Inter-Rater Variability of Prostate Lesion Segmentation on Multiparametric Prostate MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. MRI Scan
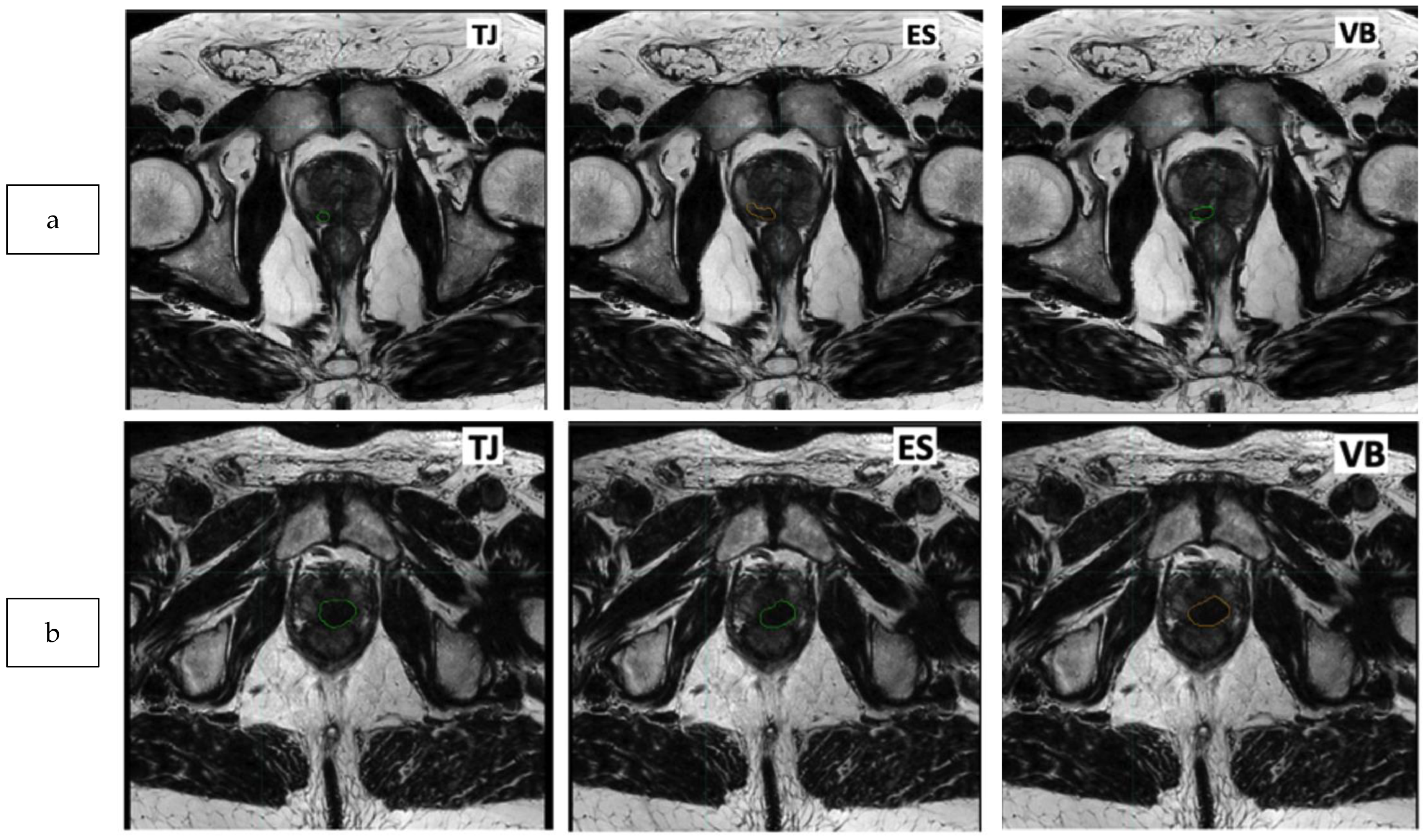
2.3. Delineation of the Dominant Index Lesion (DIL)
2.4. Similarity and Statistical Analysis
2.5. Number of Patients
3. Results
3.1. Population
3.2. Similarity Analysis: Prostate, PZ, and TZ
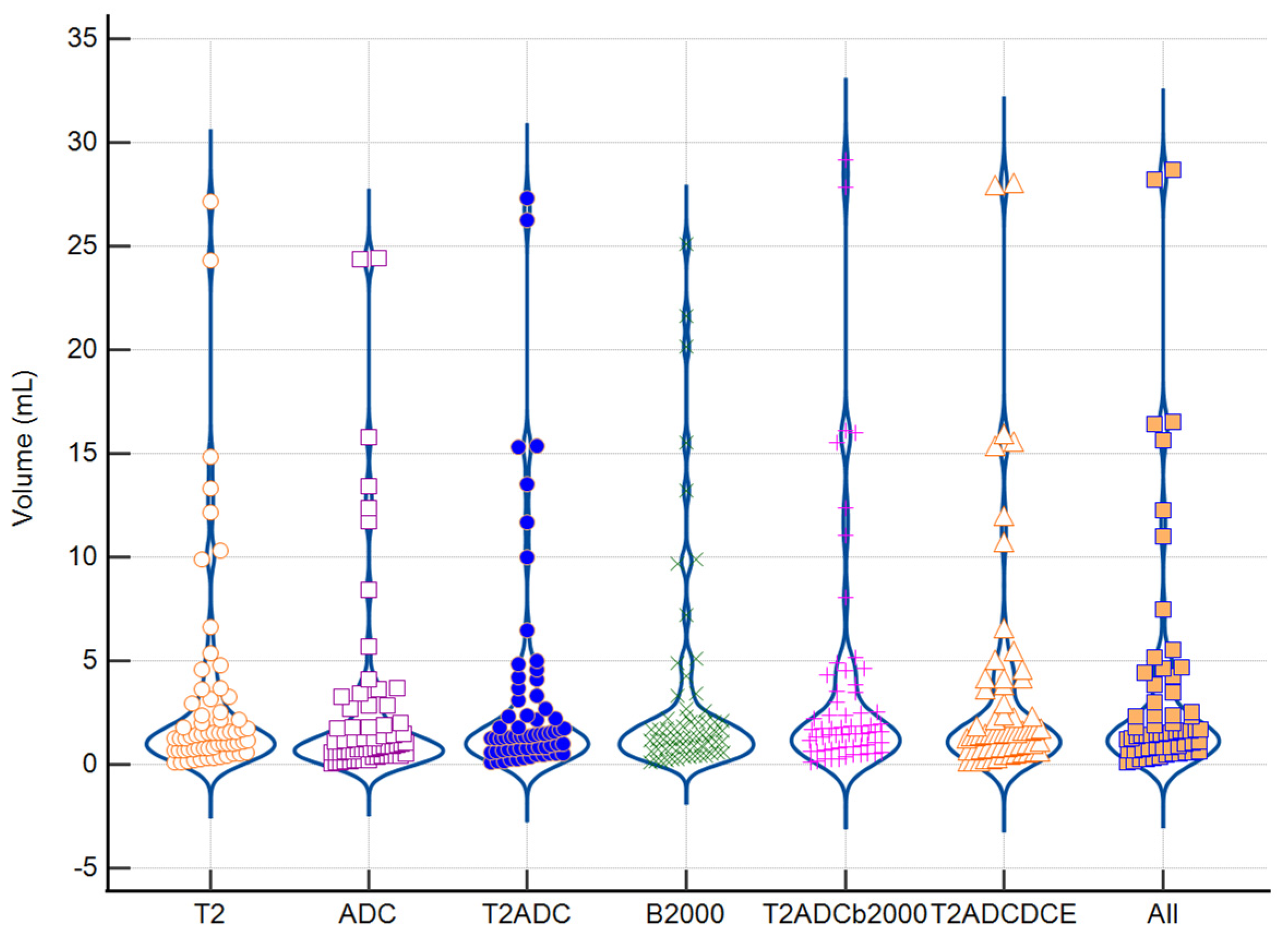
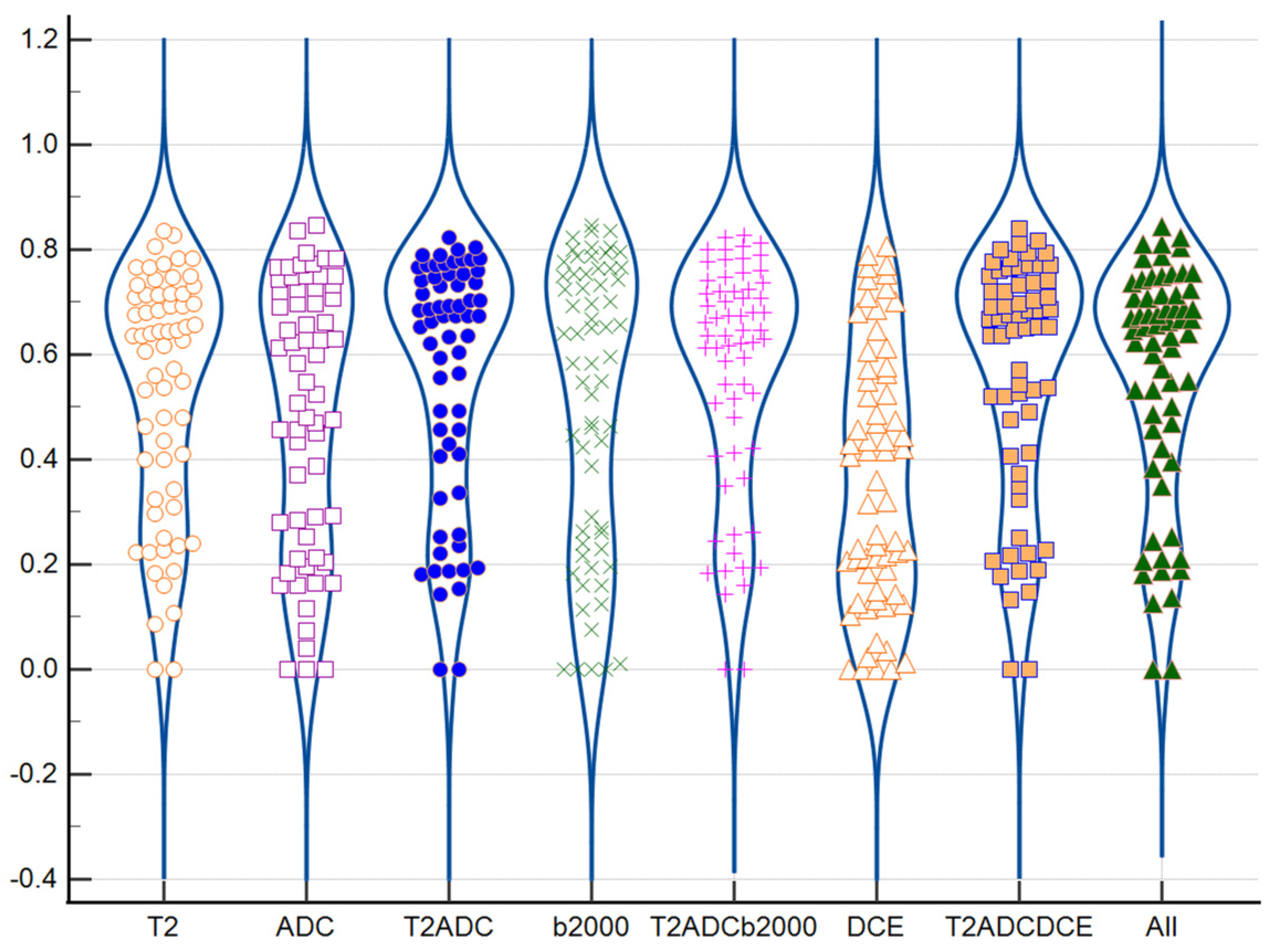
3.3. Similarity Analysis: Index Lesion
3.4. Impact of Tumoral Volume and PI-RADS Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Padala, S.A.; Vakiti, A.; Mohammed, A.; Saginala, K.; Thandra, K.C.; Rawla, P.; Barsouk, A. Epidemiology, Staging and Management of Prostate Cancer. Med. Sci. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-Dose versus Control-Dose Conformal Radiotherapy for Prostate Cancer: Long-Term Results from the MRC RT01 Randomised Controlled Trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Kuban, D.A.; Tucker, S.L.; Dong, L.; Starkschall, G.; Huang, E.H.; Cheung, M.R.; Lee, A.K.; Pollack, A. Long-Term Results of the M. D. Anderson Randomized Dose-Escalation Trial for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Beckendorf, V.; Guerif, S.; Le Prisé, E.; Cosset, J.-M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.-M.; et al. 70 Gy versus 80 Gy in Localized Prostate Cancer: 5-Year Results of GETUG 06 Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Prada, P.J.; Cardenal, J.; García Blanco, A.; Andreescu, J.; Ferri, M.; Anchuelo, J.; Diaz de Cerio, I.; Sierrasesumaga, N.; Vázquez, A.; Pacheco, M.; et al. Focal High-Dose-Rate Brachytherapy for Localized Prostate Cancer: Toxicity and Preliminary Biochemical Results. Strahlenther. Onkol. Organ Dtsch. Rontgenges. Al 2020, 196, 222–228. [Google Scholar] [CrossRef]
- Zhong, A.Y.; Lui, A.J.; Katz, M.S.; Berlin, A.; Kamran, S.C.; Kishan, A.U.; Murthy, V.; Nagar, H.; Seible, D.; Stish, B.J.; et al. Use of Focal Radiotherapy Boost for Prostate Cancer: Radiation Oncologists’ Perspectives and Perceived Barriers to Implementation. Radiat. Oncol. Lond Engl. 2023, 18, 188. [Google Scholar] [CrossRef]
- Turkbey, B.; Mani, H.; Aras, O.; Rastinehad, A.R.; Shah, V.; Bernardo, M.; Pohida, T.; Daar, D.; Benjamin, C.; McKinney, Y.L.; et al. Correlation of Magnetic Resonance Imaging Tumor Volume with Histopathology. J. Urol. 2012, 188, 1157–1163. [Google Scholar] [CrossRef]
- Isebaert, S.; Van den Bergh, L.; Haustermans, K.; Joniau, S.; Lerut, E.; De Wever, L.; De Keyzer, F.; Budiharto, T.; Slagmolen, P.; Van Poppel, H.; et al. Multiparametric MRI for Prostate Cancer Localization in Correlation to Whole-Mount Histopathology. J. Magn. Reson. Imaging JMRI 2013, 37, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; van der Kwast, T.H.; Tanguay, J.; Evans, A.J.; Hashmi, A.-T.; Lockwood, G.; Trachtenberg, J. Combined T2-Weighted and Diffusion-Weighted MRI for Localization of Prostate Cancer. AJR Am. J. Roentgenol. 2007, 189, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bratan, F.; Melodelima, C.; Souchon, R.; Hoang Dinh, A.; Mège-Lechevallier, F.; Crouzet, S.; Colombel, M.; Gelet, A.; Rouvière, O. How Accurate Is Multiparametric MR Imaging in Evaluation of Prostate Cancer Volume? Radiology 2015, 275, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T.; et al. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chatterjee, A.; Yousuf, A.; Antic, T.; Eggener, S.; Karczmar, G.S.; Oto, A. Comparison of T2-Weighted Imaging, DWI, and Dynamic Contrast-Enhanced MRI for Calculation of Prostate Cancer Index Lesion Volume: Correlation with Whole-Mount Pathology. AJR Am. J. Roentgenol. 2019, 212, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lui, A.J.; Kallis, K.; Zhong, A.Y.; Hussain, T.S.; Conlin, C.; Digma, L.A.; Phan, N.; Mathews, I.T.; Do, D.D.; Domingo, M.R.; et al. ReIGNITE Radiation Therapy Boost: A Prospective, International Study of Radiation Oncologists’ Accuracy in Contouring Prostate Tumors for Focal Radiation Therapy Boost on Conventional Magnetic Resonance Imaging Alone or With Assistance of Restriction Spectrum Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 1145–1152. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR Prostate MR Guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.; et al. PI-RADS Prostate Imaging-Reporting and Data System, v2.1. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2-1.pdf?la=en (accessed on 25 September 2023).
- Mazaheri, Y.; Akin, O.; Hricak, H. Dynamic Contrast-Enhanced Magnetic Resonance Imaging of Prostate Cancer: A Review of Current Methods and Applications. World J. Radiol. 2017, 9, 416–425. [Google Scholar] [CrossRef]
- Chen, M.Y.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Variability in Accuracy of Prostate Cancer Segmentation among Radiologists, Urologists, and Scientists. Cancer Med. 2020, 9, 7172–7182. [Google Scholar] [CrossRef]
- van Schie, M.A.; Dinh, C.V.; van Houdt, P.J.; Pos, F.J.; Heijmink, S.W.T.J.P.; Kerkmeijer, L.G.W.; Kotte, A.N.T.J.; Oyen, R.; Haustermans, K.; van der Heide, U.A. Contouring of Prostate Tumors on Multiparametric MRI: Evaluation of Clinical Delineations in a Multicenter Radiotherapy Trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 128, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Ushinsky, A.; Bardis, M.; Glavis-Bloom, J.; Uchio, E.; Chantaduly, C.; Nguyentat, M.; Chow, D.; Chang, P.D.; Houshyar, R. A 3D-2D Hybrid U-Net Convolutional Neural Network Approach to Prostate Organ Segmentation of Multiparametric MRI. AJR Am. J. Roentgenol. 2021, 216, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bardis, M.; Houshyar, R.; Chantaduly, C.; Tran-Harding, K.; Ushinsky, A.; Chahine, C.; Rupasinghe, M.; Chow, D.; Chang, P. Segmentation of the Prostate Transition Zone and Peripheral Zone on MR Images with Deep Learning. Radiol. Imaging Cancer 2021, 3, e200024. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Schoots, I.G.; Castillo Tovar, J.; Bangma, C.H.; Krestin, G.P.; Roobol, M.J.; Niessen, W.; Veenland, J.F. Clinically Significant Prostate Cancer Detection and Segmentation in Low-Risk Patients Using a Convolutional Neural Network on Multi-Parametric MRI. Eur. Radiol. 2020, 30, 6582–6592. [Google Scholar] [CrossRef] [PubMed]
- Hoar, D.; Lee, P.Q.; Guida, A.; Patterson, S.; Bowen, C.V.; Merrimen, J.; Wang, C.; Rendon, R.; Beyea, S.D.; Clarke, S.E. Combined Transfer Learning and Test-Time Augmentation Improves Convolutional Neural Network-Based Semantic Segmentation of Prostate Cancer from Multi-Parametric MR Images. Comput. Methods Programs Biomed. 2021, 210, 106375. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.M.G.; Kehayias, C.E.; Leeman, J.E.; Nguyen, P.L.; Peng, L.; Yang, D.D.; Moningi, S.; Martin, N.; Orio, P.F.; D’Amico, A.V.; et al. Assessing the Feasibility of Using Artificial Intelligence-Segmented Dominant Intraprostatic Lesion for Focal Intraprostatic Boost With External Beam Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Chang, D.; Hardcastle, N.; Jackson, P.; Kron, T.; Hofman, M.S.; Siva, S. Feasibility of Biology-Guided Radiotherapy Using PSMA-PET to Boost to Dominant Intraprostatic Tumour. Clin. Transl. Radiat. Oncol. 2022, 35, 84–89. [Google Scholar] [CrossRef]
- Draulans, C.; De Roover, R.; van der Heide, U.A.; Kerkmeijer, L.; Smeenk, R.J.; Pos, F.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Isebaert, S.; et al. Optimal 68Ga-PSMA and 18F-PSMA PET Window Levelling for Gross Tumour Volume Delineation in Primary Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1211–1218. [Google Scholar] [CrossRef]
- Guzene, L.; Beddok, A.; Nioche, C.; Modzelewski, R.; Loiseau, C.; Salleron, J.; Thariat, J. Assessing Interobserver Variability in the Delineation of Structures in Radiation Oncology: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1047–1060. [Google Scholar] [CrossRef]
- Saha, A.; Twilt, J.J.; Bosma, J.S.; van Ginneken, B.; Yakar, D.; Elschot, M.; Veltman, J.; Fütterer, J.; de Rooij, M.; Huisman, H. Artificial Intelligence and Radiologists at Prostate Cancer Detection in MRI: The PI-CAI Challenge (Study Protocol) (1.1). Zenodo 2022. [Google Scholar] [CrossRef]
- Stanzione, A.; Imbriaco, M.; Cocozza, S.; Fusco, F.; Rusconi, G.; Nappi, C.; Mirone, V.; Mangiapia, F.; Brunetti, A.; Ragozzino, A.; et al. Biparametric 3T Magnetic Resonance Imaging for Prostatic Cancer Detection in a Biopsy-Naïve Patient Population: A Further Improvement of PI-RADS V2? Eur. J. Radiol. 2016, 85, 2269–2274. [Google Scholar] [CrossRef]
- Hansford, B.G.; Peng, Y.; Jiang, Y.; Vannier, M.W.; Antic, T.; Thomas, S.; McCann, S.; Oto, A. Dynamic Contrast-Enhanced MR Imaging Curve-Type Analysis: Is It Helpful in the Differentiation of Prostate Cancer from Healthy Peripheral Zone? Radiology 2015, 275, 448–457. [Google Scholar] [CrossRef]
- Scialpi, M.; Prosperi, E.; D’Andrea, A.; Martorana, E.; Malaspina, C.; Palumbo, B.; Orlandi, A.; Falcone, G.; Milizia, M.; Mearini, L.; et al. Biparametric versus Multiparametric MRI with Non-Endorectal Coil at 3T in the Detection and Localization of Prostate Cancer. Anticancer Res. 2017, 37, 1263–1271. [Google Scholar] [CrossRef]
| Characteristics of Patients | Number of Patients = 64 |
|---|---|
| Age (median (range)) | 69 (46–90) |
| PI-RADS Classification | |
| PI-RADS 3 | 7/64 (10.9) |
| PI-RADS 4 | 12/64 (18.8) |
| PI-RADS 5 | 45/64 (70.3) |
| Mean PSA (ng/mL) | 9.1 |
| PSA range (ng/mL) | |
| <10 | 41/64 (64.1%) |
| 10–20 | 20/64 (31.3%) |
| >20 | 3/64 (4.7%) |
| ISUP Class | |
| 5 | 9/64 (14/1%) |
| 4 | 9/64 (14/1%) |
| 3 | 9/64 (14/1%) |
| 2 | 19/64 (29.7%) |
| 1 | 18/64 (28.1%) |
| Prostate | Transitional Zone | Peripherical Zone | |||||
|---|---|---|---|---|---|---|---|
| Pair of Readers | Variable | Mean | SD | Mean | SD | Mean | SD |
| TJ/ES | DICE | 0.89 | 0.03 | 0.77 | 0.13 | 0.72 | 0.12 |
| TJ/VB | DICE | 0.89 | 0.03 | 0.79 | 0.11 | 0.75 | 0.06 |
| VB/ES | DICE | 0.90 | 0.04 | 0.78 | 0.11 | 0.72 | 0.12 |
| All | DICE | 0.90 | 0.03 | 0.77 | 0.13 | 0.72 | 0.10 |
| T2 | ADC | T2ADC | b2000 | T2ADCb2000 | DCE | T2ADCDCE | All | T2 + ADC | T2 + ADC + b2000 | T2 + ADC + DCE | T2 + ADC + b2000 + DCE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2 | 1.00 | 0.27 | 0.25 | 0.73 | 0.25 | <0.0001 † | 0.23 | 0.27 | 0.15 | 0.15 | 0.15 | 0.02 † |
| ADC | 0.27 | 1.00 | 0.03 † | 0.49 | 0.03 † | 0.01 † | 0.02 † | 0.03 † | 0.01 † | 0.01 † | 0.01 † | 0.01 † |
| T2ADC | 0.25 | 0.03 † | 1.00 | 0.16 | 1.00 | <0.0001 † | 0.95 | 0.90 | 0.77 | 0.77 | 0.80 | 0.28 |
| B2000 | 0.73 | 0.49 | 0.16 | 1.00 | 0.16 | <0.0001 † | 0.14 | 0.19 | 0.09 | 0.09 | 0.10 | 0.02 † |
| T2ADCb2000 | 0.25 | 0.03 † | 1.00 | 0.16 | 1.00 | <0.0001 † | 0.95 | 0.90 | 0.77 | 0.77 | 0.80 | 0.27 |
| DCE | <0.0001 † | 0.00 † | <0.0001 † | <0.0001 † | <0.0001 † | 1.00 | <0.0001 † | <0.0001 † | <0.0001 † | <0.0001 † | <0.0001 † | <0.0001 † |
| T2ADCDCE | 0.23 | 0.02 † | 0.95 | 0.14 | 0.95 | <0.0001 † | 1.00 | 0.85 | 0.81 | 0.81 | 0.84 | 0.30 |
| All | 0.27 | 0.03 † | 0.90 | 0.19 | 0.90 | <0.0001 † | 0.85 | 1.00 | 0.67 | 0.67 | 0.70 | 0.22 |
| T2 + ADC | 0.15 | 0.01 † | 0.77 | 0.09 | 0.77 | <0.0001 † | 0.81 | 0.67 | 1.00 | 1.00 | 0.96 | 0.43 |
| T2 + ADC + b2000 | 0.15 | 0.01 † | 0.77 | 0.09 | 0.77 | <0.0001 † | 0.81 | 0.67 | 1.00 | 1.00 | 0.96 | 0.43 |
| T2 + ADC + DCE | 0.15 | 0.01 † | 0.80 | 0.10 | 0.80 | <0.0001 † | 0.84 | 0.70 | 0.96 | 0.96 | 1.00 | 0.40 |
| T2 + ADC + b2000 + DCE | 0.02 † | 0.00 † | 0.28 | 0.02 † | 0.27 | <0.0001 † | 0.30 | 0.22 | 0.43 | 0.43 | 0.40 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeganathan, T.; Salgues, E.; Schick, U.; Tissot, V.; Fournier, G.; Valéri, A.; Nguyen, T.-A.; Bourbonne, V. Inter-Rater Variability of Prostate Lesion Segmentation on Multiparametric Prostate MRI. Biomedicines 2023, 11, 3309. https://doi.org/10.3390/biomedicines11123309
Jeganathan T, Salgues E, Schick U, Tissot V, Fournier G, Valéri A, Nguyen T-A, Bourbonne V. Inter-Rater Variability of Prostate Lesion Segmentation on Multiparametric Prostate MRI. Biomedicines. 2023; 11(12):3309. https://doi.org/10.3390/biomedicines11123309
Chicago/Turabian StyleJeganathan, Thibaut, Emile Salgues, Ulrike Schick, Valentin Tissot, Georges Fournier, Antoine Valéri, Truong-An Nguyen, and Vincent Bourbonne. 2023. "Inter-Rater Variability of Prostate Lesion Segmentation on Multiparametric Prostate MRI" Biomedicines 11, no. 12: 3309. https://doi.org/10.3390/biomedicines11123309





