Acute Effects of Aerobic Exercise on Somatosensory-Evoked Potentials in Patients with Mild Cognitive Impairment
Abstract
:1. Introduction
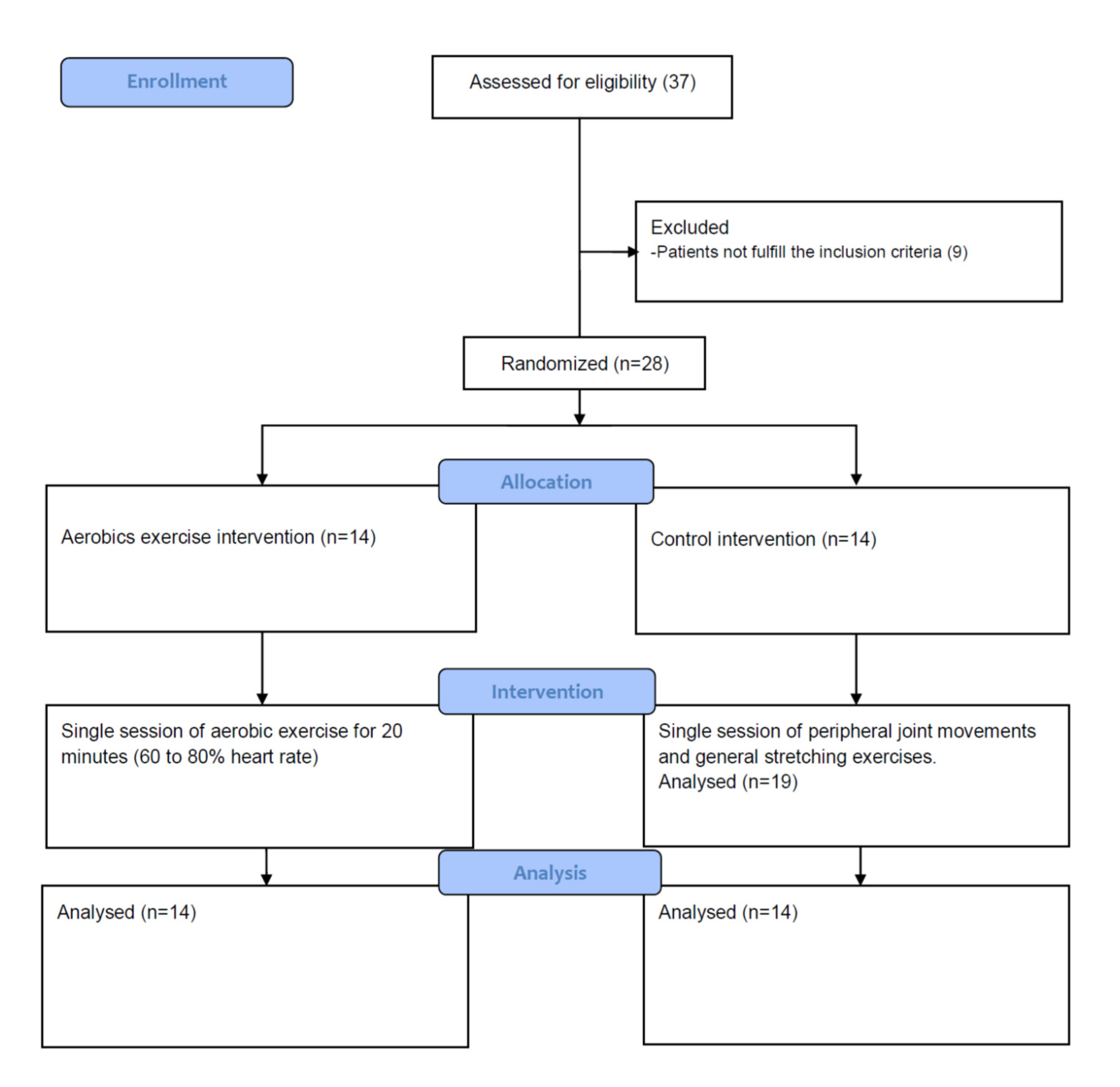
2. Material and Method
2.1. Subjects
2.2. Experimental Intervention
2.3. Control Intervention
2.4. Recordings
2.5. Stimulations
2.6. Data Analysis
2.6.1. Pre-Processing
2.6.2. Extraction of Somatosensory-Evoked Potential (SEP) Parameters
2.6.3. Statistics
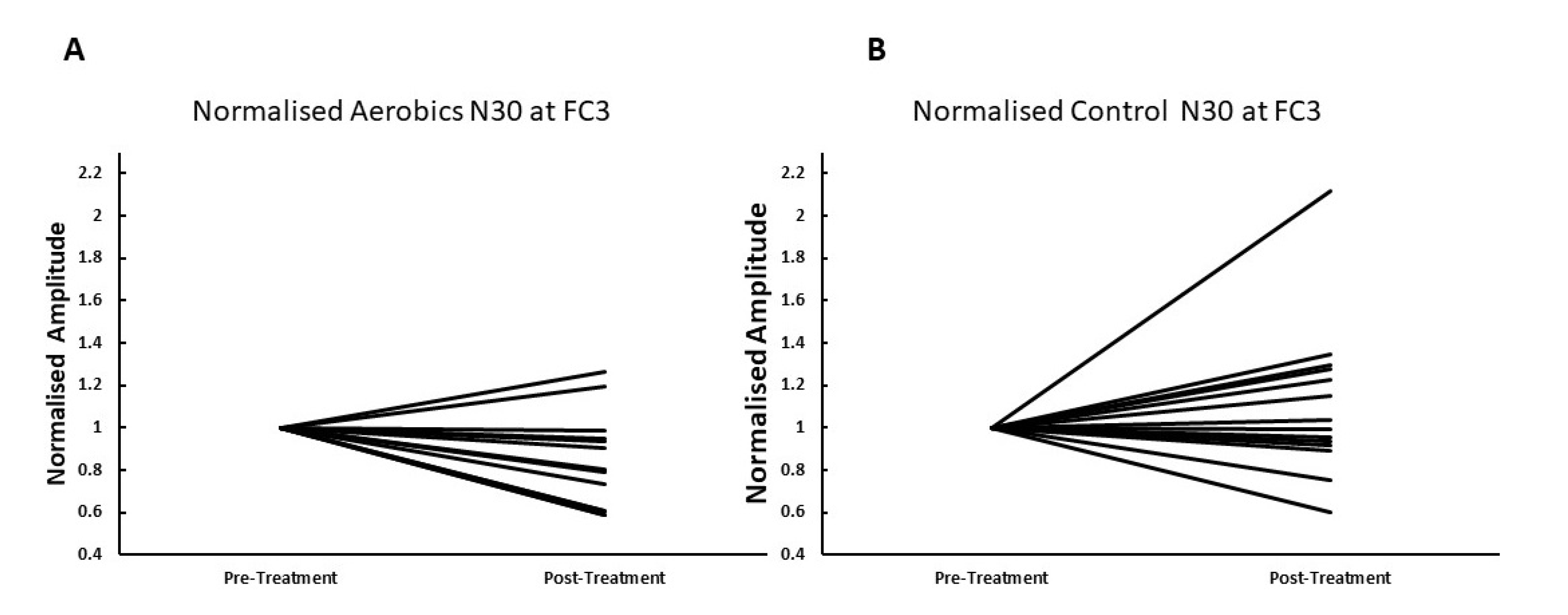
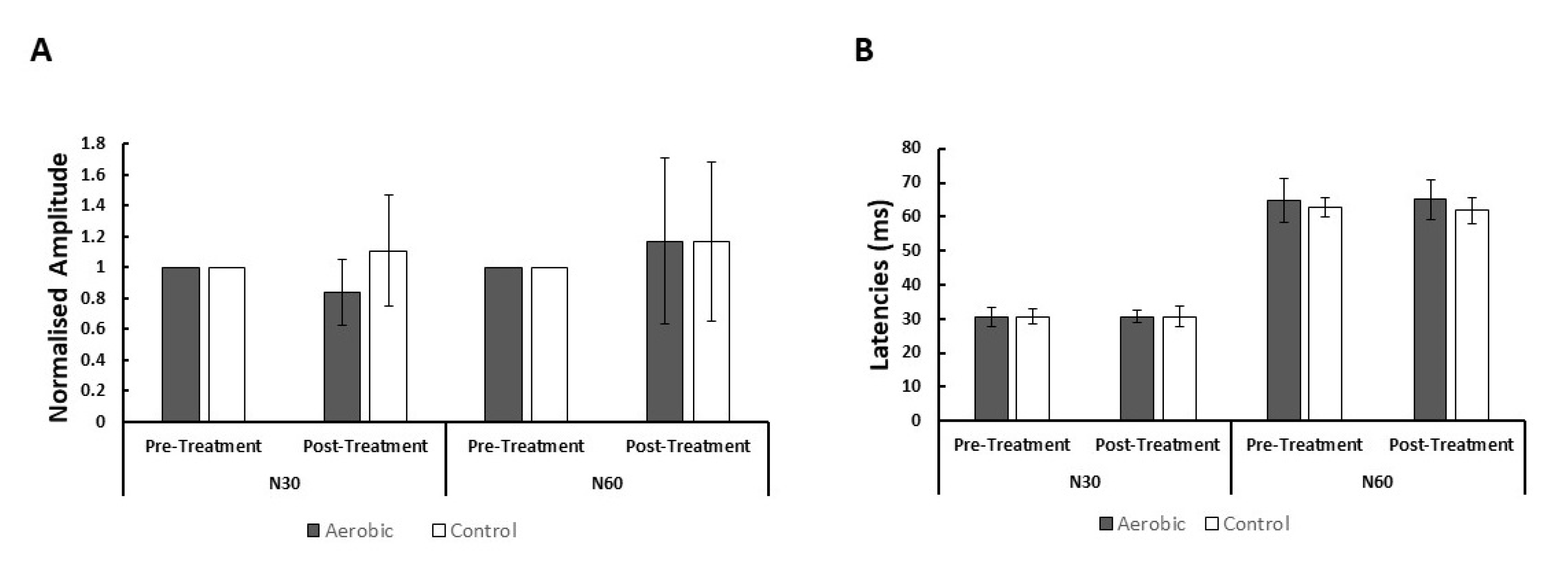
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bloom, D.E. 7 billion and counting. Science 2011, 333, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A. A problem for our age. Nature 2011, 475, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Deyn, P.; Goeman, J.; Vervaet, A.; Dourcy-Belle-Rose, B.; Van Dam, D.; Geerts, E. Prevalence and incidence of dementia among 75–80-year-old community-dwelling elderly in different districts of Antwerp, Belgium: The Antwerp Cognition (ANCOG) Study. Clin. Neurol. Neurosurg. 2011, 113, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Stokin, G.B.; Goldstein, L.S. Axonal transport and Alzheimer’s disease. Annu. Rev. Biochem. 2006, 75, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Klafki, H.W.; Staufenbiel, M.; Kornhuber, J.; Wiltfang, J. Therapeutic approaches to Alzheimer’s disease. Brain 2006, 129, 2840–2855. [Google Scholar] [CrossRef]
- Levey, A.; Lah, J.; Goldstein, F.; Steenland, K.; Bliwise, D. Mild cognitive impairment: An opportunity to identify patients at high risk for progression to Alzheimer’s disease. Clin. Ther. 2006, 28, 991–1001. [Google Scholar] [CrossRef]
- Gauthier, S. Alzheimer’s disease: The benefits of early treatment. Eur. J. Neurol. 2005, 12, 11–16. [Google Scholar] [CrossRef]
- Suva, D.; Favre, I.; Kraftsik, R.; Esteban, M.; Lobrinus, A.; Miklossy, J. Primary motor cortex involvement in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999, 58, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Geula, C.; Mesulam, M.M. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cerebral Cortex 1996, 6, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Teipel, S.J.; Stahl, R.; Dietrich, O.; Schoenberg, S.O.; Perneczky, R.; Bokde, A.L.; Reiser, M.F.; Möller, H.J.; Hampel, H. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. Neuroimage 2007, 34, 985–995. [Google Scholar] [CrossRef]
- Uylings, H.B.; De Brabander, J. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002, 49, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Kovach, C.R. Sensoristasis and imbalance in persons with dementia. J. Nurs. Scholarsh. 2000, 32, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Mansfield, J.; Dakheel-Ali, M.; Marx, M.S.; Thein, K.; Regier, N.G. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015, 228, 59–64. [Google Scholar] [PubMed] [Green Version]
- Allison, T.; McCarthy, G.; Wood, C.C. The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalogr. Clin. Neurophysiol. 1992, 84, 301–314. [Google Scholar]
- Rossi, S.; della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef]
- Bulut, S.; Özmerdivenli, R.; Bayer, H. Effects of exercise on somatosensory-evoked potentials. Int. J. Neurosci. 2003, 113, 315–322. [Google Scholar] [CrossRef]
- Nakata, H.; Oshiro, M.; Namba, M.; Shibasaki, M. Effects of aerobic exercise under different thermal conditions on human somatosensory processing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R629–R636. [Google Scholar]
- Walsh, P.; Kane, N.; Butler, S. The clinical role of evoked potentials. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. 2), ii16–ii22. [Google Scholar]
- Allison, T.; McCarthy, G.; Wood, C.C.; Darcey, T.M.; Spencer, D.D.; Williamson, P.D. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating short-latency activity. J. Neurophysiol. 1989, 62, 694–710. [Google Scholar] [CrossRef]
- Allison, T.; McCarthy, G.; Wood, C.C.; Williamson, P.D.; Spencer, D.D. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J. Neurophysiol. 1989, 62, 711–722. [Google Scholar] [PubMed]
- Inui, K.; Wang, X.; Tamura, Y.; Kaneoke, Y.; Kakigi, R. Serial processing in the human somatosensory system. Cereb. Cortex 2004, 14, 851–857. [Google Scholar] [PubMed]
- Chéron, G.; Borenstein, S. Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 537–548. [Google Scholar] [PubMed]
- Chéron, G.; Borenstein, S. Gating of the early components of the frontal and parietal somatosensory evoked potentials in different sensory-motor interference modalities. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1991, 80, 522–530. [Google Scholar]
- Kida, T.; Wasaka, T.; Nakata, H.; Kakigi, R. Centrifugal regulation of task-relevant somatosensory signals to trigger a voluntary movement. Exp. Brain Res. 2006, 169, 289–301. [Google Scholar] [CrossRef]
- Cebolla, A.M.; Palmero-Soler, E.; Dan, B.; Cheron, G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. NeuroImage 2011, 54, 1297–1306. [Google Scholar] [CrossRef]
- Mauguiere, F.; Desmedt, J.; Courjon, J. Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions: Detailed correlations with clinical signs and computerized tomographic scanning. Brain 1983, 106, 271–311. [Google Scholar] [CrossRef]
- Urushihara, R.; Murase, N.; Rothwell, J.C.; Harada, M.; Hosono, Y.; Asanuma, K.; Shimazu, H.; Nakamura, K.; Chikahisa, S.; Kitaoka, K. Effect of repetitive transcranial magnetic stimulation applied over the premotor cortex on somatosensory-evoked potentials and regional cerebral blood flow. Neuroimage 2006, 31, 699–709. [Google Scholar]
- Kaňovský, P.; Bareš, M.; Rektor, I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: Evidence from intracerebral recordings. Clin. Neurophysiol. 2003, 114, 981–991. [Google Scholar] [CrossRef]
- Rossini, P.; Gigli, G.; Marciani, M.; Zarola, F.; Caramia, M. Non-invasive evaluation of input-output characteristics of sensorimotor cerebral areas in healthy humans. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1987, 68, 88–100. [Google Scholar]
- Allison, T.; McCarthy, G.; Wood, C.C.; Jones, S.J. Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve: A review of scalp and intracranial recordings. Brain 1991, 114, 2465–2503. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Huy, N.T.; Bourguet, M. The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest electrical signs of sensory processing in man. Electroencephalogr. Clin. Neurophysiol. 1983, 56, 272–282. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Reni, L.; Cocito, L.; Ratto, S.; Abbruzzese, M.; Favale, E. Short-latency somatosensory evoked potentials in degenerative and vascular dementia. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1034–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen-Dahm, C.; Madsen, C.S.; Waldemar, G.; Ballegaard, M.; Hejl, A.M.; Johnsen, B.; Jensen, T.S. Contact heat evoked potentials (CHEPs) in patients with mild-moderate Alzheimer’s disease and matched control—A pilot study. Pain Med. 2015, 17, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, R.; Del Gracco, S.; Elia, M.; Musumeci, S.; Spada, R.; Stefanini, M. Scalp topographic mapping of middle-latency somatosensory evoked potentials in normal aging and dementia. Neurophysiol. Clin./Clin. Neurophysiol. 1996, 26, 311–319. [Google Scholar] [CrossRef]
- Stephen, J.M.; Montaño, R.; Donahue, C.H.; Adair, J.C.; Knoefel, J.K.; Qualls, C.; Hart, B.; Ranken, D.; Aine, C.J. Somatosensory responses in normal aging, mild cognitive impairment, and Alzheimer’s disease. J. Neural Transm. 2010, 117, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, H.; Takeda, M.; Okuda, B.; Kawabata, K.; Nishimura, H.; Kodama, N.; Iwamoto, Y.; Sugita, M. Multimodal evoked potentials in Alzheimer’s disease and Binswanger’s disease. J. Geriatr. Psychiatry Neurol. 1996, 9, 7–12. [Google Scholar] [CrossRef]
- Ito, J. Somatosensory event-related potentials (ERPs) in patients with different types of dementia. J. Neurol. Sci. 1994, 121, 139–146. [Google Scholar] [CrossRef]
- O’Leary, K.C.; Pontifex, M.B.; Scudder, M.R.; Brown, M.L.; Hillman, C.H. The effects of single bouts of aerobic exercise, exergaming, and videogame play on cognitive control. Clin. Neurophysiol. 2011, 122, 1518–1525. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.; Gapin, J.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, R.; Darby, L.A.; Fullenkamp, A.; Morgan, A.L. Effects of acute aerobic exercise on executive function in older women. J. Sports Sci. Med. 2015, 14, 574. [Google Scholar] [PubMed]
- Perciavalle, V.; Alagona, G.; De Maria, G.; Rapisarda, G.; Costanzo, E.; Perciavalle, V.; Coco, M. Somatosensory evoked potentials and blood lactate levels. Neurol. Sci. 2015, 36, 1597–1601. [Google Scholar] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. 2019. Available online: https://www.mocatest.org/ (accessed on 22 September 2020).
- She, J.; Nakamura, H.; Makino, K.; Ohyama, Y.; Hashimoto, H. Selection of suitable maximum-heart-rate formulas for use with Karvonen formula to calculate exercise intensity. Int. J. Autom. Comput. 2015, 12, 62–69. [Google Scholar]
- Kamijo, K.; Hayashi, Y.; Sakai, T.; Yahiro, T.; Tanaka, K.; Nishihira, Y. Acute effects of aerobic exercise on cognitive function in older adults. J. Gerontol. Ser. B 2009, 64, 356–363. [Google Scholar]
- Nakata, H.; Oshiro, M.; Namba, M.; Shibasaki, M. Effects of passive heat stress on human somatosensory processing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R1387–R1396. [Google Scholar]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 569–579. [Google Scholar]
- Amjad, I.; Toor, H.; Niazi, I.K.; Afzal, H.; Jochumsen, M.; Shafique, M.; Allen, K.; Haavik, H.; Ahmed, T. Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int. J. Neurosci. 2019, 129, 551–562. [Google Scholar]
- Tian, Q.; Chastan, N.; Bair, W.N.; Resnick, S.M.; Ferrucci, L.; Studenski, S.A. The brain map of gait variability in aging, cognitive impairment and dementia—A systematic review. Neurosci. Biobehav. Rev. 2017, 74, 149–162. [Google Scholar]
- Nuwer, M.R. Fundamentals of evoked potentials and common clinical applications today. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 142–148. [Google Scholar]
- Jones, S. An ‘interference’approach to the study of somatosensory evoked potentials in man. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 517–530. [Google Scholar] [CrossRef]
- Kakigi, R.; Jones, S. Effects on median nerve SEPs of tactile stimulation applied to adjacent and remote areas of the body surface. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1985, 62, 252–265. [Google Scholar] [CrossRef]
- Kida, T.; Wasaka, T.; Nakata, H.; Akatsuka, K.; Kakigi, R. Centrifugal regulation of a task-relevant somatosensory signal triggering voluntary movement without a preceding warning signal. Exp. Brain Res. 2006, 173, 733. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Inui, K.; Wasaka, T.; Nishihira, Y.; Kakigi, R. Mechanisms of differences in gating effects on short-and long-latency somatosensory evoked potentials relating to movement. Brain Topogr. 2003, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Yamashiro, K.; Onishi, H.; Shimoyama, Y.; Yoshida, T.; Maruyama, A. The effect of water immersion on short-latency somatosensory evoked potentials in human. BMC Neurosci. 2012, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, P.W.; Moseley, G.L. Pain and motor control of the lumbopelvic region: Effect and possible mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef]
- Kluger, A.; Gianutsos, J.G.; Golomb, J.; Ferris, S.H.; George, A.E.; Franssen, E.; Reisberg, B. Patterns of motor impairment in normal aging, mild cognitive decline, and early Alzheimer’Disease. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1997, 52, P28–P39. [Google Scholar]
- Schröter, A.; Mergl, R.; Bürger, K.; Hampel, H.; Möller, H.J.; Hegerl, U. Kinematic analysis of handwriting movements in patients with Alzheimer’s disease, mild cognitive impairment, depression and healthy subjects. Dement. Geriatr. Cogn. Disord. 2003, 15, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Suzumura, S.; Osawa, A.; Maeda, N.; Sano, Y.; Kandori, A.; Mizuguchi, T.; Yin, Y.; Kondo, I. Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr. Gerontol. Int. 2018, 18, 907–914. [Google Scholar]
- Mustroph, M.L.; Chen, S.; Desai, S.C.; Cay, E.B.; DeYoung, E.K.; Rhodes, J.S. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 2012, 219, 62–71. [Google Scholar]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of dysfunctional spinal joints affects sensorimotor integration in the prefrontal cortex: A brain source localization study. Neural Plast. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, H.H.; Murphy, B. Altered sensorimotor integration with cervical spine manipulation. J. Manip. Physiol. Ther. 2008, 31, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.; Murphy, B. The Effect of Sacroiliac Joint Manipulation on Feed-Forward Activation Times of the Deep Abdominal Musculature. J. Manip. Physiol. Ther. 2006, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Cheron, G. Somatosensory evoked potentials to finger stimulation in healthy octogenarians and in young adults: Wave forms, scalp topography and transit times of pariental and frontal components. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 404–425. [Google Scholar] [CrossRef]
- Kakigi, R.; Shibasaki, H. Effects of age, gender, and stimulus side on scalp topography of somatosensory evoked potentials following median nerve stimulation. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1991, 8, 320–330. [Google Scholar] [CrossRef]
- Klunk, W.; Panchalingam, K.; Moossy, J.; McClure, R.; Pettegrew, J. N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: A preliminary proton nuclear magnetic resonance study. Neurology 1992, 42, 1578–1585. [Google Scholar] [CrossRef]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R.; Barnes, C.L. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science 1984, 225, 1168–1170. [Google Scholar] [CrossRef]
- Nitsch, R.; Frotscher, M. Reduction of posttraumatic transneuronal” early gene” activation and dendritic atrophy by the N-methyl-D-aspartate receptor antagonist MK-801. Proc. Natl. Acad. Sci. USA 1992, 89, 5197–5200. [Google Scholar] [CrossRef] [Green Version]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; dos Santos Pedroso, G.; Nesi, R.T.; de Andrade, V.M. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef]
- Singh, A.M.; Staines, W.R. The effects of acute aerobic exercise on the primary motor cortex. J. Mot. Behav. 2015, 47, 328–339. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amjad, I.; Niazi, I.K.; Toor, H.G.; Nedergaard, R.B.; Shafique, M.; Holt, K.; Haavik, H.; Ahmed, T. Acute Effects of Aerobic Exercise on Somatosensory-Evoked Potentials in Patients with Mild Cognitive Impairment. Brain Sci. 2020, 10, 663. https://doi.org/10.3390/brainsci10100663
Amjad I, Niazi IK, Toor HG, Nedergaard RB, Shafique M, Holt K, Haavik H, Ahmed T. Acute Effects of Aerobic Exercise on Somatosensory-Evoked Potentials in Patients with Mild Cognitive Impairment. Brain Sciences. 2020; 10(10):663. https://doi.org/10.3390/brainsci10100663
Chicago/Turabian StyleAmjad, Imran, Imran Khan Niazi, Hamza Ghazanfar Toor, Rasmus Bach Nedergaard, Muhammad Shafique, Kelly Holt, Heidi Haavik, and Touqeer Ahmed. 2020. "Acute Effects of Aerobic Exercise on Somatosensory-Evoked Potentials in Patients with Mild Cognitive Impairment" Brain Sciences 10, no. 10: 663. https://doi.org/10.3390/brainsci10100663







