Motor–Cognitive Interventions May Effectively Improve Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
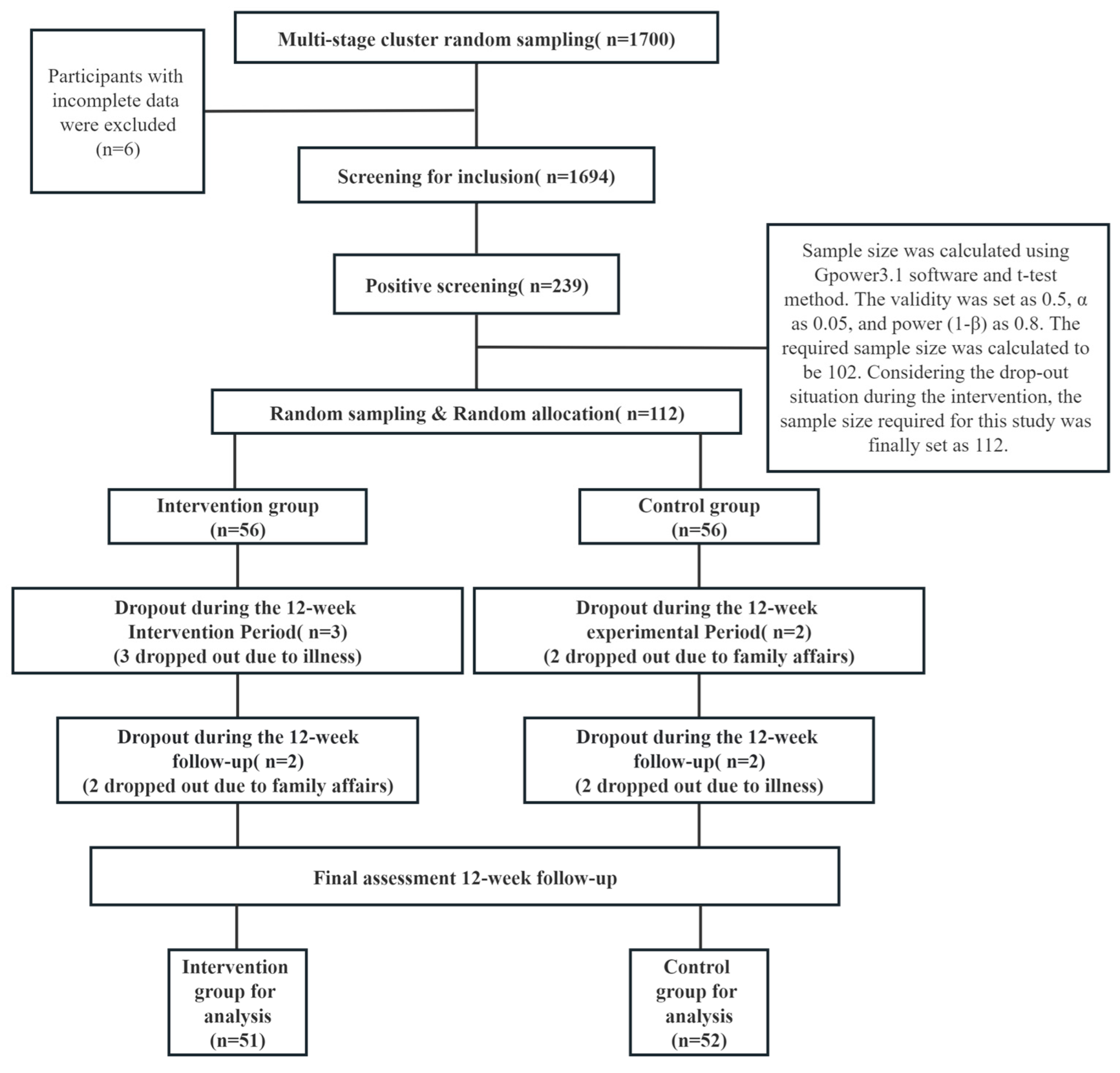
2.1. Participants
2.2. Measures
2.2.1. Demographics
2.2.2. Mini-Mental State Examination (MMSE)
2.2.3. Montreal Cognitive Assessment Scale (MoCA)
2.2.4. Activity of Daily Living Scale (ADLs)
2.2.5. Intervention Methods
2.3. Procedure
2.4. Sample Size Calculation
2.5. Data Analysis
3. Results
3.1. Comparison of the Demographic Characteristics of the Two Groups
3.2. Comparison of Initial Daily Living Ability between the Two Groups
3.3. Analysis of the Effect of the Intervention
Comparison of the Total MoCA and MMSE Scores of the Two Groups
4. Discussion
4.1. Analysis of General Information for the Intervention and Control Groups
4.2. Analysis of the Implementation of the Intervention Plan
4.3. Intervention Effects of the Exercise-Cognitive Intervention
4.3.1. Improving Cognitive Function
4.3.2. Trends in Cognitive Function
4.3.3. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Lyu, X.Y.; Lyu, S.J.; Zhao, R. The effect of social participation on income-related ineguality in health outcome among Chinese older adults. Int Health. 2021, 13, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Matyas, N.; Auer, S.; Gisinger, C.; Kil, M.; Aschenberger, F.K.; Klerings, I.; Gartlehner, G. Continuing education for the prevention of mild cognitive impairment and Alzheimer’s-type dementia: A systematic review protocol. Syst. Rev. 2017, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Bature, F.; Guinn, B.A.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open. 2017, 7, e015746. [Google Scholar] [CrossRef]
- Duara, R.; Loewenstein, D.A.; Greig-Custo, M.T.; Raj, A.; Barker, W.; Potter, E.; Schofield, E.; Small, B.; Schinka, J.; Wu, Y.G.; et al. Diagnosis and staging of mild cognitive impairment, using a modification of the clinical dementia rating scale: The mCDR. Int. J. Geriatr. Psychiatry 2010, 25, 282–289. [Google Scholar] [CrossRef]
- Langa, K.M.; Levine, D.A. The Diagnosis and Management of Mild Cognitive Impairment A Clinical Review. Jama-J. Am. Med. Assoc. 2014, 312, 2551–2561. [Google Scholar] [CrossRef]
- Brasure, M.; Desai, P.; Davila, H.; Nelson, V.A.; Calvert, C.; Jutkowitz, E.; Butler, M.; Fink, H.A.; Ratner, E.; Hemmy, L.S.; et al. Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia a Systematic Review. Ann. Intern. Med. 2018, 168, 30–38. [Google Scholar] [CrossRef]
- Butler, M.; Nelson, V.A.; Davila, H.; Ratner, E.; Fink, H.A.; Hemmy, L.S.; McCarten, J.R.; Barclay, T.R.; Brasure, M.; Kane, R.L. Over-the-Counter Supplement Interventions to Prevent Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer-Type Dementia a Systematic Review. Ann. Intern. Med. 2018, 168, 52–62. [Google Scholar] [CrossRef]
- Delbroek, T.; Vermeylen, W.; Spildooren, J. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: A randomized controlled trial. J. Phys. Ther. Sci. 2017, 29, 1137–1143. [Google Scholar] [CrossRef]
- Daffner, K.R. Promoting Successful Cognitive Aging: A Comprehensive Review. J. Alzheimer’s Dis. 2010, 19, 1101–1122. [Google Scholar] [CrossRef]
- Singh, M.A.F.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. The Study of Mental and Resistance Training (SMART) Study-Resistance Training and/or Cognitive Training in Mild Cognitive Impairment: A Randomized, Double-Blind, Double-Sham Controlled Trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Xie, M.; Tao, J.; Fang, Y.H.; Chen, S.J.; Chen, L.D. Study and application of the Simple Intelligence Mental State Examination Scale. Chin. J. Rehabil. Med. 2016, 31, 694–696+706. [Google Scholar] [CrossRef]
- China Dementia and Cognitive Disorders Guidelines Writing Group; Cognitive Disorders Committee of the Neurologist Branch of the Chinese Medical Association. 2018 Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment (I): Dementia and its classification and diagnostic criteria. Natl. Med. J. China 2018, 98, 965–970. [Google Scholar] [CrossRef]
- China Dementia and Cognitive Disorders Guidelines Writing Group; Cognitive Disorders Committee of the Neurologist Branch of the Chinese Medical Association. 2018 Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment (V): Diagnosis and treatment of mild cognitive impairment. Natl. Med. J. China 2018, 98, 1294–1301. [Google Scholar] [CrossRef]
- Katzman, R.; Zhang, M.Y.; Ouang Ya, Q.; Wang, Z.Y.; Liu, W.T.; Yu, E.; Wong, S.C.; Salmon, D.P.; Grant, I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Li, F.; Zhou, A.H.; Wang, F.; Zuo, X.M.; Jia, X.F.; Song, H.Q.; Jia, J.P. Montreal Cognitive Assessment in Detecting Cognitive Impairment in Chinese Elderly Individuals: A Population-Based Study. J. Geriatr. Psychiatry Neurol. 2011, 24, 184–190. [Google Scholar] [CrossRef]
- Wade, D.T.; Colin, C. The Barthel ADL index: A standard measure of physical disability? Int. Disabil. Stud. 1988, 10, 64–67. [Google Scholar] [CrossRef]
- Zhang, L.X.; Liu, X.Q. Determination of the Cut-off Point of the Chinese Version of the Montreal Cognitive Assessment among Chinese Elderly in Guangzhou. Chin. Ment. Health J. 2008, 22, 123–125+151. [Google Scholar] [CrossRef]
- Cancela, J.M.; Suarez, M.H.V.; Vasconcelos, J.; Lima, A.; Ayan, C. Efficacy of Brain Gym Training on the Cognitive Performance and Fitness Level of Active Older Adults: A Preliminary Study. J. Aging Phys. Act. 2015, 23, 653–658. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, W. Aerobic exercise intervention for elderly people with mild cognitive impairment in a nursing home. Chin. J. Gerontol. 2019, 39, 3176–3178. [Google Scholar]
- Hayden, R.; Clair, A.A.; Johnson, G. The effect of rhythmic auditory stimulation (RAS) on physical therapy outcomes for patients in gait training following stroke: A feasibility study. Int. J. Neurosci. 2009, 119, 2183–2195. [Google Scholar] [CrossRef]
- Reid, K.F.; Pasha, E.; Doros, G.; Clark, D.J.; Patten, C.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: Influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur. J. Appl. Physiol. 2014, 114, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Ogawa, J.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. Physical Exercise with Music Maintains Activities of Daily Living in Patients with Dementia: Mihama-Kiho Project Part 2. J. Alzheimer’s Dis. 2017, 57, 85–96. [Google Scholar] [CrossRef]
- Tabei, K.; Satoh, M.; Ogawa, J.; Tokita, T.; Nakaguchi, N.; Nakao, K.; Kida, H.; Tomimoto, H. Physical Exercise with Music Reduces Gray and White Matter Loss in the Frontal Cortex of Elderly People: The Mihama-Kiho Scan Project. Front. Aging Neurosci. 2017, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.S.; Shi, C.Q.; Zheng, S.Q.; Miu, Y.T.; Liu, B.B.; Zhang, T. Effects of cognitive intervention on cognitive function and quality of life ofthe elderly with mild cognitive impairment in community. Clin. Res. Pract. 2020, 5, 152–153. [Google Scholar] [CrossRef]
- Ye, L.S. Research progress of exercise training on cognitive function for patients with Alzheimer’s disease. J. Clin. Nurs. Pract. 2020, 6, 277–280. [Google Scholar]
- Werner, P.; Rabinowitz, S.; Klinger, E.; Korczyn, A.D.; Josman, N. Use of the Virtual Action Planning Supermarket for the Diagnosis of Mild Cognitive Impairment [Article]. Dement. Geriatr. Cogn. Disord. 2009, 27, 301–309. [Google Scholar] [CrossRef]
- Levaux, M.N.; Laroi, F.; Malmedier, M.; Offerlin-Meyer, I.; Danion, J.M.; Van der Linden, M. Rehabilitation of executive functions in a real-life setting: Goal management training applied to a person with schizophrenia. Case Rep. Psychiatry 2012, 2012, 503023. [Google Scholar] [CrossRef]
- Park, J.H. Does the virtual shopping training improve executive function and instrumental activities of daily living of patients with mild cognitive impairment? Asian J. Psychiatry 2022, 69, 102977. [Google Scholar] [CrossRef]
- Shi, J.P. The methods of sample size estimation in clinical study. Chin. J. Tissue Eng. Res. 2003, 7, 1569–1571. [Google Scholar]
- Dai, F.; Kong, L.L.; Chen, J.; Wang, C.X. Prevalence rate and influencing factors of mild cognitive impairment in the elderly in Qingdao community. J. Psychiatry 2019, 32, 195–199. [Google Scholar]
- Legault, C.; Jennings, J.M.; Katula, J.A.; Dagenbach, D.; Gaussoin, S.A.; Sink, K.M.; Rapp, S.R.; Rejeski, W.J.; Shumaker, S.A.; Espeland, M.A.; et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: The Seniors Health and Activity Research Program Pilot (SHARP-P) Study, a randomized controlled trial. BMC Geriatr. 2011, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.; Schumacher, V. The Interplay between Cognitive and Motor Functioning in Healthy Older Adults: Findings from Dual-Task Studies and Suggestions for Intervention. Gerontology 2011, 57, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Li, X. An introduction to the motivation of middle-aged and elderly women to participate in square dance for fitness. Contemp. Sports Technol. 2019, 9, 156–157. [Google Scholar] [CrossRef]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined Intervention of Physical Activity, Aerobic Exercise, and Cognitive Exercise Intervention to Prevent Cognitive Decline for Patients with Mild Cognitive Impairment: A Randomized Controlled Clinical Study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lin, F.V.; Salisbury, D.L.; Shah, K.N.; Chow, L.; Vock, D.; Nelson, N.W.; Porsteinsson, A.P.; Jack, C. Efficacy and mechanisms of combined aerobic exercise and cognitive training in mild cognitive impairment: Study protocol of the ACT trial. Trials 2018, 19, 700. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Chen, L.; Shen, X.F. Effects of moderate aerobic exercise on elderly patients with mild cognitive impairment: A Meta-analysis. Chinses Nurs. Res. 2021, 35, 3235–3241. [Google Scholar] [CrossRef]
- Wang, T.T.; Feng, W.B.; Zhou, G.J.; Deng, C.Y.; Sun, G.H.; Liao, Y. Research Advances in Rehabilitation Treatment for Mild Cognitive Impairment in The Elderly. Chin. J. Gerontol. 2022, 42, 1773–1778. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ocana-Fernandez, M.A.; Vega-Rivera, N.M.; Torres-Perez, O.M.; Gomez-Sanchez, A.; Estrada-Camarena, E.; Ortiz-Lopez, L. Environmental enrichment induces neuroplastic changes in middle age female BALBC mice and increases the hippocampal levels of BDNF, P-AKT AND P-MAPK1/2. Neuroscience 2014, 260, 158–170. [Google Scholar] [CrossRef]
- Hagovska, M.; Olekszyova, Z. Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment. Geriatr. Gerontol. Int. 2016, 16, 1043–1050. [Google Scholar] [CrossRef]
- Davis, R.N.; Massman, P.J.; Doody, R.S. Cognitive intervention in Alzheimer disease: A randomized placebo-controlled study. Alzheimer Dis. Assoc. Disord. 2001, 15, 1–9. [Google Scholar] [CrossRef]
- Olchik, M.R.; Farina, J.; Steibel, N.; Teixeira, A.R.; Yassuda, M.S. Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Arch. Gerontol. Geriatr. 2013, 56, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Yvon, C. A review of multi domain interventions to support healthy cognitive ageing. J. Nutr. Health Aging 2013, 17, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Colcombe, S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study-Revisited. Perspect. Psychol. Sci. 2018, 13, 213–217. [Google Scholar] [CrossRef]
- Feng, W.; Li, C.; Chen, Y.; Cheng, Y.; Wu, W. Five-year follow-up study of multi-domain cognitive training for healthy elderly community members. Shanghai Arch. Psychiatry 2014, 26, 30–41. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yin, S.F.; Lang, M.J.; He, R.Q.; Li, J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016, 31, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Chen, A.G.; Yin, H.C.; Yan, J.; Yang, Y. Effects of Acute Aerobic Exercise of Different Intensity on Executive Function. Acta Psychol. Sin. 2011, 43, 1055–1062. [Google Scholar]
- Lauenroth, A.; Ioannidis, A.E.; Teichmann, B. Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 2016, 16, 141. [Google Scholar] [CrossRef]
- Stojan, R.; Voelcker-Rehage, C. Fitness effects on the cognitive function of older adults: A meta-analytic study. J. Clin. Med. 2019, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Bisbe, M.; Fuente-Vidal, A.; Lopez, E.; Moreno, M.; Naya, M.; de Benetti, C.; Mila, R.; Bruna, O.; Boada, M.; Alegret, M. Comparative Cognitive Effects of Choreographed Exercise and Multimodal Physical Therapy in Older Adults with Amnestic Mild Cognitive Impairment: Randomized Clinical Trial. J. Alzheimer’s Dis. 2020, 73, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]


| Variables | Classification | Intervention Group (n = 51) | Control Group (n = 52) | t/χ2/Z | p |
|---|---|---|---|---|---|
| Age | 73.41 ± 7.785 | 74.90 ± 6.613 | 1.05 a | 0.297 | |
| Gender | Male | 16 (31.37%) | 20 (38.46%) | 0.341 b | 0.560 |
| Female | 35 (68.63%) | 32 (61.54%) | |||
| Marital status | No partner | 8 (15.69%) | 12 (23.08%) | 0.840 b | 0.361 |
| With partner | 43 (84.31%) | 40 (76.92%) | |||
| Living arrangement | Living alone | 3 (5.88%) | 4 (7.69%) | 0.000 b | 1.000 |
| Not living alone | 48 (94.12%) | 48 (92.31%) | |||
| Education | Below junior high school | 29 (56.86%) | 35 (67.31%) | 2.448 b | 0.121 |
| Lower secondary and above | 22 (43.14%) | 17 (32.69%) | |||
| ADL | \ | 100.00 (100.00,100.00) | 100.00 (95.00,100.00) | 1.544 c | 0.123 |
| Topics | Contents | |
|---|---|---|
| First | Getting to know each other and gaining trust | Describing the purpose of the intervention, the members of the team |
| Group members introduce themselves to each other | ||
| A proper understanding of MCI disease and dementia | Explaining the relationship between MCI disease and dementia | |
| Sports interventions | Five senses exercise, brain exercise (aerobic exercise), breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: gestures practice; map puzzles | ||
| Executive function training: simulated shopping training | ||
| Second | Relationship: cognitive impairment and mental health | What is mental health and depression? |
| Sports interventions | Five senses exercise, brain exercise, breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: song recognition; character matching | ||
| Executive function training: simulated shopping training | ||
| Third | Relationship: cognitive impairment and movement | Choosing appropriate exercises and developing an exercise plan |
| Sports interventions | Five senses exercise, brain exercise, breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: number addition and subtraction; simulated trips | ||
| Executive function training: simulated shopping training | ||
| Fourth | Relationship: cognitive impairment and sleep | Introduction to the importance of sleep |
| How to improve the quality of your sleep | ||
| Factors causing sleep disorders | ||
| Sports interventions | Five senses exercise, brain exercise, breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: animal sound recognition; map puzzles | ||
| Executive function training: simulated shopping training | ||
| Fifth | Relationship: cognitive impairment and chronic illness | Types of chronic diseases |
| Risk factors for chronic diseases | ||
| The dangers of chronic disease for MCI disease | ||
| Sports interventions | Five senses exercise, brain exercise, breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: gestures practice; character matching | ||
| Executive function training: simulated shopping training | ||
| Sixth | Experience | Effective exercise–cognitive training techniques |
| Sports interventions | Five senses exercise, brain exercise, breathing exercise | |
| Cognitive interventions | Attention training: Schulte squares | |
| Memory training: song recognition; simulated trips | ||
| Executive function training: simulated shopping training |
| T1 | T2 | T3 | Repeat Measurement F-Test | ||
|---|---|---|---|---|---|
| M ± SD | F | η2P | |||
| MoCA | |||||
| intervention group | 20.63 ± 2.088 | 23.59 ± 1.899 | 23.27 ± 1.799 | ||
| control group | 20.69 ± 2.280 | 20.44 ± 2.261 | 20.31 ± 2.147 | ||
| t | 0.151 | −7.651 | −7.643 | ||
| p | 0.881 | 0.000 | 0.000 | ||
| Group | 30.277 *** | 0.231 | |||
| Time | 28.081 *** | 0.36 | |||
| Group × Time | 39.550 *** | 0.442 | |||
| MMSE | |||||
| intervention group | 24.33 ± 1.785 | 25.73 ± 1.343 | 25.86 ± 1.456 | ||
| control group | 24.00 ± 1.715 | 23.65 ± 1.792 | 23.73 ± 1.921 | ||
| t | −0.966 | −6.648 | −6.355 | ||
| p | 0.336 | 0.000 | 0.000 | ||
| Group | 25.587 *** | 0.202 | |||
| Time | 9.821 *** | 0.164 | |||
| Group × Time | 24.614 *** | 0.33 | |||
| T1 | T2 | T3 | Time | Group | Time × Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | t | p | Mean ± SD | t | p | Mean ± SD | t | p | F | |||
| Visuospatial/Executive | |||||||||||||
| Intervention group | 51 | 3.29 ± 1.101 | 0.655 | 0.514 | 3.92 ± 0.845 | −2.354 | 0.021 * | 4.00 ± 0.894 | −2.421 | 0.017 * | 10.662 *** | 1.945 | 8.510 *** |
| Control group | 52 | 3.44 ± 1.195 | 3.44 ± 1.195 | 3.52 ± 1.111 | |||||||||
| Naming | |||||||||||||
| Intervention group | 51 | 2.57 ± 0.575 | 1.029 | 0.306 | 2.92 ± 0.272 | −2.366 | 0.021 * | 2.96 ± 0.196 | −2.443 | 0.017 * | 12.689 *** | 1.284 | 10.250 *** |
| Control group | 52 | 2.69 ± 0.643 | 2.69 ± 0.643 | 2.75 ± 0.590 | |||||||||
| Attention | |||||||||||||
| Intervention group | 51 | 3.78 ± 1.064 | −1.582 | 0.117 | 3.96 ± 1.058 | −2.286 | 0.025 * | 3.78 ± 1.119 | −2.063 | 0.042 * | 3.680 * | 4.318 * | 0.86 |
| Control group | 52 | 3.38 ± 1.471 | 3.38 ± 1.471 | 3.27 ± 1.402 | |||||||||
| Language | |||||||||||||
| Intervention group | 51 | 2.49 ± 0.674 | 2.361 | 0.02 * | 2.96 ± 0.196 | −3.486 | 0.001 *** | 2.90 ± 0.300 | −3.516 | 0.001 *** | 4.336 * | 3.284 | 13.324 *** |
| Control group | 52 | 2.79 ± 0.605 | 2.54 ± 0.851 | 2.46 ± 0.851 | |||||||||
| Abstraction | |||||||||||||
| Intervention group | 51 | 1.57 ± 0.700 | −0.089 | 0.929 | 1.78 ± 0.461 | −2.294 | 0.024 * | 1.78 ± 0.461 | −3.261 | 0.002 ** | 4.726 * | 3.988 * | 4.726 * |
| Control group | 52 | 1.56 ± 0.539 | 1.56 ± 0.539 | 1.46 ± 0.541 | |||||||||
| Delayed Recall | |||||||||||||
| Intervention group | 51 | 1.41 ± 0.983 | 1.349 | 0.181 | 2.45 ± 1.301 | −4.007 | 0.000 *** | 2.18 ± 1.144 | −3.141 | 0.002 ** | 16.319 *** | 6.309 * | 14.549 *** |
| Control group | 52 | 1.63 ± 0.658 | 1.63 ± 0.658 | 1.60 ± 0.664 | |||||||||
| Orientation | |||||||||||||
| Intervention group | 51 | 5.47 ± 0.833 | −1.489 | 0.139 | 5.59 ± 0.669 | −2.289 | 0.024 * | 5.67 ± 0.589 | −2.606 | 0.011 * | 2.221 | 4.895 * | 0.862 |
| Control group | 52 | 5.19 ± 1.049 | 5.19 ± 1.049 | 5.25 ± 0.988 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, M.; Liu, H.; Cheng, J.; Yu, C.; Zhao, L. Motor–Cognitive Interventions May Effectively Improve Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Behav. Sci. 2023, 13, 737. https://doi.org/10.3390/bs13090737
Tao M, Liu H, Cheng J, Yu C, Zhao L. Motor–Cognitive Interventions May Effectively Improve Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Behavioral Sciences. 2023; 13(9):737. https://doi.org/10.3390/bs13090737
Chicago/Turabian StyleTao, Mingda, Huajun Liu, Jinxuan Cheng, Caiyun Yu, and Lili Zhao. 2023. "Motor–Cognitive Interventions May Effectively Improve Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial" Behavioral Sciences 13, no. 9: 737. https://doi.org/10.3390/bs13090737
APA StyleTao, M., Liu, H., Cheng, J., Yu, C., & Zhao, L. (2023). Motor–Cognitive Interventions May Effectively Improve Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Behavioral Sciences, 13(9), 737. https://doi.org/10.3390/bs13090737





