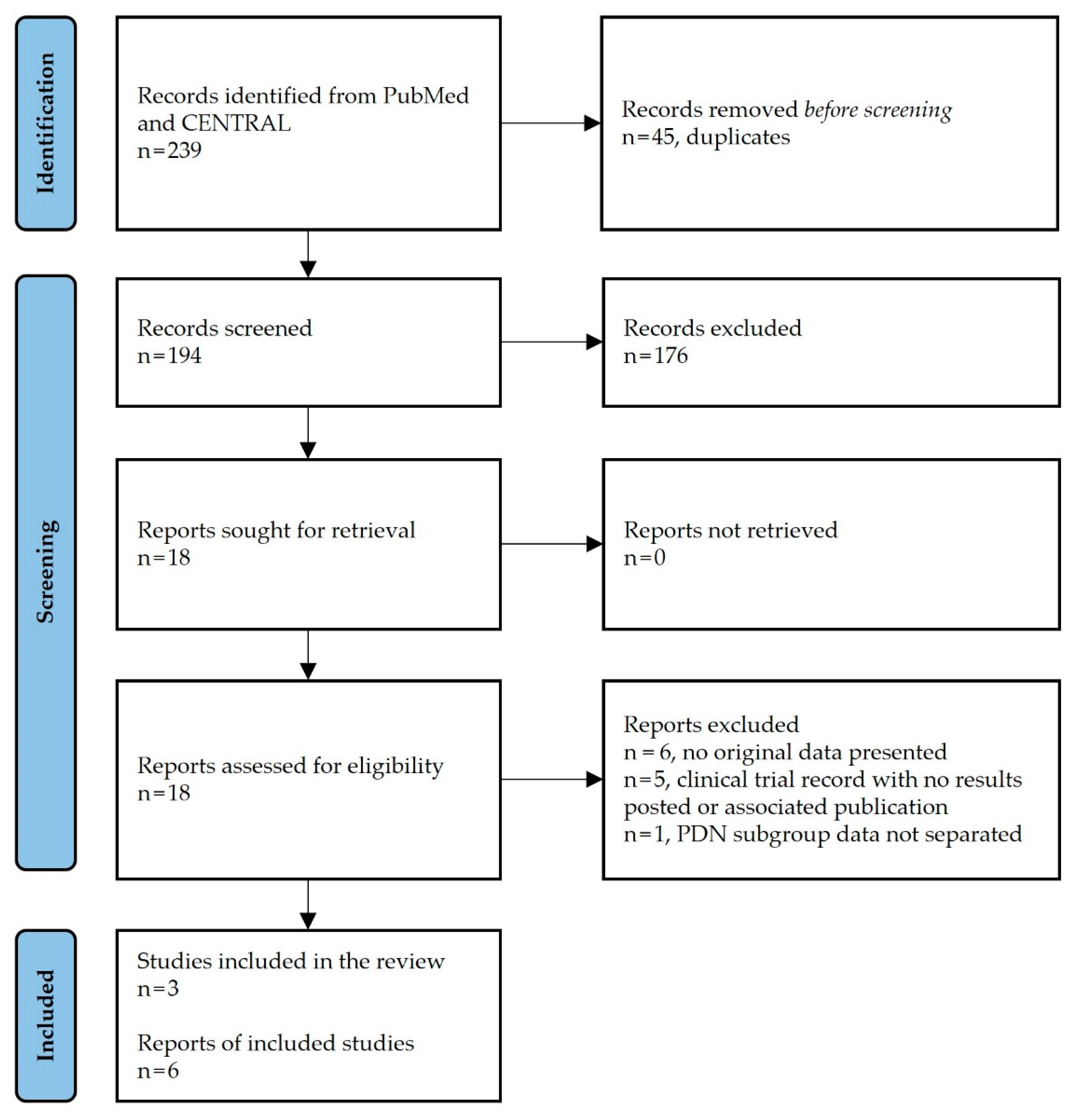
3.1. Study Selection
3.2. Study Characteristics
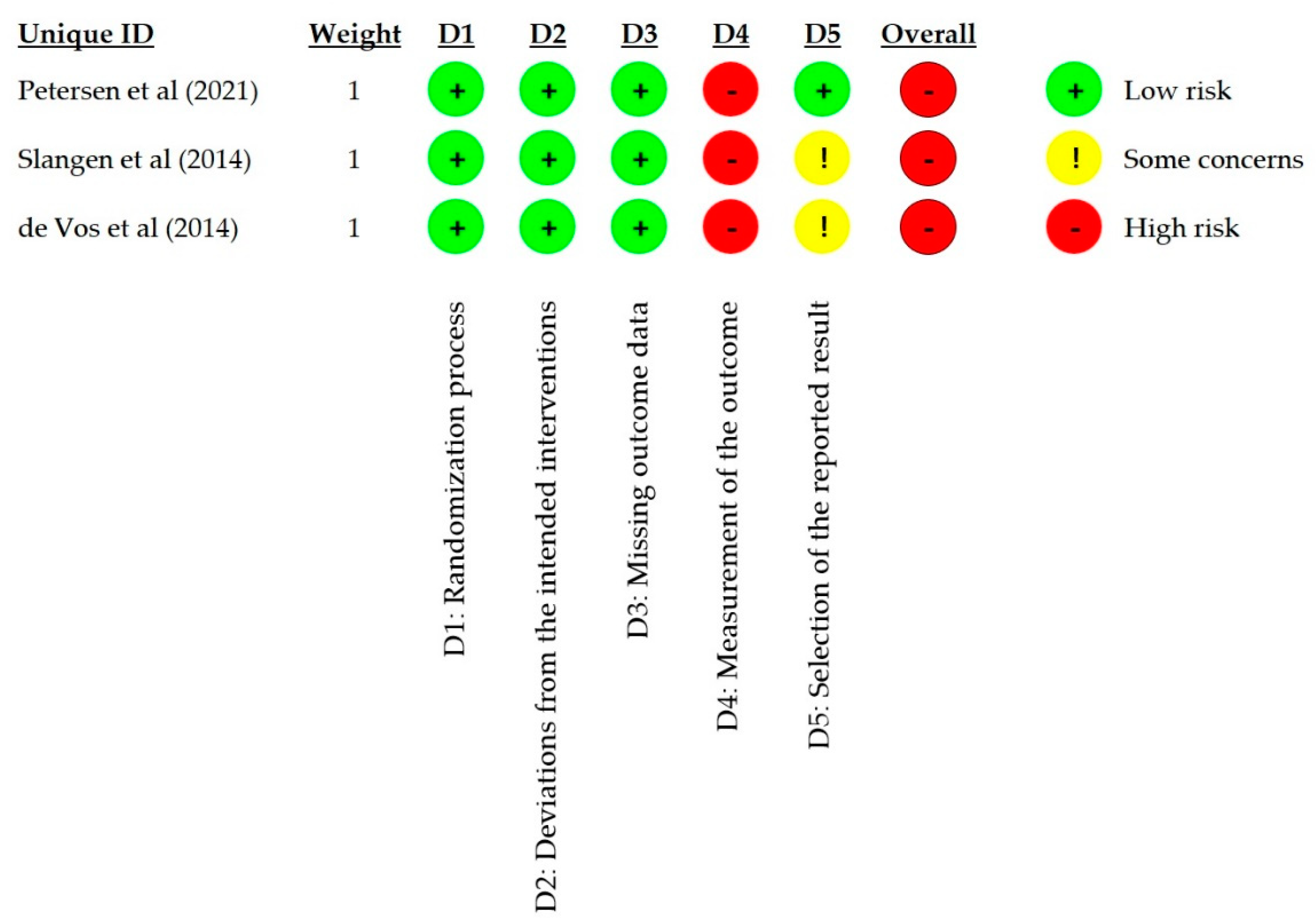
3.3. Risk of Bias in Studies
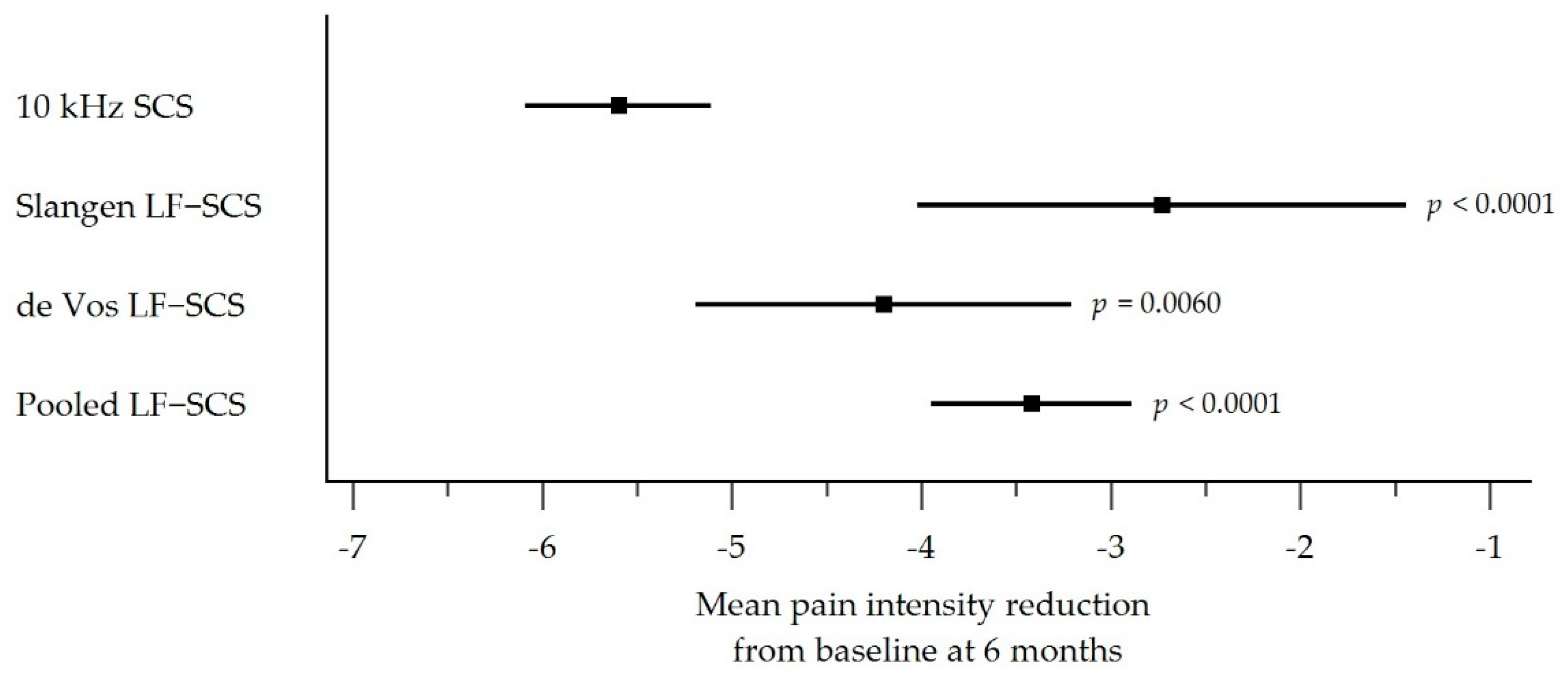
3.4. Pain Intensity Outcomes
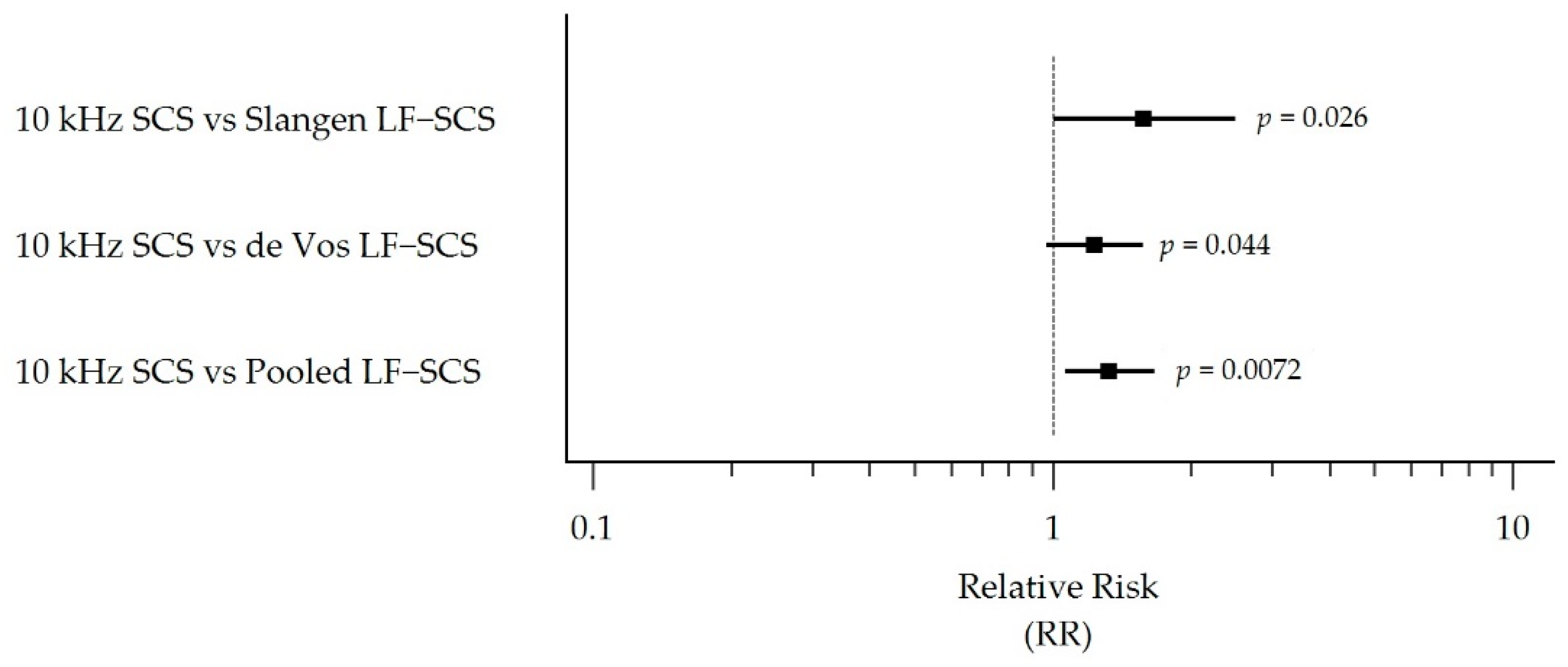
3.5. Responder Rate Outcomes
3.6. Treatment-Related Adverse Events
4. Discussion
4.1. Interpretation of Results
4.2. Treatment-Related Adverse Events
4.3. Strengths and Limitations of the Review
4.4. Implications for Practice, Policy, and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Karlsson, P.; Gylfadottir, S.S.; Andersen, S.T.; Bennett, D.L.; Tankisi, H.; Finnerup, N.B.; Terkelsen, A.J.; Khan, K.; Themistocleous, A.C.; et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021, 144, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galer, B.S.; Gianas, A.; Jensen, M.P. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res. Clin. Pract. 2000, 47, 123–128. [Google Scholar] [CrossRef]
- Kioskli, K.; Scott, W.; Winkley, K.; Kylakos, S.; McCracken, L.M. Psychosocial Factors in Painful Diabetic Neuropathy: A Systematic Review of Treatment Trials and Survey Studies. Pain Med. 2019, 20, 1756–1773. [Google Scholar] [CrossRef] [Green Version]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Waldfogel, J.M.; Nesbit, S.A.; Dy, S.M.; Sharma, R.; Zhang, A.; Wilson, L.M.; Bennett, W.L.; Yeh, H.C.; Chelladurai, Y.; Feldman, D.; et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017, 88, 1958–1967. [Google Scholar] [CrossRef]
- Yang, M.; Qian, C.; Liu, Y. Suboptimal Treatment of Diabetic Peripheral Neuropathic Pain in the United States. Pain Med. 2015, 16, 2075–2083. [Google Scholar] [CrossRef] [Green Version]
- Deer, T.R.; Grider, J.S.; Lamer, T.J.; Pope, J.E.; Falowski, S.; Hunter, C.W.; Provenzano, D.A.; Slavin, K.V.; Russo, M.; Carayannopoulos, A.; et al. A Systematic Literature Review of Spine Neurostimulation Therapies for the Treatment of Pain. Pain Med. 2020, 21, 1421–1432. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Brown, L.L.; Yearwood, T.L.; et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015, 123, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.M.; Veizi, E.; Hanes, M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience from an Academic Center Database. Neuromodulation 2015, 18, 603–608. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery 2005, 56, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery 2008, 63, 762–770. [Google Scholar] [CrossRef]
- De Carolis, G.; Paroli, M.; Tollapi, L.; Doust, M.W.; Burgher, A.H.; Yu, C.; Yang, T.; Morgan, D.M.; Amirdelfan, K.; Kapural, L.; et al. Paresthesia-Independence: An Assessment of Technical Factors Related to 10 kHz Paresthesia-Free Spinal Cord Stimulation. Pain Physician 2017, 20, 331–341. [Google Scholar]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results from a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- El Majdoub, F.; Neudorfer, C.; Richter, R.; Schieferdecker, S.; Maarouf, M. 10 kHz cervical SCS for chronic neck and upper limb pain: 12 months’ results. Ann. Clin. Transl. Neurol. 2019, 6, 2223–2229. [Google Scholar] [CrossRef] [Green Version]
- Kapural, L.; Gupta, M.; Paicius, R.; Strodtbeck, W.; Vorenkamp, K.E.; Gilmore, C.; Gliner, B.; Rotte, A.; Subbaroyan, J.; Province-Azalde, R. Treatment of Chronic Abdominal Pain with 10-kHz Spinal Cord Stimulation: Safety and Efficacy Results from a 12-Month Prospective, Multicenter, Feasibility Study. Clin. Transl. Gastroenterol. 2020, 11, e00133. [Google Scholar] [CrossRef]
- Gupta, M.; Scowcroft, J.; Kloster, D.; Guirguis, M.; Carlson, J.; McJunkin, T.; Chaiban, G.; Israel, A.; Subbaroyan, J. 10-kHz Spinal Cord Stimulation for Chronic Postsurgical Pain: Results from a 12-Month Prospective, Multicenter Study. Pain Pract. 2020, 20, 908–918. [Google Scholar] [CrossRef]
- Tate, J.L.; Stauss, T.; Li, S.; Rotte, A.; Subbaroyan, J. A Prospective, Multi-Center, Clinical Trial of a 10-kHz Spinal Cord Stimulation System in the Treatment of Chronic Pelvic Pain. Pain Pract. 2021, 21, 45–53. [Google Scholar] [CrossRef]
- Amirdelfan, K.; Vallejo, R.; Benyamin, R.; Yu, C.; Yang, T.; Bundschu, R.; Yearwood, T.L.; Sitzman, B.T.; Gliner, B.; Subbaroyan, J.; et al. High-Frequency Spinal Cord Stimulation at 10 kHz for the Treatment of Combined Neck and Arm Pain: Results from a Prospective Multicenter Study. Neurosurgery 2020, 87, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Verrills, P.; Salmon, J.; Russo, M.; Gliner, B.; Barnard, A.; Caraway, D. 10 kHz spinal cord stimulation for chronic upper limb and neck pain: Australian experience. Eur. Spine J. 2020, 29, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Burgher, A.; Kosek, P.; Surrett, S.; Rosen, S.M.; Bromberg, T.; Gulve, A.; Kansal, A.; Wu, P.; McRoberts, W.P.; Udeshi, A.; et al. Ten kilohertz SCS for Treatment of Chronic Upper Extremity Pain (UEP): Results from Prospective Observational Study. J. Pain Res. 2020, 13, 2837–2851. [Google Scholar] [CrossRef]
- Kapural, L.; Jameson, J.; Johnson, C.; Kloster, D.; Calodney, A.; Kosek, P.; Pilitsis, J.; Bendel, M.; Petersen, E.; Wu, C.; et al. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10 kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. J. Neurosurg. Spine 2022, 37, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Billet, B.; Hanssens, K.; De Coster, O.; Santos, A.; Rotte, A.; Minne, V. High-frequency (10 kHz) spinal cord stimulation for the treatment of focal, chronic postsurgical neuropathic pain: Results from a prospective study in Belgium. Pain Manag. 2022, 12, 75–85. [Google Scholar] [CrossRef]
- Kumar, K.; Toth, C.; Nath, R.K. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg. Neurol. 1996, 46, 363–369. [Google Scholar] [CrossRef]
- Kumar, K.; Hunter, G.; Demeria, D. Spinal cord stimulation in treatment of chronic benign pain: Challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006, 58, 481–496. [Google Scholar] [CrossRef]
- Tesfaye, S.; Watt, J.; Benbow, S.J.; Pang, K.A.; Miles, J.; MacFarlane, I.A. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet 1996, 348, 1698–1701. [Google Scholar] [CrossRef]
- Daousi, C.; Benbow, S.J.; MacFarlane, I.A. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabet. Med. 2005, 22, 393–398. [Google Scholar] [CrossRef]
- de Vos, C.C.; Rajan, V.; Steenbergen, W.; van der Aa, H.E.; Buschman, H.P. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J. Diabetes Complicat. 2009, 23, 40–45. [Google Scholar] [CrossRef]
- Pluijms, W.A.; Slangen, R.; Bakkers, M.; Faber, C.G.; Merkies, I.S.; Kessels, A.G.; Dirksen, C.D.; Joosten, E.A.; Reulen, J.P.; van Dongen, R.T.; et al. Pain relief and quality-of-life improvement after spinal cord stimulation in painful diabetic polyneuropathy: A pilot study. Br. J. Anaesth. 2012, 109, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Slangen, R.; Pluijms, W.A.; Faber, C.G.; Dirksen, C.D.; Kessels, A.G.; van Kleef, M. Sustained effect of spinal cord stimulation on pain and quality of life in painful diabetic peripheral neuropathy. Br. J. Anaesth. 2013, 111, 1030–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galan, V.; Scowcroft, J.; Chang, P.; Li, S.; Staats, P.; Rotte, A.; Subbaroyan, J. 10-kHz spinal cord stimulation treatment for painful diabetic neuropathy: Results from post-hoc analysis of the SENZA-PPN study. Pain Manag. 2020, 10, 291–300. [Google Scholar] [CrossRef]
- Sills, S. Treatment of painful polyneuropathies of diabetic and other origins with 10 kHz SCS: A case series. Postgrad. Med. 2020, 132, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Hesseltine, A.W.; Nashi, S.E.; Sills, S.M.; McJunkin, T.L.; Patil, S.; Bharara, M.; Caraway, D.L.; Brooks, E.S. A Real-World Analysis of High-Frequency 10 kHz Spinal Cord Stimulation for the Treatment of Painful Diabetic Peripheral Neuropathy. J. Diabetes Sci. Technol. 2022, 16, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, S.; Espinet, A.; Wahlstedt, A.; Kang, P.; Liem, L.; Patel, N.K.; Vesper, J.; Kimber, A.; Cusack, W.; Kramer, J. Retrospective Case Series on the Treatment of Painful Diabetic Peripheral Neuropathy with Dorsal Root Ganglion Stimulation. Neuromodulation 2018, 21, 787–792. [Google Scholar] [CrossRef]
- de Vos, C.C.; Bom, M.J.; Vanneste, S.; Lenders, M.W.; de Ridder, D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2014, 17, 152–159. [Google Scholar] [CrossRef]
- Petersen, E.A.; Stauss, T.G.; Scowcroft, J.A.; Brooks, E.S.; White, J.L.; Sills, S.M.; Amirdelfan, K.; Guirguis, M.N.; Xu, J.; Yu, C.; et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients with Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 687–698. [Google Scholar] [CrossRef]
- Slangen, R.; Schaper, N.C.; Faber, C.G.; Joosten, E.A.; Dirksen, C.D.; van Dongen, R.T.; Kessels, A.G.; van Kleef, M. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: A prospective two-center randomized controlled trial. Diabetes Care 2014, 37, 3016–3024. [Google Scholar] [CrossRef] [Green Version]
- de Vos, C.C.; Meier, K.; Zaalberg, P.B.; Nijhuis, H.J.; Duyvendak, W.; Vesper, J.; Enggaard, T.P.; Lenders, M.W. Spinal cord stimulation in patients with painful diabetic neuropathy: A multicentre randomized clinical trial. Pain 2014, 155, 2426–2431. [Google Scholar] [CrossRef]
- van Beek, M.; Slangen, R.; Schaper, N.C.; Faber, C.G.; Joosten, E.A.; Dirksen, C.D.; van Dongen, R.T.; Kessels, A.G.; van Kleef, M. Sustained Treatment Effect of Spinal Cord Stimulation in Painful Diabetic Peripheral Neuropathy: 24-Month Follow-up of a Prospective Two-Center Randomized Controlled Trial. Diabetes Care 2015, 38, e132–e134. [Google Scholar] [CrossRef] [PubMed]
- van Beek, M.; Geurts, J.W.; Slangen, R.; Schaper, N.C.; Faber, C.G.; Joosten, E.A.; Dirksen, C.D.; van Dongen, R.T.; van Kuijk, S.M.J.; van Kleef, M. Severity of Neuropathy Is Associated with Long-term Spinal Cord Stimulation Outcome in Painful Diabetic Peripheral Neuropathy: Five-Year Follow-up of a Prospective Two-Center Clinical Trial. Diabetes Care 2018, 41, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sears, N.C.; Machado, A.G.; Nagel, S.J.; Deogaonkar, M.; Stanton-Hicks, M.; Rezai, A.R.; Henderson, J.M. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation 2011, 14, 312–318. [Google Scholar] [CrossRef]
- Kemler, M.A.; De Vet, H.C.; Barendse, G.A.; Van den Wildenberg, F.A.; Van Kleef, M. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: Five-year final follow-up of patients in a randomized controlled trial. J. Neurosurg. 2008, 108, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alo, K.M.; Redko, V.; Charnov, J. Four Year Follow-up of Dual Electrode Spinal Cord Stimulation for Chronic Pain. Neuromodulation 2002, 5, 79–88. [Google Scholar] [CrossRef]
- Ohnmeiss, D.D.; Rashbaum, R.F.; Bogdanffy, G.M. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine 1996, 21, 1344–1350, discussion 51. [Google Scholar] [CrossRef] [PubMed]
- Burchiel, K.J.; Anderson, V.C.; Brown, F.D.; Fessler, R.G.; Friedman, W.A.; Pelofsky, S.; Weiner, R.L.; Oakley, J.; Shatin, D. Prospective, Multicenter Study of Spinal Cord Stimulation for Relief of Chronic Back and Extremity Pain. Spine 1996, 21, 2786–2794. [Google Scholar] [CrossRef]
- Kupers, R.C.; Van den Oever, R.; Van Houdenhove, B.; Vanmechelcn, W.; Hepp, B.; Nuttin, B.; Gybels, J.M. Spinal cord stimulation in Belgium: A nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain 1994, 56, 211–216. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Zahurak, M.; James, C.S.; Long, D.M. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery 1993, 32, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.A.; Stauss, T.G.; Scowcroft, J.A.; Brooks, E.S.; White, J.L.; Sills, S.M.; Amirdelfan, K.; Guirguis, M.N.; Xu, J.; Yu, C.; et al. Durability of High-Frequency 10-kHz Spinal Cord Stimulation for Patients with Painful Diabetic Neuropathy Refractory to Conventional Treatments: 12-Month Results from a Randomized Controlled Trial. Diabetes Care 2022, 45, e3–e6. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Palmisani, S.; Smith, T.E.; Carganillo, R.; Houghton, R.; Pang, D.; Burgoyne, W.; Lam, K.; Lucas, J. Long-Term Improvements in Chronic Axial Low Back Pain Patients without Previous Spinal Surgery: A Cohort Analysis of 10-kHz High-Frequency Spinal Cord Stimulation over 36 Months. Pain Med. 2018, 19, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Palmisani, S.; Smith, T.E.; Pang, D.; Lam, K.; Burgoyne, W.; Houghton, R.; Hudson, E.; Lucas, J. 10 kHz High-Frequency Spinal Cord Stimulation for Chronic Axial Low Back Pain in Patients with No History of Spinal Surgery: A Preliminary, Prospective, Open Label and Proof-of-Concept Study. Neuromodulation 2017, 20, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Van Buyten, J.P.; Al-Kaisy, A.; Smet, I.; Palmisani, S.; Smith, T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: Results of a prospective multicenter European clinical study. Neuromodulation 2013, 16, 59–65. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Van Buyten, J.P.; Smet, I.; Palmisani, S.; Pang, D.; Smith, T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.V.; Nevitt, S.; Maden, M.; Meier, K.; Taylor, R.S.; Eldabe, S.; de Vos, C.C. Spinal cord stimulation for the management of painful diabetic neuropathy: A systematic review and meta-analysis of individual patient and aggregate data. Pain 2021, 162, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.; Noel-Storr, A.; Rader, T.; Shokraneh, F. Technical Supplement to Chapter 4: Searching for and Selecting Studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 6; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M.S., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane. 2019. Available online: www.training.cochrane.org/handbook (accessed on 6 September 2022).
- Cooper, C.; Varley-Campbell, J.; Carter, P. Established search filters may miss studies when identifying randomized controlled trials. J. Clin. Epidemiol. 2019, 112, 12–19. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane. 2022. Available online: https://training.cochrane.org/handbook (accessed on 6 September 2022).
- Mekhail, N.A.; Argoff, C.E.; Taylor, R.S.; Nasr, C.; Caraway, D.L.; Gliner, B.E.; Subbaroyan, J.; Brooks, E.S. High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: Design of a multicenter, randomized controlled trial (SENZA-PDN). Trials 2020, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- CA2016-5 US SENZA-PDN-1 Statistical Analysis Plan, Version 3.0; Nevro Corp. 2019. Available online: https://clinicaltrials.gov/ProvidedDocs/20/NCT03228420/SAP_001.pdf (accessed on 10 June 2022).
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.; Franken, G. Spinal cord stimulation in chronic neuropathic pain: Mechanisms of action, new locations, new paradigms. Pain 2020, 161 (Suppl. 1), S104–S113. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, L.; Joosten, E.A. Mechanisms and mode of action of spinal cord stimulation in chronic neuropathic pain. Postgrad. Med. 2020, 132 (Suppl. 3), 17–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Bae, C.; Lee, D.; Kagan, Z.; Bradley, K.; Chung, J.M.; La, J.H. Low-intensity, Kilohertz Frequency Spinal Cord Stimulation Differently Affects Excitatory and Inhibitory Neurons in the Rodent Superficial Dorsal Horn. Neuroscience 2020, 428, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.T.; Tseng, C.C.; Chia, W.T.; Lin, C.R. High-frequency spinal cord stimulation treatment attenuates the increase in spinal glutamate release and spinal miniature excitatory postsynaptic currents in rats with spared nerve injury-induced neuropathic pain. Brain Res. Bull. 2020, 164, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lee, K.Y.; Lee, D.; Kagan, Z.B.; Bradley, K. Low-Intensity 10 kHz Spinal Cord Stimulation Reduces Behavioral and Neural Hypersensitivity in a Rat Model of Painful Diabetic Neuropathy. J. Pain Res. 2022, 15, 1503–1513. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, D.; Kagan, Z.B.; Wang, D.; Bradley, K. Differential Modulation of Dorsal Horn Neurons by Various Spinal Cord Stimulation Strategies. Biomedicines 2021, 9, 568. [Google Scholar] [CrossRef]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. 2016, 17, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowski, M.N.; Kim, C.H.; Deer, T.R. Complications of Spinal Cord Stimulation. Neuromodulation 2018, 657–668. [Google Scholar] [CrossRef]
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared with the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follett, K.A.; Boortz-Marx, R.L.; Drake, J.M.; DuPen, S.; Schneider, S.J.; Turner, M.S.; Coffey, R.J. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology 2004, 100, 1582–1594. [Google Scholar] [CrossRef]
- Hoelzer, B.C.; Bendel, M.A.; Deer, T.R.; Eldrige, J.S.; Walega, D.R.; Wang, Z.; Costandi, S.; Azer, G.; Qu, W.; Falowski, S.M.; et al. Spinal Cord Stimulator Implant Infection Rates and Risk Factors: A Multicenter Retrospective Study. Neuromodul. Technol. Neural Interface 2017, 20, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011, 11, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.A.; Stauss, T.G.; Scowcroft, J.A.; Brooks, E.S.; White, J.L.; Sills, S.M.; Amirdelfan, K.; Guirguis, M.N.; Xu, J.; Yu, C.; et al. High-Frequency 10-kHz Spinal Cord Stimulation Improves Health-Related Quality of Life in Patients with Refractory Painful Diabetic Neuropathy: 12-Month Results from a Randomized Controlled Trial. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 347–360. [Google Scholar] [CrossRef] [PubMed]

| Study | |||
|---|---|---|---|
| Characteristic | Petersen et al. [38] | Slangen et al. [39] | de Vos et al. [40] |
| Centers (Countries) | 18 (USA) | 2 (NL) | 7 (NL, DK, BE, DE) |
| SCS modality | 10 kHz SCS Paresthesia-free (Nevro) | LF-SCS Continuous paresthesia (Medtronic) | LF-SCS Continuous paresthesia (St. Jude Medical) |
| Comparison | 10 kHz SCS + CMM vs. CMM | LF-SCS + CMM vs. CMM | LF-SCS + CMM vs. CMM |
| Blinding | No | No | No |
| Follow-up duration | 6 mo | 6 mo | 6 mo |
| Indication, pain area | PDN, LL | PDN, LL | PDN, LE |
| Pain rating scale | VAS (0–10 cm) | NRS (0–10 points) | VAS (0–100 points) |
| Pain inclusion criteria | LL VAS ≥ 5 cm | LL NRS ≥ 5 points | LE VAS ≥ 50 points |
| Pain exclusion criteria | UL VAS ≥ 3 cm | UL NRS > 3 points | UE Pain > 20 points |
| Randomization ratio | 1:1 | 3:2 | 2:1 |
| Mean age by group | 10 kHz SCS + CMM: 60.7 ± 11.4 y CMM: 60.8 ± 9.9 y | LF-SCS + CMM: 57.1 ± 12.4 y CMM: 56.5 ± 8.0 y | LF-SCS + CMM: 58 ± 11 y CMM: 61 ± 12 y |
| Female/male proportion by group | 10 kHz SCS + CMM: 38%/62% CMM: 36%/64% | LF-SCS + CMM: 32%/68% CMM: 36%/64% | LF-SCS + CMM: 37.5%/62.5% CMM: 35%/65% |
| Diabetes Type I/II proportion by group | 10 kHz SCS + CMM: 7%/93% CMM: 3%/97% | LF-SCS + CMM: 14%/86% CMM: 7%/93% | LF-SCS + CMM: 25%/75% CMM: 25%/75% |
| Diabetes durationby group | 10 kHz SCS + CMM: 12.9 ± 8.5 y CMM: 12.2 ± 8.5 y | LF-SCS + CMM: 12.7 ± 10.1 y CMM: 12.6 ± 7.2 y | LF-SCS + CMM: 16 ± 11 y CMM: 17 ± 12 y |
| Duration of pain or peripheral neuropathyby group | 10 kHz SCS + CMM: 7.4 ± 5.7 y ‡ CMM: 7.1 ± 5.1 y ‡ | LF-SCS + CMM: 6.0 ± 5.1 y † CMM: 4.9 ± 3.6 y † | LF-SCS + CMM: 7 ± 6 y † CMM: 7 ± 6 y † |
| Duration of temporary stimulation trial | 5–7 d | 2 w | Up to 1 w |
| n randomized by group | 10 kHz SCS + CMM: 113 CMM: 103 | LF-SCS + CMM: 22 CMM: 14 | LF-SCS + CMM: 40 CMM: 20 |
| SCS group n trialed n with ≤50% pain relief n with ≥50% pain relief n implanted | 104 6 98 90 # | 22 * 4 17 17 | 40 3 37 37 |
| SCS group Proportion with a successful trial | 94% | 77% | 92.5% |
| SCS group Trial-to-permanent implant proportion | 87% | 77% | 92.5% |
| LF-SCS Group | ||||
|---|---|---|---|---|
| Statistic | 10 kHz SCS [38] | Slangen LF-SCS ‡ [39] | de Vos LF-SCS [40] | Pooled LF-SCS † [39,40] |
| mITT population ∫ | 104 | 22 | 40 | 62 |
| Mean baseline pain score | 7.6 | 7.1 | 7.3 | 7.2 |
| Mean reduction in pain score at 6 months (95% CI) * | −5.60 (−6.09, −5.11) | −2.73 (−4.02, −1.44) | −4.20 (−5.19, −3.21) | −3.42 (−3.95, −2.89) |
| p-value § 10 kHz SCS vs. LF-SCS group | p < 0.0001 | p = 0.0060 | p < 0.0001 | |
| Percentage reduction in pain relative to baseline | 73.7% | 38.7% | 57.5% | 47.5% |
| LF-SCS Group | ||||
|---|---|---|---|---|
| Statistic | 10 kHz SCS [38] | Slangen LF-SCS [39] | de Vos LF-SCS [40] | Pooled LF-SCS [39,40] |
| Number of responders * | 75 | 9 | 25 | 34 |
| mITT population † | 104 | 22 | 40 | 62 |
| Proportion of responders (%) ‡ | 72.12% | 40.91% | 62.50% | 54.84% |
| RR (95% CI) 10 kHz SCS vs. LF-SCS group | 1.76 (1.05, 2.95) | 1.15 (0.88, 1.51) | 1.32 (1.02, 1.70) | |
| p-value § | p = 0.016 | p = 0.1478 | p = 0.018 | |
| LF-SCS Group | ||||
|---|---|---|---|---|
| Statistic | 10 kHz SCS [38] | Slangen LF-SCS [39] | de Vos LF-SCS [40] | Pooled LF-SCS [39,40] |
| Number of responders * | 75 | 9 | 25 | 34 |
| Permanent implant Population † | 90 | 17 | 37 | 54 |
| Proportion of responders (%) ‡ | 83.33% | 52.94% | 67.57% | 62.96% |
| RR (95% CI) 10 kHz SCS vs. LF-SCS group | 1.57 (1.00, 2.49) | 1.23 (0.97, 1.57) | 1.32 (1.06, 1.66) | |
| p-value § | p = 0.026 | p = 0.044 | p = 0.0072 | |
| Study | ||||||
|---|---|---|---|---|---|---|
| Petersen et al. [38] | Petersen et al. † [50] | Slangen et al. [39] | van Beek et al. § [41] | van Beek et al. §‡ [42] | de Vos et al. [40] | |
| Follow-up Duration | 6 mo | 12 mo | 6 mo | 24 mo | 60 mo | 6 mo |
| n | 90 implanted | 154 implanted * | 17 implanted | 17 implanted | 40 implanted | 37 implanted |
| Postimplant infection n patients (%) | 3 (3.3%) | 8 (5.2%) | 6 w: 1 (5.9%) | 6 w: 1 (5.9%) | 6 w and 2 mo: 2 (5.0%) | 0 ⌃ |
| Lead migration n patients (%) | 1 (1.1%) | 1 (0.6%) | - | - | - | 1 (2.7%) |
| IPG site pain n patients | 1 (1.1%) | - | - | - | 10 (25.0%) | 2 (5.4%) |
| Uncomfortable stimulation n patients (%) | 1 (1.1%) | - | - | - | 9 (22.5%) | - |
| Lead revision n patients (%) | - | 1 (0.6%) | - | 4 (23.5%) | 4 (10.0%) | Due to lead migration: 1 (2.7%) Due to incomplete paresthesia overlap: 2 (5.4%) |
| Lead replacement n patients (%) | - | - | - | - | 3 (7.5%) | - |
| IPG revision n patients (%) | 2 (1.3%) | - | » | Due to prolonged pocket pain (20 mo): 1 (2.5%) | Due to IPG site pain: 2 (5.4%) | |
| IPG explant due to AE n patients (%) | 2 (2.2%) | Due to infection: 5 (3.2%) | Due to postimplant infection: 1 (5.9%) | Due to postimplant infection: 1 (5.9%) | Due to infection: 2 (5.0%) Due to lack of efficacy: 6 (15.0%) | - |
| IPG replacement n patients (%) | 0 ψ | 0 ψ | 0 ψ | 1 × replacement: 2 (11.8%) | 1 × replacement: 8 (20.0%) 2 × replacements: 5 (12.5%) | |
| Other n patients (%) | Wound dehiscence: 2 (2.2%) Impaired healing: 1 (1.1%) Device extrusion: 1 (1.1%) Incision site pain: 1 (1.1%) Other: 7 (7.8%) ∫ | - | Dural puncture with subdural hematoma sequala, leading to death: 1 (5.9%) | - | - | Coagulopathy during implantation: 1 (2.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoelzer, B.C.; Edgar, D.; Lu, S.-P.; Taylor, R.S. Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials. Biomedicines 2022, 10, 2630. https://doi.org/10.3390/biomedicines10102630
Hoelzer BC, Edgar D, Lu S-P, Taylor RS. Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials. Biomedicines. 2022; 10(10):2630. https://doi.org/10.3390/biomedicines10102630
Chicago/Turabian StyleHoelzer, Bryan C., Deborah Edgar, Shiao-Ping Lu, and Rod S. Taylor. 2022. "Indirect Comparison of 10 kHz Spinal Cord Stimulation (SCS) versus Traditional Low-Frequency SCS for the Treatment of Painful Diabetic Neuropathy: A Systematic Review of Randomized Controlled Trials" Biomedicines 10, no. 10: 2630. https://doi.org/10.3390/biomedicines10102630




