Metabolomics of Red Wines Aged Traditionally, with Chips or Staves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Standards
2.2. Ageing Conditions
2.3. Identification and Quantification of Aroma Compounds
2.3.1. Major Volatiles
2.3.2. Minor Volatiles
Extraction of Minor Aroma
Determination of Minor Aroma
2.4. Odour Activity Values (OAVs)
2.5. Aroma Series
2.6. Statistical Treatment
3. Results and Discussion
3.1. Volatile Compounds
3.2. Evolution of Chemical Families and Aroma Compounds Aged with Alternative and Traditional Systems
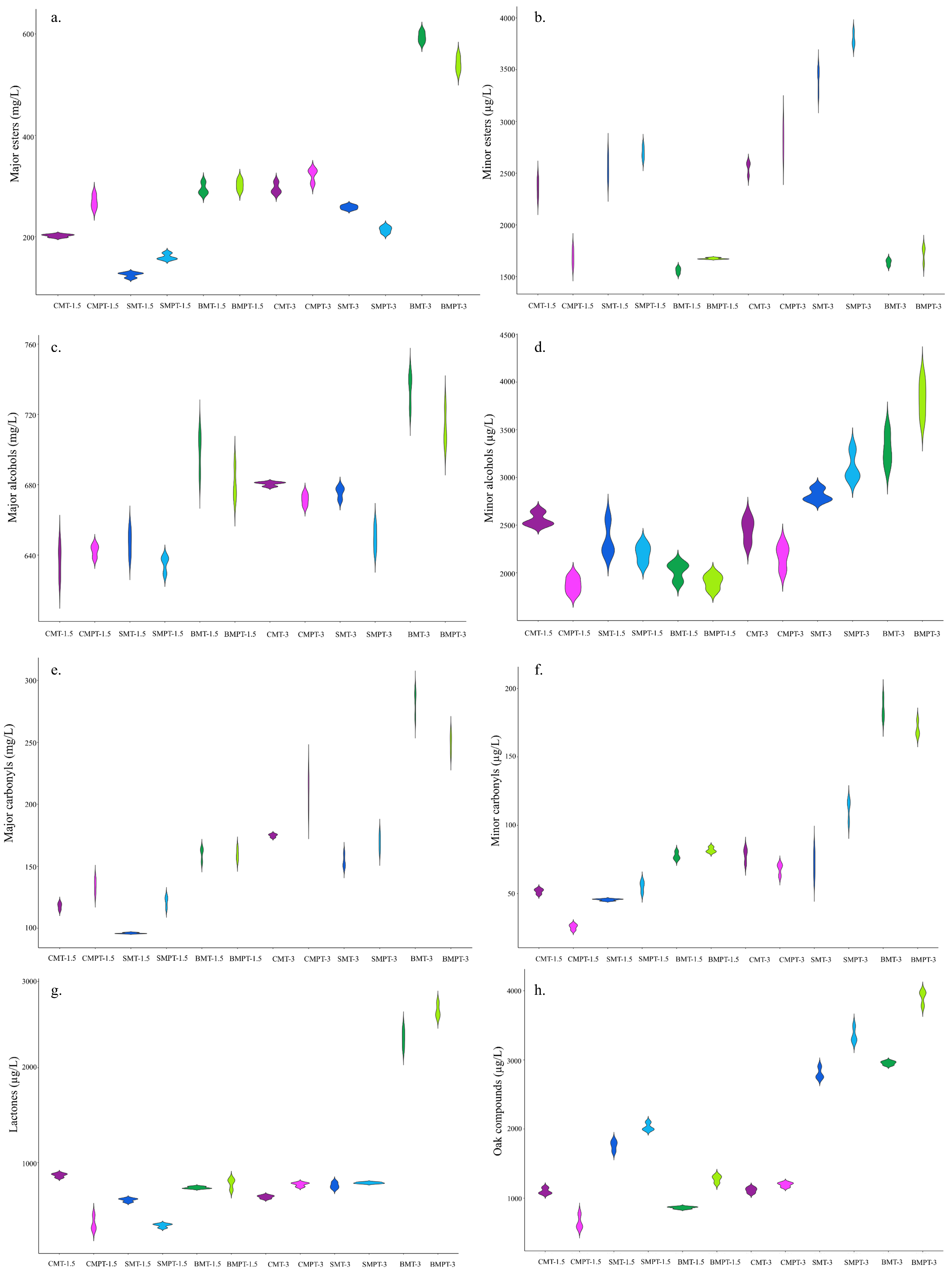
3.2.1. Esters
3.2.2. Alcohols
3.2.3. Carbonyls
3.2.4. Lactones
3.2.5. Oak Compounds
3.3. Odour Activity Values (OAVs)
3.4. Aromatic Series
3.5. Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
3.6. Cluster with Aroma Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chira, K.; Teissedre, P.-L. Chemical and sensory evaluation of wine matured in oak barrel: Effect of oak species involved and toasting process. Eur. Food Res. Technol. 2015, 240, 533–547. [Google Scholar] [CrossRef]
- Federico Casassa, L.; Ceja, G.M.; Vega-Osorno, A.; du Fresne, F.; Llodra, D. Detailed chemical composition of Cabernet Sauvignon wines aged in French oak barrels coopered with three different stave bending techniques. Food Chem. 2020, 340, 127573. [Google Scholar] [CrossRef] [PubMed]
- del Alamo-Sanza, M.; Nevares, I. Oak wine barrel as an active vessel: A critical review of past and current knowledge. Crit. Rev. Food Sci. Nutr. 2017, 58, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Fraile, P.; Garrido, J.; Ancin, C. Ancín Influence of a Saccharomyces selected strain in the volatile composition of Rosé wines. Evolution during fermentation. J. Agric. Food Chem. 2000, 48, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.D.; de Lerma, N.L.; Cotea, V.V.; Zamfir, C.I.; Peinado, R.A. Effect of aging time, dosage and toasting level of oak chips on the color parameters, phenolic compounds and antioxidant activity of red wines (var. Fetească neagră). Eur. Food Res. Technol. 2016, 242, 2171–2180. [Google Scholar] [CrossRef]
- García-Alcaraz, J.L.; Flor Montalvo, F.J.; Martínez Cámara, E.; Pérez de la Parte, M.M.; Jiménez-Macías, E.; Blanco-Fernández, J. Economic-environmental impact analysis of alternative systems for red wine ageing in re-used barrels. J. Clean. Prod. 2020, 244, 118783. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Anjos, O.; Lino, J.; Soares, A.; Belchior, P.A. Physicochemical and sensory evaluation of wine brandies aged using oak and chestnut wood simultaneously in wooden barrels and in stainless steel tanks with staves. Int. J. Food Sci. Technol. 2016, 51, 2537–2545. [Google Scholar] [CrossRef]
- Canas, S.; Anjos, O.; Caldeira, I.; Fernandes, T.A.; Santos, N.; Lourenço, S.; Granja-Soares, J.; Fargeton, L.; Boissier, B.; Catarino, S. Micro-oxygenation level as a key to explain the variation in the colour and chemical composition of wine spirits aged with chestnut wood staves. LWT-Food Sci. Technol. 2022, 154, 112658. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; Muiño, I.; Del Álamo, M.; Nevares, I. Volatile composition of toasted oak chips and staves and of red wine aged with them. Am. J. Enol. Vitic. 2010, 61, 157–165. [Google Scholar] [CrossRef]
- Spillman, P.J.; Sefton, M.; Gawel, R. The contribution of volatile compounds derived during oak barrel maturation to the aroma of a Chardonnay and Cabernet Sauvignon wine. Aust. J. Grape Wine Res. 2004, 10, 227–235. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E. Tratamiento de la Madera de Roble para Tonelería; Informe Técnico. IV Encuentro Enológico. Crianza en barricas y otras alternativas; Fundación para la Cultura del Vino: Madrid, Spain, 2007; pp. 9–23. [Google Scholar]
- Singleton, V.L. Maturation of wines and spirits: Comparisons, facts, and hypotheses. Am. J. Enol. Vitic. 1995, 46, 98–115. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Peinado, R.A.; Cotea, V.V.; López de Lerma, N. Volatilome fingerprint of red wines aged with chips or staves: Influence of the aging time and toasting degree. Food Chem. 2020, 310, 125801. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Boidron, J.N.; Pons, M. Incidence du traitement thermique du bois de chêne sur sa composition chimique. 2e Partie: Evolution de certains composés en fonction de l’intensité de brulage. OENO One 1989, 23, 223–250. [Google Scholar] [CrossRef]
- Koussissi, E.; Dourtoglou, V.G.; Ageloussis, G.; Paraskevopoulos, Y.; Dourtoglou, T.; Paterson, A. Influence of toasting of oak chips on red wine maturation from sensory and gas chromatographic headspace analysis. Food Chem. 2009, 114, 1503–1509. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- González Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC–MS. Food Chem. 2011, 129, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.-D.; de Lerma, N.L.; Luchian, C.E.; Cotea, V.V.; Peinado, R.A. Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols. Food Chem. 2018, 240, 751–758. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 5 May 2022).
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascues, E.; Benito, S. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Clarke, R.J. Wine: Flavour Chemistry, 2nd ed.; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Barrera-García, V.D.; Gougeon, R.D.; Di Majo, D.; De Aguirre, C.; Voilley, A.; Chassagne, D. Different sorption behaviors for wine polyphenols in contact with oak wood. J. Agric. Food Chem. 2007, 55, 7021–7027. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Domingues, L.; Teixeira, J.A.; Oliveira, J.M.; Tavares, T. Understanding wine sorption by oak wood: Modeling of wine uptake and characterization of volatile compounds retention. Food Res. Int. 2019, 116, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Martínez-García, R.; Roldán-Romero, Y.; Moreno, J.; Puig-Pujol, A.; Mauricio, J.C.; García-Martínez, T. Use of a flor yeast strain for the second fermentation of sparkling wines: Effect of endogenous CO2 over-pressure on the volatilome. Food Chem. 2019, 308, 125555. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J. Influencia del Tipo de Envejecimiento Sobre el Perfil Aromático de Vinos Generosos Andaluces. Ph.D. Thesis, University of Córdoba, Córdoba, Spain, 2005; pp. 25–150. [Google Scholar]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Pulgati, F.H.; Zini, C.A. Main differences between volatiles of sparkling and base wines accessed through comprehensive two dimensional gas chromatography with time-of-flight mass spectrometric detection and chemometric tools. Food Chem. 2014, 164, 427–437. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Etiévant, P.X. Wine. In Volatile Compounds of Food and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Buttery, B.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Cullere, L.; Ferreira, V.; Cacho, J. Analysis occurrence and potential sensory significance of aliphatic aldehydes in white wine. Food Chem. 2011, 127, 1397–1403. [Google Scholar] [CrossRef]
- Available online: http://www.leffingwell.com/esters.htm (accessed on 15 May 2023).
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Gómez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J. Quantitative determination of the odorants of Young red wine from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Peinado, R.; Moreno, J.; Medina, M.; Mauricio, J.C. Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol. Lett. 2004, 26, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, F.J.; Palacios-Fernández, S.; López de Lerma, N.; García-Martínez, T.; Mauricio, J.C.; Peinado, R.A. The Effect of Yeast, Sugar and Sulfur Dioxide on the Volatile Compounds in Wine. Fermentation 2023, 9, 541. [Google Scholar] [CrossRef]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014, 2014, 863019. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Sefton, M.A.; Taylor, D.K.; Elsey, G.M. An odour detection threshold determination of all four possible stereoisomers of oak lactone in a white and a red wine. Aust. J. Grape Wine Res. 2006, 12, 115–118. [Google Scholar] [CrossRef]
- Rocha, S.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine assessment of the identification of the would-be impact odourants. Anal. Chim. Act. 2004, 513, 257–262. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- López de Lerma, N.; Bellicontro, A.; Mencarelli, F.; Moreno, J.; Peinado, R.A. Use of electronic nose, validated by GC–MS, to establish the optimum off-vine dehydration time of wine grapes. Food Chem. 2012, 130, 447–452. [Google Scholar] [CrossRef]
- Jarauta, I.; Cacho, J.; Ferreira, V. Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: An analytical study. J. Agric. Food Chem. 2005, 53, 4166–4177. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Bretón, P.; Lorenzo, C.; Salinas, M.R.; Martínez, J.; Garde-Cerdán, T. Influence of oak barrel aging on the quality of red wines. In Oak: Ecology, Types and Management; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 59–86. [Google Scholar]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, M.R. Study of the evolution of nitrogen compounds during grape ripening. application to differentiate grape varieties and cultivated systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Bellincontro, A.; De Santis, D.; Botondi, R.; Villa, I.; Mencarelli, F. Different postharvest dehydration rates affect quality characteristics and volatile compounds of Malvasia, Trebbiano and Sangiovese grape for wine production. J. Sci. Food Agric. 2004, 84, 1791–1800. [Google Scholar] [CrossRef]
- Ubeda, C.; Cortiella, M.G.; Barrio Galán, R.; Peña-Neira, A. Influence of maturity and vineyard location on free and bound aroma compounds of grapes from the país cultivar. S. Afr. J. Enol. Vitic. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Silva Ferreira, C.; Reis, S.; Rodrigues, C.; Oliveira, C.; Guedes de Pinho, P. Simultaneous determination of ketoacids and dicarbonyl compounds, key Maillard intermediat intermediates on the generation of aged wine aroma. J. Food Sci. 2007, 72, S314–S318. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Lan, Y.; Han, S.; Liang, N.; Zhu, B.; Shi, Y.; Duan, C. Comprehensive investigation of lactones and furanones in ice wines and dry wines using gas chromatography-triple quadrupole mass spectrometry. Food Res. Int. 2020, 137, 109650. [Google Scholar] [CrossRef]
- Allamy, L.; Darriet, P.; Pons, A. Molecular interpretation of dried-fruit aromas in Merlot and Cabernet Sauvignon musts and young wines: Impact of over-ripening. Food Chem. 2018, 266, 245–253. [Google Scholar] [CrossRef]
- Hevia, K.; Castro, R.; Natera, R.; González-García, J.A.; Barroso, C.G.; Durán-Guerrero, E. Optimization of head space sorptive extraction to determine volatile compounds from oak wood in fortified wines. Chromatographia 2016, 79, 763–771. [Google Scholar] [CrossRef]
- Siebert, T.E.; Barter, S.R.; de Barros Lopes, M.A.; Herderich, M.J.; Leigh Francis, I. Investigation of ‘stone fruit’ aroma in Chardonnay, Viognier and botrytis Semillon wines. Food Chem. 2018, 256, 286–296. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Belchior, A.P. Comparison of alternative systems for the ageing of wine brandy: Wood shape and wood botanical species effect. Ciência E Técnica Vitivinícola 2009, 24, 90–99. [Google Scholar]
- Canas, S.; Caldeira, I.; Belchior, A.P. Extraction/oxidation kinetics of low molecular weight compounds in wine brandy resulting from different ageing technologies. Food Chem. 2013, 138, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Anjos, O.; Portal, V.; Belchior, A.P.; Canas, S. Sensory and chemical modifications of wine–brandy aged with chestnut and oak wood fragments in comparison to wooden barrels. Anal. Chim. Acta 2010, 660, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Santos, R.; Ricardo-da-Silva, J.M.; Anjos, O.; Mira, H.; Belchior, A.P.; Canas, S. Kinetics of odorant compounds in wine brandies aged in different systems. Food Chem. 2016, 11, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Bruno de Sousa, R.; Belchior, A.P.; Clímaco, M.C. A sensory and chemical approach to the aroma of wooden aged Lourinhã wine brandy. Ciência Técnica Vitivinic. 2008, 23, 97–110. [Google Scholar]
- Herrera, P.; Durán-Guerrero, E.; Sánchez-Guillén, M.M.; García-Moreno, M.V.; Guillén, D.A.; Barroso, C.G.; Castro, R. Effect of the type of wood used for ageing on the volatile composition of Pedro Ximénez sweet wine. J. Sci. Food Agric. 2020, 100, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Bi, S.; Sun, S.; Lao, F.; Liao, X.; Wu, J. Gas chromatography–mass spectrometry combined with multivariate data analysis as a tool for differentiating between processed orange juice samples on the basis of their volatile markers. Food Chem. 2020, 311, 125913. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma–active compounds of American, French, Hungarian and Russian oak woods, studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Towey, J.P. Oak lactone isomer ratio distinguishes between wine fermented in American and French Oak Barrels. J. Agric. Food Chem. 1994, 42, 1971–1974. [Google Scholar] [CrossRef]
- Cerdan, T.G.; Rodriguez, M.S.; Ancin, A.C. Volatile composition of aged wine in used barrels of French oak and of American oak. Food Res. Int. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- Bautista-Ortn, A.B.; Lencina, A.G.; Cano-Lopez, M.; Pardo-Minguez, F.; Lopez-Roca, J.M.; Gomez-Plaza, E. The use of oak chips during the ageing of a red wine in stainless steel tanks or used barrels: Effect of the contact time and size of the oak chips on aroma compounds. Aust. J. Grape Wine Res. 2008, 14, 63–70. [Google Scholar] [CrossRef]
- Tikunov, Y.; Lommen, A.; Ric de Vos, C.H.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef]
- Li, Y.N.; Zhang, Y.Y.; Peng, Z.; Tan, B.; Lin, H.Y. The difference of quality components of Fuzhuan tea and Qian-liang tea based on the orthogonal partial least squares discriminant analysis model. J. Food Saf. Qual. 2017, 8, 4382–4387. [Google Scholar]
- Wang, L.; Liu, R.; Xing, S.; Zhang, Y.; Xu, B.H. Comparative study on UPLC/Q-TOF-MS lon flow diagram, OPLS-DA/PCS and differential compounds between radis polygoni and rhizoma pinelliae and pinellia ternata and pinellia ternate. J. Sichuan Tradit. Chin. Med. 2020, 38, 58–62. [Google Scholar]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape VarietiesWine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Jin, W.G.; Zhao, P.; Liu, J.X.; Geng, J.Z.; Chen, X.H.; Pei, J.J.; Jiang, P.F. Volatile flavor components analysis of Giant salamander (Andria davidiauns) meat during roasting process based on gas chromatography-ion mobility spectroscopy and chemometrics. Food Ferment. Ind. 2021, 47, 231–239. [Google Scholar]




| Compounds | Formula | CAS a | Odour Descriptor | Odour Threshold b (μg/L) | Aroma Series c |
|---|---|---|---|---|---|
| Methanol | CH4O | 67-56-1 | Chemical, medicinal, fruity, pungent | 668,000 [29] | 1 |
| Propanol | C3H8O | 71-23-8 | Ripe fruit, fusel alcohol | 830,000 [30] | 1 |
| Isobutanol | C4H10O | 78-83-1 | Nail polish, bitter | 40,000 [30] | 1 |
| Isoamyl alcohols | C5H12O | 123-51-3 | Burnt, alcohol, nail polish, whisky, ripe fruit | 30,000 [30] | 1 |
| 2-phenylethanol | C8H10O | 60-12-8 | Rose, honey, lilac | 10,000 [30] | 2 |
| Hexanol | C6H14O | 111-27-3 | Green, grass, oily | 8000 [30] | 3 |
| E-3-hexenol | C6H12O | 928-97-2 | Green, grass | 400 [31] | 3 |
| E-2-hexenol | C6H12O | 928-95-0 | Green tomato | 15,000 [32] | 3 |
| Furfuryl alcohol | C5H6O2 | 98-00-0 | Paint, burnt, coffee | 8000 [30] | 1, 4 |
| Benzyl alcohol | C7H8O | 100-51-6 | Floral, phenolic, sweet | 200,000 [33] | 2, 5 |
| Acetaldehyde | C2H4O | 75-07-0 | Pungent, ripe apple | 110,000 [30] | 1, 3 |
| Acetoin | C4H8O2 | 513-86-0 | Yogurt, butterscotch | 150,000 [30] | 6 |
| Heptanal | C7H14O | 111-71-7 | Herbal, coriander | 3 [34] | 3 |
| Octanal | C8H16O | 124-13-0 | Citrus, green, fresh | 2.5 [35] | 7 |
| Benzaldehyde | C7H6O | 100-52-7 | Bitter almond, smoked, walnut | 350 [34] | 5 |
| Ethyl propionate | C5H10O2 | 105-37-3 | Fruity, grape, pineapple | 10 [36] | 5 |
| Ethyl isobutanoate | C6H12O2 | 97-62-1 | Ripe melon, apple, strawberry | 15 [30] | 5 |
| Ethyl butanoate | C6H12O2 | 105-54-4 | Fruity, floral, sweet, apple | 20 [37] | 5 |
| Isoamyl acetate | C7H14O2 | 123-92-2 | Banana | 30 [38] | 5 |
| Ethyl octanoate | C10H20O2 | 106-32-1 | Banana, pineapple, floral, pear, soapy | 5 [38] | 5, 9 |
| Phenylethyl acetate | C10H12O2 | 103-45-7 | Fruity, honey, floral | 250 [37] | 8 |
| Ethyl decanoate | C12H24O2 | 110-38-3 | Sweet, fruity, caramel, nuts, and dried fruit | 200 [38] | 5, 9 |
| Ethyl tetradecanoate | C16H32O2 | 124-06-1 | Waxy, buttery, fatty odour | 4000 [30] | 9 |
| Ethyl vanillate | C10H12O4 | 617-05-0 | Smoky, burnt | 990 [33] | 1, 6 |
| Ethyl dodecanoate | C14H28O2 | 106-33-2 | Creamy, floral | 500 [30] | 9 |
| Ethyl hexadecanoate | C18H36O2 | 628-97-7 | Fatty, rancid, fruity, sweet, caramel | 2000 [30] | 9 |
| Phenethyl phenyl acetate | C16H16O2 | 102-20-5 | Floral, honey | 250 * | 2, 8 |
| Ethyl cinnamate | C11H12O2 | 4610-69-9 | Fruity, soapy, cinnamon-like, spice | 1.1 [39] | 10 |
| Ethyl acetate | C4H8O2 | 141-78-6 | Fruity, glue, pineapple, varnish, balsamic | 7500 [30] | 1 |
| Ethyl lactate | C5H10O3 | 97-64-3 | Lactic, yogurt, buttery | 150,000 [40] | 6 |
| Diethyl succinate | C8H14O4 | 123-25-1 | Overripe melon | 200,000 [33] | 5 |
| Crotonolactone | C4H4O2 | 497-23-4 | Toasty, buttery | 35,000 [41] | 6 |
| Butyrolactone | C4H6O2 | 96-48-0 | Sweet, caramel | 35,000 [42] | 6 |
| Nonalactone | C9H16O2 | 6008-27-1 | Coconut, creamy | 30 [39] | 5, 6 |
| Decalactone | C10H18O2 | 706-14-9 | Peach, milky | 47 [39] | 5 |
| trans-whisky lactone | C9H16O2 | 39638-67-0 | Woody, coconut notes, vanilla | 370 [43] | 10, 11 |
| cis-whisky lactone | C9H16O2 | 55013-32-6 | Woody, coconut notes, vanilla | 54 [43] | 10, 11 |
| Guaiacol | C7H8O2 | 90-05-1 | Medicine, smoke, woody | 75 [44] | 1, 4 |
| 2-methoxy-4-vinylphenol | C9H10O2 | 7786-61-0 | Spices, clove, woody | 40 [45] | 1, 4 |
| Furfural | C5H4O2 | 98-01-1 | Burned almonds, caramel, nutty | 770 [46] | 4 |
| 5-methylfurfural | C6H6O2 | 620-02-0 | Caramel, roasted almond, loral, sweet, and bready | 1100 [46] | 4 |
| 5-HMF | C6H6O3 | 67-47-0 | Caramel, butter, waxy, tobacco | 8000 [13] | 4 |
| Compounds | CMT | CMPT | SMT | SMPT | BMT | BMPT | CMT | CMPT | SMT | SMPT | BMT | BMPT | MANOVA | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 Months | 3 Months | AS | TG | AT | In | |||||||||||
| Methanol | 0.329 ± 0.01 | 0.343 ± 0.00 | 0.339 ± 0.00 | 0.338 ± 0.01 | 0.346 ± 0.01 | 0.343 ± 0.02 | 0.369 ± 0.01 | 0.372 ± 0.00 | 0.357 ± 0.00 | 0.341 ± 0.01 | 0.379 ± 0.01 | 0.368 ± 0.02 | ** | ns | *** | ns |
| Propanol | 0.043 ± 0.00 | 0.044 ± 0.00 | 0.044 ± 0.00 | 0.044 ± 0.00 | 0.052 ± 0.00 | 0.054 ± 0.00 | 0.046 ± 0.00 | 0.046 ± 0.00 | 0.046 ± 0.00 | 0.045 ± 0.00 | 0.054 ± 0.00 | 0.057 ± 0.00 | *** | * | *** | ns |
| Isobutanol | 1.170 ± 0.03 | 1.150 ± 0.02 | 1.206 ± 0.01 | 1.197 ± 0.02 | 1.220 ± 0.01 | 1.243 ± 0.02 | 1.226 ± 0.02 | 1.206 ± 0.03 | 1.216 ± 0.02 | 1.200 ± 0.03 | 1.249 ± 0.01 | 1.273 ± 0.02 | *** | ns | *** | ns |
| Isoamyl alcohols | 8.850 ± 0.13 | 8.945 ± 0.07 | 8.996 ± 0.08 | 8.738 ± 0.23 | 9.464 ± 0.09 | 9.600 ± 0.18 | 9.212 ± 0.18 | 8.985 ± 0.07 | 9.229 ± 0.12 | 9.053 ± 0.15 | 9.793 ± 0.10 | 9.916 ± 0.20 | *** | ns | *** | ns |
| 2-phenylethanol | 6.901 ± 0.06 | 6.276 ± 0.22 | 6.567 ± 0.49 | 6.265 ± 0.08 | 9.264 ± 0.89 | 6.848 ± 0.36 | 7.004 ± 0.08 | 6.711 ± 0.13 | 7.297 ± 0.42 | 6.526 ± 0.18 | 9.316 ± 0.34 | 7.072 ± 0.37 | *** | *** | * | ns |
| Hexanol | 0.226 ± 0.01 | 0.166 ± 0.01 | 0.215 ± 0.02 | 0.201 ± 0.01 | 0.201 ± 0.01 | 0.180 ± 0.01 | 0.222 ± 0.01 | 0.169 ± 0.01 | 0.245 ± 0.01 | 0.279 ± 0.01 | 0.273 ± 0.02 | 0.333 ± 0.02 | *** | ** | *** | *** |
| E-3-hexenol | 0.586 ± 0.01 | 0.346 ± 0.01 | 0.365 ± 0.02 | 0.386 ± 0.02 | 0.012 ± 0.00 | 0.004 ± 0.00 | 0.426 ± 0.03 | 0.431 ± 0.02 | 0.465 ± 0.01 | 0.560 ± 0.03 | 0.036 ± 0.00 | 0.045 ± 0.00 | *** | ns | *** | *** |
| E-2-hexenol | 0.031 ± 0.00 | 0.022 ± 0.00 | 0.027 ± 0.00 | 0.025 ± 0.00 | 0.023 ± 0.00 | 0.025 ± 0.00 | 0.028 ± 0.00 | 0.035 ± 0.00 | 0.039 ± 0.00 | 0.039 ± 0.00 | 0.027 ± 0.00 | 0.033 ± 0.00 | *** | ns | *** | ** |
| Furfuryl alcohol | 0.006 ± 0.00 | 0.009 ± 0.00 | 0.008 ± 0.00 | 0.007 ± 0.00 | 0.007 ± 0.00 | 0.011 ± 0.00 | 0.007 ± 0.00 | 0.011 ± 0.00 | 0.007 ± 0.00 | 0.005 ± 0.00 | 0.087 ± 0.00 | 0.081 ± 0.00 | *** | *** | *** | *** |
| Benzyl alcohol | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | *** | * | *** | ns |
| Acetaldehyde | 0.470 ± 0.02 | 0.523 ± 0.01 | 0.384 ± 0.01 | 0.479 ± 0.02 | 0.575 ± 0.02 | 0.546 ± 0.02 | 0.565 ± 0.03 | 0.613 ± 0.08 | 0.537 ± 0.04 | 0.621 ± 0.02 | 0.810 ± 0.03 | 0.699 ± 0.03 | *** | ns | ns | ns |
| Acetoin | 0.441 ± 0.01 | 0.511 ± 0.04 | 0.358 ± 0.01 | 0.459 ± 0.02 | 0.646 ± 0.04 | 0.663 ± 0.02 | 0.751 ± 0.03 | 0.957 ± 0.05 | 0.633 ± 0.02 | 0.674 ± 0.04 | 1.291 ± 0.07 | 1.152 ± 0.04 | *** | ** | *** | ** |
| Heptanal | 0.567 ± 0.03 | 0.544 ± 0.02 | 0.600 ± 0.07 | 0.600 ± 0.07 | 0.159 ± 0.03 | 0.208 ± 0.06 | 0.600 ± 0.03 | 0.556 ± 0.02 | 0.633 ± 0.03 | 0.500 ± 0.03 | 0.178 ± 0.03 | 0.248 ± 0.02 | *** | ** | *** | *** |
| Octanal | 0.725 ± 0.02 | 1.120 ± 0.08 | 0.440 ± 0.04 | 0.896 ± 0.08 | 2.333 ± 0.14 | 2.679 ± 0.23 | 0.440 ± 0.04 | 0.889 ± 0.08 | 1.720 ± 0.32 | 1.000 ± 0.08 | 3.956 ± 0.36 | 7.299 ± 0.54 | *** | ** | *** | ns |
| Benzaldehyde | 0.138 ± 0.01 | 0.061 ± 0.01 | 0.122 ± 0.00 | 0.146 ± 0.01 | 0.204 ± 0.01 | 0.213 ± 0.1 | 0.215 ± 0.02 | 0.183 ± 0.01 | 0.188 ± 0.02 | 0.308 ± 0.02 | 0.498 ± 0.02 | 0.433 ± 0.02 | *** | ns | *** | ** |
| Ethyl propionate | 35.136 ± 0.3 | 18.461 ± 1.1 | 28.442 ± 2.2 | 28.076 ± 1.9 | 32.519 ± 2.3 | 36.829 ± 0.9 | 34.938 ± 1.3 | 34.633 ± 0.9 | 48.195 ± 1.5 | 51.042 ± 1.2 | 56.408 ± 2.0 | 54.500 ± 3.5 | *** | * | *** | *** |
| Ethyl isobutanoate | 0.313 ± 0.02 | 0.476 ± 0.03 | 0.800 ± 0.00 | 0.933 ± 0.00 | 1.313 ± 0.14 | 1.287 ± 0.10 | 0.023 ± 0.00 | 0.102 ± 0.01 | 0.033 ± 0.00 | 0.078 ± 0.01 | 0.342 ± 0.03 | 0.327 ± 0.01 | ns | ns | ns | ns |
| Ethyl butanoate | 17.237 ± 1.1 | 10.278 ± 0.3 | 16.238 ± 0.3 | 16.777 ± 0.7 | 23.781 ± 0.8 | 26.736 ± 1.1 | 19.886 ± 0.4 | 18.132 ± 0.9 | 22.538 ± 1.1 | 26.604 ± 0.9 | 24.307 ± 1.3 | 27.372 ± 1.3 | *** | *** | *** | *** |
| Isoamylacetate | 42.950 ± 2.53 | 30.536 ± 2.2 | 41.430 ± 3.5 | 42.939 ± 2.3 | 15.944 ± 0.3 | 16.278 ± 0.4 | 44.021 ± 1.8 | 54.086 ± 4.1 | 57.928 ± 2.5 | 66.259 ± 1.5 | 11.667 ± 0.5 | 11.611 ± 0.7 | *** | ns | *** | * |
| Ethyl octanoate | 9.202 ± 0.39 | 6.033 ± 0.33 | 8.288 ± 0.36 | 9.150 ± 0.46 | 1.853 ± 0.11 | 1.800 ± 0.09 | 12.875 ± 0.2 | 15.482 ± 0.2 | 15.582 ± 0.8 | 17.323 ± 0.5 | 2.147 ± 0.06 | 2.167 ± 0.06 | *** | *** | *** | *** |
| Phenylethyl acetate | 0.316 ± 0.02 | 0.403 ± 0.01 | 0.411 ± 0.02 | 0.421 ± 0.02 | 0.533 ± 0.03 | 0.541 ± 0.02 | 0.508 ± 0.01 | 0.479 ± 0.02 | 0.408 ± 0.01 | 0.468 ± 0.02 | 0.370 ± 0.00 | 0.535 ± 0.03 | *** | *** | *** | *** |
| Ethyl decanoate | 0.417 ± 0.03 | 0.313 ± 0.02 | 0.629 ± 0.03 | 0.782 ± 0.03 | 0.128 ± 0.01 | 0.120 ± 0.01 | 0.615 ± 0.02 | 0.512 ± 0.02 | 0.822 ± 0.04 | 0.720 ± 0.02 | 0.087 ± 0.00 | 0.092 ± 0.01 | *** | ns | *** | * |
| Ethyl tetradecanoate | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.005 ± 0.00 | 0.005 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.007 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | *** | ns | ns | ns |
| Ethyl vanillate | 0.085 ± 0.00 | 0.084 ± 0.00 | 0.127 ± 0.00 | 0.123 ± 0.00 | 0.085 ± 0.00 | 0.088 ± 0.00 | 0.085 ± 0.00 | 0.090 ± 0.00 | 0.164 ± 0.00 | 0.195 ± 0.00 | 0.087 ± 0.00 | 0.088 ± 0.00 | *** | ns | *** | ns |
| Ethyl dodecanoate | 0.068 ± 0.01 | 0.116 ± 0.01 | 0.304 ± 0.02 | 0.487 ± 0.01 | 0.017 ± 0.00 | 0.017 ± 0.00 | 0.090 ± 0.01 | 0.112 ± 0.00 | 0.158 ± 0.01 | 0.097 ± 0.01 | 0.016 ± 0.00 | 0.016 ± 0.00 | ns | *** | ** | ns |
| Ethyl hexadecanoate | 0.010 ± 0.00 | 0.008 ± 0.00 | 0.008 ± 0.00 | 0.008 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.009 ± 0.00 | 0.008 ± 0.00 | 0.011 ± 0.00 | 0.013 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | *** | *** | *** | ns |
| Phenethyl phenyl acetate | 0.008 ± 0.00 | 0.001 ± 0.00 | 0.412 ± 0.00 | 0.282 ± 0.24 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.002 ± 0.00 | 0.001 ± 0.00 | 0.424 ± 0.00 | 0.492 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | *** | *** | *** | * |
| Ethyl cinnamate | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.892 ± 0.02 | 0.910 ± 0.07 | *** | ns | *** | ** |
| Ethyl acetate | 4.694 ± 0.12 | 3.791 ± 0.08 | 4.348 ± 0.34 | 4.271 ± 0.18 | 4.412 ± 0.11 | 4.471 ± 0.08 | 4.726 ± 0.01 | 4.332 ± 0.02 | 4.424 ± 0.26 | 4.328 ± 0.23 | 4.590 ± 0.12 | 4.710 ± 0.07 | *** | ns | *** | *** |
| Ethyl lactate | 0.922 ± 0.03 | 1.435 ± 0.09 | 0.459 ± 0.02 | 0.695 ± 0.04 | 1.352 ± 0.07 | 1.488 ± 0.07 | 1.500 ± 0.07 | 1.696 ± 0.09 | 1.293 ± 0.04 | 1.018 ± 0.05 | 3.206 ± 0.06 | 2.962 ± 0.09 | *** | *** | ** | ns |
| Diethyl succinate | 0.142 ± 0.01 | 0.127 ± 0.01 | 0.116 ± 0.01 | 0.120 ± 0.01 | 0.292 ± 0.00 | 0.228 ± 0.01 | 0.174 ± 0.01 | 0.173 ± 0.01 | 0.155 ± 0.01 | 0.143 ± 0.01 | 0.393 ± 0.01 | 0.307 ± 0.02 | *** | ns | *** | *** |
| Crotonolactone | 0.012 ± 0.00 | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.005 ± 0.00 | 0.006 ± 0.00 | 0.008 ± 0.00 | 0.007 ± 0.00 | 0.007 ± 0.00 | 0.009 ± 0.00 | 0.020 ± 0.00 | 0.022 ± 0.00 | *** | *** | *** | *** |
| Butyrolactone | 0.012 ± 0.00 | 0.004 ± 0.00 | 0.010 ± 0.00 | 0.002 ± 0.00 | 0.015 ± 0.00 | 0.015 ± 0.00 | 0.009 ± 0.00 | 0.014 ± 0.00 | 0.014 ± 0.00 | 0.012 ± 0.00 | 0.046 ± 0.00 | 0.052 ± 0.00 | *** | *** | *** | *** |
| Nonalactone | 1.133 ± 0.01 | 0.873 ± 0.07 | 1.088 ± 0.07 | 1.101 ± 0.13 | 0.940 ± 0.07 | 0.984 ± 0.09 | 1.186 ± 0.10 | 1.222 ± 0.04 | 1.310 ± 0.09 | 1.523 ± 0.08 | 0.901 ± 0.09 | 0.688 ± 0.04 | *** | * | *** | ns |
| Decalactone | 0.050 ± 0.00 | 0.052 ± 0.01 | 0.060 ± 0.01 | 0.048 ± 0.00 | 0.048 ± 0.00 | 0.064 ± 0.00 | 0.048 ± 0.00 | 0.061 ± 0.00 | 0.048 ± 0.00 | 0.049 ± 0.00 | 0.063 ± 0.00 | 0.083 ± 0.00 | *** | *** | *** | ns |
| trans-whisky lactone | 0.067 ± 0.00 | 0.079 ± 0.00 | 0.110 ± 0.01 | 0.064 ± 0.00 | 0.122 ± 0.01 | 0.123 ± 0.01 | 0.057 ± 0.00 | 0.078 ± 0.00 | 0.149 ± 0.00 | 0.090 ± 0.00 | 0.285 ± 0.00 | 0.353 ± 0.02 | ** | ns | *** | ns |
| cis-whisky lactone | 0.630 ± 0.06 | 0.111 ± 0.02 | 5.648 ± 0.36 | 3.044 ± 0.07 | 1.439 ± 0.10 | 1.673 ± 0.04 | 1.204 ± 0.09 | 0.148 ± 0.02 | 7.431 ± 0.38 | 5.152 ± 0.17 | 3.895 ± 0.16 | 5.494 ± 0.19 | *** | * | *** | ns |
| Guaiacol | 0.374 ± 0.03 | 0.097 ± 0.01 | 0.138 ± 0.01 | 0.174 ± 0.03 | 0.747 ± 0.00 | 0.773 ± 0.00 | 0.358 ± 0.03 | 0.153 ± 0.01 | 0.300 ± 0.02 | 0.285 ± 0.02 | 1.080 ± 0.00 | 1.013 ± 0.00 | *** | ns | *** | ns |
| 2-methoxy-4-vinylphenol | 1.881 ± 0.13 | 0.665 ± 0.06 | 0.383 ± 0.04 | 0.590 ± 0.03 | 1.354 ± 0.07 | 1.496 ± 0.10 | 1.453 ± 0.07 | 1.155 ± 0.07 | 1.174 ± 0.05 | 1.244 ± 0.05 | 2.066 ± 0.13 | 2.131 ± 0.13 | *** | ns | *** | ns |
| Furfural | 0.005 ± 0.00 | 0.002 ± 0.00 | 0.006 ± 0.00 | 0.006 ± 0.00 | 0.003 ± 0.00 | 0.005 ± 0.00 | 0.004 ± 0.00 | 0.004 ± 0.00 | 0.010 ± 0.00 | 0.011 ± 0.00 | 0.014 ± 0.00 | 0.017 ± 0.00 | *** | *** | * | ns |
| 5-methylfurfural | 0.006 ± 0.00 | 0.010 ± 0.00 | 0.020 ± 0.00 | 0.044 ± 0.00 | 0.006 ± 0.00 | 0.014 ± 0.00 | 0.013 ± 0.00 | 0.021 ± 0.00 | 0.038 ± 0.00 | 0.065 ± 0.00 | 0.013 ± 0.00 | 0.030 ± 0.00 | *** | ns | *** | * |
| 5-HMF | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.001 ± 0.00 | 0.001 ± 0.00 | *** | ** | *** | *** |
| Aroma Series | CMT | CMPT | SMT | SMPT | BMT | BMPT | CMT | CMP | SMT | SMPT | BMT | BMPT | MANOVA | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 Months | 3 Months | AS | TG | AT | In | |||||||||||
| Chemistry | 17.90 ± 0.10 | 15.65 ± 0.15 | 15.97 ± 0.30 | 15.96 ± 0.38 | 18.26 ± 0.11 | 18.63 ± 0.29 | 18.05 ± 0.15 | 16.96 ± 0.11 | 17.45 ± 0.44 | 17.32 ± 0.27 | 20.20 ± 0.20 | 20.33 ± 0.26 | *** | *** | *** | *** |
| Floral | 6.91 ± 0.06 | 6.28 ± 0.22 | 6.98 ± 0.49 | 6.55 ± 0.31 | 9.26 ± 0.89 | 6.85 ± 0.36 | 7.01 ± 0.08 | 6.71 ± 0.13 | 7.72 ± 0.42 | 7.02 ± 0.18 | 9.32 ± 0.34 | 7.07 ± 0.37 | *** | *** | * | ns |
| Green | 1.88 ± 0.04 | 1.60 ± 0.00 | 1.59 ± 0.06 | 1.69 ± 0.05 | 0.97 ± 0.02 | 0.96 ± 0.06 | 1.84 ± 0.05 | 1.80 ± 0.05 | 1.92 ± 0.05 | 2.00 ± 0.07 | 1.32 ± 0.05 | 1.36 ± 0.05 | *** | ns | *** | ** |
| Caramelly | 2.27 ± 0.12 | 0.78 ± 0.07 | 0.56 ± 0.05 | 0.82 ± 0.06 | 2.12 ± 0.07 | 2.30 ± 0.09 | 1.84 ± 0.05 | 1.34 ± 0.07 | 1.53 ± 0.04 | 1.61 ± 0.04 | 3.26 ± 0.12 | 3.27 ± 0.14 | *** | *** | *** | *** |
| Fruity | 106.72 ± 3.71 | 67.21 ± 3.98 | 97.21 ± 6.07 | 100.07 ± 4.28 | 77.02 ± 1.91 | 84.54 ± 0.69 | 113.98 ± 2.82 | 124.59 ± 6.14 | 146.80 ± 5.84 | 164.05 ± 3.55 | 96.81 ± 2.92 | 97.58 ± 5.42 | *** | ns | *** | *** |
| Creamy | 2.60 ± 0.05 | 2.91 ± 0.14 | 2.05 ± 0.09 | 2.39 ± 0.17 | 3.04 ± 0.11 | 3.24 ± 0.06 | 3.54 ± 0.13 | 3.99 ± 0.09 | 3.42 ± 0.07 | 3.43 ± 0.15 | 5.55 ± 0.22 | 4.96 ± 0.10 | *** | ** | *** | *** |
| Citrus | 0.73 ± 0.02 | 1.12 ± 0.08 | 0.44 ± 0.04 | 0.90 ± 0.08 | 2.33 ± 0.14 | 2.68 ± 0.23 | 0.44 ± 0.04 | 0.89 ± 0.08 | 1.72 ± 2.32 | 1.00 ± 0.08 | 3.96 ± 0.36 | 7.30 ± 0.54 | *** | ** | *** | ** |
| Honey | 0.32 ± 0.02 | 0.40 ± 0.01 | 0.82 ± 0.02 | 0.70 ± 0.26 | 0.53 ± 0.03 | 0.54 ± 0.02 | 0.51 ± 0.01 | 0.48 ± 0.02 | 0.83 ± 0.01 | 0.80 ± 0.26 | 0.37 ± 0.00 | 0.54 ± 0.03 | ns | ns | ns | ns |
| Waxy | 9.70 ± 0.42 | 6.48 ± 0.34 | 9.23 ± 0.32 | 10.43 ± 0.45 | 2.00 ± 0.12 | 1.94 ± 0.09 | 13.59 ± 0.19 | 16.12 ± 0.21 | 16.58 ± 0.87 | 18.16 ± 0.47 | 2.25 ± 0.06 | 2.27 ± 0.07 | *** | * | *** | *** |
| Spicy | 0.70 ± 0.05 | 0.19 ± 0.02 | 5.76 ± 0.36 | 3.11 ± 0.07 | 1.56 ± 0.11 | 1.80 ± 0.05 | 1.26 ± 0.09 | 0.23 ± 0.02 | 7.58 ± 0.39 | 5.24 ± 0.17 | 5.07 ± 0.17 | 6.76 ± 0.27 | *** | *** | *** | *** |
| Woody | 0.70 ± 0.05 | 0.19 ± 0.02 | 5.76 ± 0.36 | 3.11 ± 0.07 | 1.56 ± 0.11 | 1.80 ± 0.05 | 1.26 ± 0.09 | 0.23 ± 0.02 | 7.58 ± 0.39 | 5.24 ± 0.17 | 4.18 ± 0.16 | 5.85 ± 0.21 | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitriu, G.-D.; Sánchez-Suárez, F.; Peinado, R.A.; Cotea, V.V.; de Lerma, N.L.; Gabur, I.; Simioniuc, V. Metabolomics of Red Wines Aged Traditionally, with Chips or Staves. Foods 2024, 13, 196. https://doi.org/10.3390/foods13020196
Dumitriu G-D, Sánchez-Suárez F, Peinado RA, Cotea VV, de Lerma NL, Gabur I, Simioniuc V. Metabolomics of Red Wines Aged Traditionally, with Chips or Staves. Foods. 2024; 13(2):196. https://doi.org/10.3390/foods13020196
Chicago/Turabian StyleDumitriu (Gabur), Georgiana-Diana, Fernando Sánchez-Suárez, Rafael A. Peinado, Valeriu V. Cotea, Nieves López de Lerma, Iulian Gabur, and Violeta Simioniuc. 2024. "Metabolomics of Red Wines Aged Traditionally, with Chips or Staves" Foods 13, no. 2: 196. https://doi.org/10.3390/foods13020196
APA StyleDumitriu, G.-D., Sánchez-Suárez, F., Peinado, R. A., Cotea, V. V., de Lerma, N. L., Gabur, I., & Simioniuc, V. (2024). Metabolomics of Red Wines Aged Traditionally, with Chips or Staves. Foods, 13(2), 196. https://doi.org/10.3390/foods13020196








