How the “Olive Oil Polyphenols” Health Claim Depends on Anthracnose and Olive Fly on Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Characterization
2.2. Olive Oil Extraction
2.3. Chemical and Sensory Characterization of Olive Oil
2.4. Statistical Analysis
3. Results and Discussion
3.1. Quality Criteria of the Oils
3.2. “Olive Oil Polyphenols” Health Claim vs. Olive Damage by Olive Fly and Anthracnose Disease
3.3. Phenolic Profiles of Olive Oils with Different Incidences of Anthracnose Disease
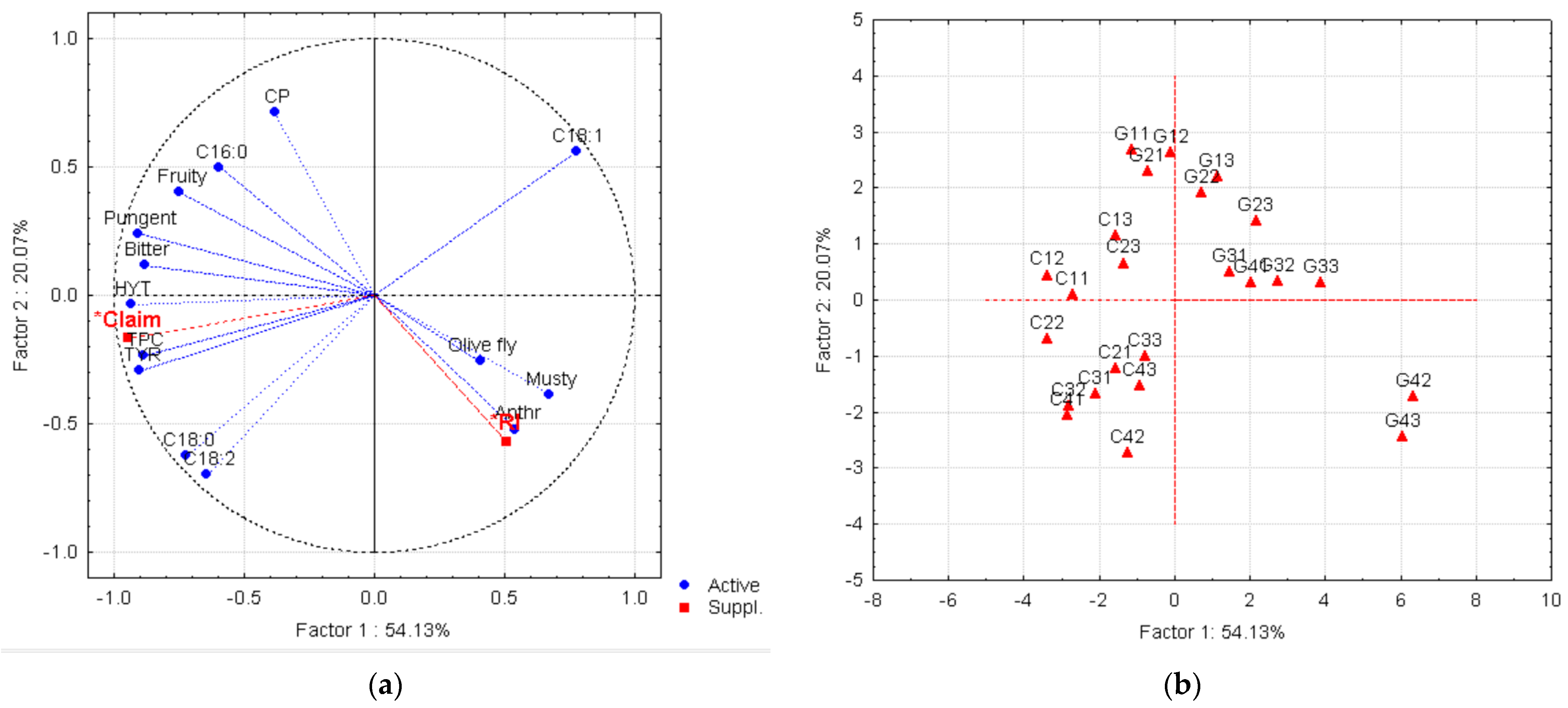
3.4. Multivariate Characterizations of the Olive Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trichopoulou, A.; Dilis, V. Olive Oil and Longevity. Mol. Nutr. Food Res. 2007, 51, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- La Lastra, C.; Barranco, M.D.; Motilva, V.; Herrerias, J.M. Mediterrranean Diet and Health Biological Importance of Olive Oil. Curr. Pharm. Des. 2001, 7, 933–950. [Google Scholar]
- Roche, H.M.; Gibney, M.J.; Kafatos, A.; Zampelas, A.; Williams, C.M. Beneficial properties of olive oil. Food Res. Int. 2000, 33, 227–231. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health Effects of Olive Oil Polyphenols: Recent Advances and Possibilities for the Use of Health Claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Brenes, M.; García, P.; Garrido, A. Hydroxytyrosol 4-β-d-Glucoside, an Important Phenolic Compound in Olive Fruits and Derived Products. J. Agric. Food Chem. 2002, 50, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive Oil and its Principal Bioactive Compound: Hydroxytyrosol—A Review of the Recent Literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Jimenez, A.; Sanchez-Villasclaras, S.; Uceda, M.; Beltran, G. Modulation of bitterness and pungency in virgin olive oil from unripe “Picual” fruits. Eur. J. Lipid Sci. Technol. 2015, 117, 1463–1472. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Caravaca, A.M.G.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. 2012. Available online: http://data.europa.eu/eli/reg/2012/432/oj (accessed on 1 March 2024).
- Aparicio, R.; Ferreiro, L.; Alonso, V. Effect of climate on the chemical composition of virgin olive oil. Anal. Chim. Acta 1994, 292, 235–241. [Google Scholar] [CrossRef]
- Guerfel, M.; Ouni, Y.; Taamalli, A.; Boujnah, D.; Stefanoudaki, E.; Zarrouk, M. Effect of location on virgin olive oils of the two main Tunisian olive cultivars. Eur. J. Lipid Sci. Technol. 2009, 111, 926–932. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Environmental and seasonal influence on virgin olive (Olea europaea L.) oil volatiles in northern Italy. Sci. Hortic. 2009, 122, 385–392. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Beltrán, G.; Aguilera, M.P.; Rio, C.; Sanchez, S.; Martinez, L. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem. 2005, 89, 207–215. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Škevin, D.; Belobrajić, I. Chemical and sensorial changes of Croatian monovarietal olive oils during ripening. Eur. J. Lipid Sci. Technol. 2012, 114, 1400–1408. [Google Scholar] [CrossRef]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. EFSA Health Claims-Based Virgin Olive Oil Shelf-Life. Antioxidants 2023, 12, 1563. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Talhinhas, P.; Afonso, H.; Alegre, H.; Oliveira, H.; Ferreira-Dias, S. Olive Oils from Fruits Infected with Different Anthracnose Pathogens Show Sensory Defects Earlier Than Chemical Degradation. Agronomy 2021, 11, 1041. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Dminić, I.; Kosić, U.; Majetić, V.; Godena, S.; Valenčič, V. Dynamics of oil quality parameters changes related to olive fruit fly attack. Eur. J. Lipid Sci. Technol. 2010, 112, 1033–1040. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Rojnić, I.D.; Bažok, R.; Barčić, J.I. Reduction of olive fruit fly damage by early harvesting and impact on oil quality parameters. Eur. J. Lipid Sci. Technol. 2015, 117, 103–111. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Palmeri, V. Molecular analysis of the fungal microbiome associated with the olive fruit fly Bactrocera oleae. Fungal Ecol. 2015, 18, 67–74. [Google Scholar] [CrossRef]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, G.; Moral, J.; Strano, M.C.; Caruso, P.; Sciara, M.; Bella, P.; Sorrentino, G.; Di Silvestro, S. Characterization of Colletotrichum strains associated with olive anthracnose in Sicily. Phytopathol. Mediterr. 2022, 61, 139–151. [Google Scholar] [CrossRef]
- Cabral, A.; Nascimento, T.; Azinheira, H.G.; Loureiro, A.; Talhinhas, P.; Oliveira, H. Olive Anthracnose in Portugal Is Still Mostly Caused by Colletotrichum nymphaeae, but C. acutatum Is Spreading and C. alienum and C. cigarro Are Reported for the First Time. Horticulturae 2024, 10, 434. [Google Scholar] [CrossRef]
- Moral, J.; Jurado-Bello, J.; Sánchez, M.I.; de Oliveira, R.; Trapero, A. Effect of Temperature, Wetness Duration, and Planting Density on Olive Anthracnose Caused by Colletotrichum spp. Phytopathology 2012, 102, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Talhinhas, P.; Gonçalves, E.; Sreenivasaprasad, S.; Oliveira, H. Virulence diversity of anthracnose pathogens (Colletotrichum acutatum and C. gloeosporioides species complexes) on eight olive cultivars commonly grown in Portugal. Eur. J. Plant Pathol. 2015, 142, 73–83. [Google Scholar] [CrossRef]
- Moral, J.; Xaviér, C.; Roca, L.F.; Romero, J.; Moreda, W.; Trapero, A. Olive Anthracnose and its effect on oil quality. Grasas Y Aceites 2014, 65, e028. [Google Scholar] [CrossRef]
- Leoni, C.; Bruzzone, J.; Villamil, J.J.; Martínez, C.; Montelongo, M.J.; Bentancur, O.; Conde-Innamorato, P. Percentage of anthracnose (Colletotrichum acutatum s.s.) acceptable in olives for the production of extra virgin olive oil. Crop Prot. 2018, 108, 47–53. [Google Scholar] [CrossRef]
- IOC. Guide for the Determination of the Characteristics of Oil-Olives; COI/OH/Doc. Nº.1; International Olive Council: Madrid, Spain, 2011. [Google Scholar]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-scale optimization of olive oil extraction: Simultaneous addition of enzymes and microtalc improves the yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU). 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012; Official Journal of the European Union: Brussels, Belgium, 2022. [Google Scholar]
- International Olive Council. List of Laboratories Undertaking the Sensory Analysis of Virgin Olive Oils Recognised by the International Olive Council for the Period from 1 December 2022 to 30 November2023; T.28/Doc. No 3/Rev. 25; International Olive Council: Madrid, Spain, 2022. [Google Scholar]
- IOC. Sensory Analysis of Olive Oil—Method for the Organoleptic Assessment of Virgin Olive Oil; COI/T.20/Doc. No 15; International Olive Council: Madrid, Spain, 2018. [Google Scholar]
- Pokorny, J.; Kalinová, L.; Dysseler, P. Determination of Chlorophyll pigments in Crude Vegetable Oils. Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing Analytical Requirements to Support Health Claims on “Olive Oil Polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Sotiroglou, M.; Mastralexi, A.; Nenadis, N.; García-González, D.L.; Gallina Toschi, T. In House Validated UHPLC Protocol for the Determination of the Total Hydroxytyrosol and Tyrosol Content in Virgin Olive Oil Fit for the Purpose of the Health Claim Introduced by the EC Regulation 432/2012 for “Olive Oil Polyphenols”. Molecules 2019, 24, 1044. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z.; Vichi, S.; Quintanilla-Casas, B.; Donarski, J.; Bailey-Horne, V.; Butinar, B.; Miklavčič, M.; García González, D.-L.; et al. Toward a Harmonized and Standardized Protocol for the Determination of Total Hydroxytyrosol and Tyrosol Content in Virgin Olive Oil (VOO). Extraction Solvent. Eur. J. Lipid Sci. Technol. 2018, 120, 1800099. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Valli, E.; Bendini, A.; Di Lecce, G.; Simal-Gándara, J.; Gallina Toschi, T. A widely used spectrophotometric assay to quantify olive oil biophenols according to the health claim (EU Reg. 432/2012). Eur. J. Lipid Sci. Technol. 2016, 118, 1593–1599. [Google Scholar] [CrossRef]
- Peres, F.; Marques, M.P.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules 2023, 28, 6898. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95442-2. [Google Scholar]
- Gomes, S.; Bacelar, E.; Martins-Lopes, P.; Carvalho, T.; Guedes-Pinto, H. Infection process of olive fruits by Colletotrichum acutatum and the protective role of the cuticle and epidermis. J. Agric. Sci. 2012, 4, 101. [Google Scholar] [CrossRef]
- Abacıgil, T.Ö.; Kıralan, M.; Ramadan, M.F. Quality parameters of olive oils at different ripening periods as affected by olive fruit fly infestation and olive anthracnose. Rend. Lincei. Sci. Fis. E Nat. 2023, 34, 595–603. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Gorb, E.; Gorb, S. Role of Fruit Epicuticular Waxes in Preventing Bactrocera oleae (Diptera: Tephritidae) Attachment in Different Cultivars of Olea europaea. Insects 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- López-Huertas, E.; Lozano-Sánchez, J.; Segura-Carretero, A. Olive oil varieties and ripening stages containing the antioxidants hydroxytyrosol and derivatives in compliance with EFSA health claim. Food Chem. 2021, 342, 128291. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of Fly Attack (Bactrocera oleae) on the Phenolic Profile and Selected Chemical Parameters of Olive Oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Medjkouh, L.; Tamendjari, A.; Alves, R.C.; Laribi, R.; Oliveira, M.B.P.P. Phenolic profiles of eight olive cultivars from Algeria: Effect of Bactrocera oleae attack. Food Funct. 2018, 9, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Antunes, P.; Ferreira-Dias, S. Phenolic compounds of ‘Galega Vulgar’ and ‘Cobrançosa’ olive oils along early ripening stages. Food Chem. 2016, 211, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Ferreira-Dias, S. Bioactive compounds of Portuguese virgin olive oils discriminate cultivar and ripening stage. J. Am. Oil Chem. Soc. 2016, 93, 1137–1147. [Google Scholar] [CrossRef]
- Romero, J.; Santa-Bárbara, A.E.; Moral, J.; Agustí-Brisach, C.; Roca, L.F.; Trapero, A. Effect of latent and symptomatic infections by Colletotrichum godetiae on oil quality. Eur. J. Plant Pathol. 2022, 163, 545–556. [Google Scholar] [CrossRef]
- Notario, A.; Sánchez, R.; Luaces, P.; Sanz, C.; Pérez, A.G. The Infestation of Olive Fruits by Bactrocera oleae (Rossi) Modifies the Expression of Key Genes in the Biosynthesis of Volatile and Phenolic Compounds and Alters the Composition of Virgin Olive Oil. Molecules 2022, 27, 1650. [Google Scholar] [CrossRef]


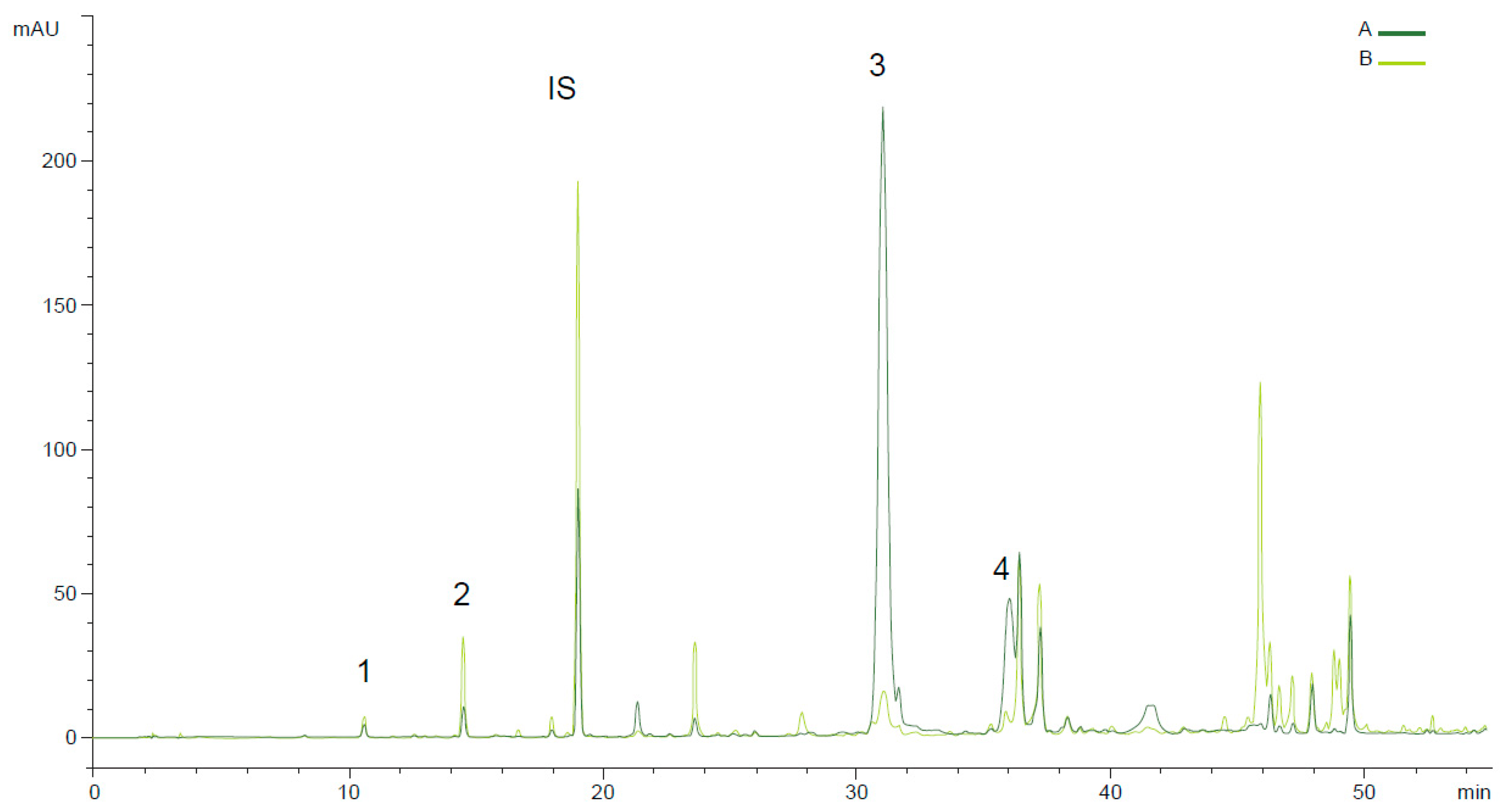
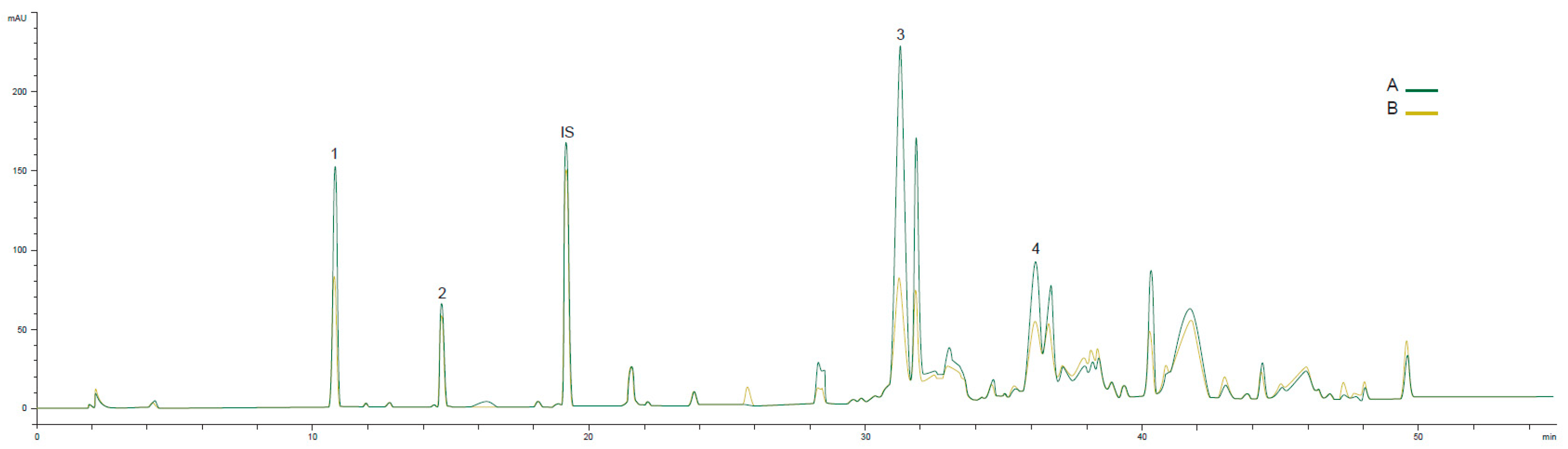
| Year | Ant. (%) | Fly (%) | RI | FFA (%) | PV (meqO2 kg−1) | K232 | K270 | Fruity | Bitter | Pung. | Musty | Cat. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 9 | 47 | 3.1 | 0.38 ± 0.00 | 8.60 ± 0.24 | 1.65 ± 0.02 | 0.17 ± 0.01 | 6.1 | 7.7 | 7.7 | 0 | EVOO |
| 5 | 57 | 3.1 | 0.37 ± 0.00 | 9.92 ± 0.13 | 1.76 ± 0.01 | 0.13 ± 0.01 | 5.2 | 7.3 | 7.8 | 0 | EVOO | |
| 51 | 82 | 4.1 | 0.50 ± 0.00 | 7.44 ± 0.08 | 1.57 ± 0.01 | 0.13 ± 0.01 | 4.6 | 4.9 | 4.6 | 0 | EVOO | |
| 70 | 35 | 5.8 | 0.64 ± 0.00 | 6.53 ± 0.07 | 1.50 ± 0.01 | 0.12 ± 0.01 | 3.2 | 6.0 | 5.0 | 0 | EVOO | |
| 2020 | 3 | 44 | 1.8 | 0.34 ± 0.00 | 9.95 ± 0.08 | 1.69 ± 0.02 | 0.18 ± 0.01 | 6.1 | 9.1 | 8.9 | 0 | EVOO |
| 5 | 16 | 2.9 | 0.23 ± 0.01 | 8.61 ± 0.25 | 1.58 ± 0.03 | 0.12 ± 0.00 | 4.6 | 7.3 | 5.2 | 0 | EVOO | |
| 25 | 42 | 4.8 | 0.23 ± 0.00 | 4.30 ± 0.18 | 1.50 ± 0.02 | 0.10 ± 0.01 | 3.4 | 4.6 | 2.8 | 0 | EVOO | |
| 91 | 69 | 6.4 | 1.52 ± 0.01 | 3.18 ± 0.38 | 1.46 ± 0.01 | 0.10 ± 0.01 | 0.1 | 0 | 0 | 1.2 | VOO | |
| 2021 | 0 | 15 | 2.1 | 0.32 ± 0.00 | 9.02 ± 0.04 | 1.71 ± 0.01 | 0.13 ± 0.01 | 3.9 | 2.8 | 4.3 | 0 | EVOO |
| 0 | 41 | 3.5 | 0.33 ± 0.00 | 6.50 ± 0.03 | 1.53 ± 0.02 | 0.11 ± 0.01 | 3.4 | 2.2 | 3.7 | 0 | EVOO | |
| 3 | 59 | 3.8 | 0.42 ± 0.00 | 4.23 ± 0.15 | 1.38 ± 0.01 | 0.07 ± 0.01 | 1.6 | 0 | 0.6 | 0 | EVOO | |
| 91 | 72 | 5.1 | 2.12 ± 0.01 | 4.33 ± 0.10 | 1.56 ± 0.01 | 0.13 ± 0.01 | 0.1 | 0 | 0 | 1.9 | L |
| Year | Ant. (%) | Fly (%) | RI | FFA (%) | PV meqO2 kg−1 | K232 | K270 | Fruity | Bitter | Pung. | Cat. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 0 | 36 | 1.6 | 0.26 ± 0.00 | 15.23 ± 0.12 | 2.05 ± 0.01 | 0.19 ± 0.01 | 7.0 | 8.1 | 7.9 | EVOO |
| 30 | 83 | 3.8 | 0.30 ± 0.00 | 11.63 ± 0.15 | 1.97 ± 0.01 | 0.16 ± 0.01 | 7.0 | 7.3 | 7.6 | EVOO | |
| 44 | 63 | 3.5 | 0.45 ± 0.00 | 10.93 ± 0.07 | 1.85 ± 0.01 | 0.14 ± 0.00 | 5.2 | 6.8 | 6.0 | EVOO | |
| 64 | 36 | 5.2 | 0.42 ± 0.00 | 11.43 ± 0.13 | 1.86 ± 0.01 | 0.12 ± 0.00 | 5.9 | 8.7 | 6.2 | EVOO | |
| 2020 | 6 | 29 | 2.4 | 0.34 ± 0.001 | 15.51 ± 0.16 | 1.96 ± 0.01 | 0.19 ± 0.01 | 4.3 | 9.4 | 9.8 | EVOO |
| 4 | 14 | 3.4 | 0.28 ± 0.00 | 13.06 ± 0.11 | 2.02 ± 0.01 | 0.17 ± 0.01 | 4.3 | 9,1 | 9.5 | EVOO | |
| 4 | 26 | 3.9 | 0.28 ± 0.00 | 11.20 ± 0.10 | 1.93 ± 0.01 | 0.10 ± 0.00 | 5.1 | 8.1 | 7.3 | EVOO | |
| 12 | 40 | 4.7 | 0.22 ± 0.00 | 7.73 ± 0.13 | 1.78 ± 0.01 | 0.09 ± 0.00 | 2.7 | 7.6 | 6.7 | EVOO | |
| 2021 | 0 | 27 | 1.0 | 0.32 ± 0.00 | 12.55 ± 2.17 | 1.87 ± 0.02 | 0.18 ± 0.04 | 6.5 | 6.1 | 7.2 | EVOO |
| 0 | 46 | 1.9 | 0.34 ± 0.00 | 11.21 ± 0.09 | 1.77 ± 0.02 | 0.15 ± 0.01 | 6.3 | 5.7 | 7.1 | EVOO | |
| 24 | 73 | 2.7 | 0.34 ± 0.00 | 10.91 ± 0.15 | 1.73 ± 0.01 | 0.14 ± 0.01 | 5.5 | 5.6 | 6.5 | EVOO | |
| 9 | 28 | 3.8 | 0.39 ± 0.00 | 10.13 ± 0.77 | 1.71 ± 0.02 | 0.10 ± 0.03 | 4.0 | 4.6 | 4.5 | EVOO |
| Year | C16:0 (%) | C18:0 (%) | C18:2 (%) | C18:1 (%) | TPC mg GAE/kg | CP mg/kg |
|---|---|---|---|---|---|---|
| 2019 | 15.76 ± 0.04 | 2.52 ± 0.04 | 4.94 ± 0.07 | 74.98 ± 0.14 | 626.41 ± 27.21 | 65.49 ± 0.04 |
| 15.33 ± 0.04 | 2.67 ± 0.01 | 4.96 ± 0.07 | 75.04 ± 0.07 | 504.82 ± 12.68 | 50.60 ± 0.05 | |
| 14.52 ± 0.03 | 2.59 ± 0.01 | 5.22 ± 0.03 | 75.29 ± 0.05 | 458.49 ± 2.20 | 28.44 ± 0.25 | |
| 14.04 ± 0.01 | 2.39 ± 0.01 | 4.95 ± 0.02 | 76.54 ± 0.02 | 468.29 ± 10.72 | 1.72 ± 0.02 | |
| 2020 | 13.95 ± 0.02 | 2.22 ± 0.02 | 4.90 ± 0.02 | 76.49 ± 0.04 | 681.43 ± 4.16 | 61.24 ± 0.01 |
| 14.25 ± 0.06 | 2.42 ± 0.05 | 4.76 ± 0.08 | 75.90 ± 0.14 | 539.03 ± 9.85 | 34.43 ± 0.02 | |
| 13.33 ± 0.07 | 2.44 ± 0.02 | 5.32 ± 0.07 | 76.16 ± 0.14 | 337.09 ± 12.67 | 4.96 ± 0.01 | |
| 12.60 ± 0.04 | 2.53 ± 0.03 | 4.70 ± 0.05 | 77.28 ± 0.07 | 87.05 ± 4.71 | 5.37 ± 0.01 | |
| 2021 | 14.35 ± 0.01 | 2.30 ± 0.03 | 5.42 ± 0.04 | 75.18 ± 0.06 | 352.22 ± 6.76 | 64.88 ± 0.59 |
| 14.08 ± 0.01 | 2.22 ± 0.01 | 5.54 ± 0.03 | 75.60 ± 0.03 | 274.85 ± 5.13 | 30.58 ± 0.10 | |
| 13.58 ± 0.04 | 2.30 ± 0.04 | 5.55 ± 0.02 | 75.75 ± 0.06 | 210.15 ± 5.73 | 8.55 ± 0.02 | |
| 13.27 ± 0.05 | 2.42 ± 0.04 | 6.43 ± 0.03 | 75.17 ± 0.07 | 137.90 ± 2.77 | 2.50 ± 0.02 |
| Year | C16:0 (%) | C18:0 (%) | C18:2 (%) | C18:1 (%) | TPC mg GAE/kg | CP mg/kg |
|---|---|---|---|---|---|---|
| 2019 | 14.78 ± 0.02 | 3.12 ± 0.03 | 7.71 ± 0.02 | 72.42 ± 0.03 | 762.33 ± 16.23 | 28.39 ± 0.01 |
| 14.28 ± 0.03 | 3.13 ± 0.01 | 8.82 ± 0.03 | 71.48 ± 0.01 | 718.72 ± 2.53 | 21.21 ± 0.04 | |
| 14.66 ± 0.02 | 3.11 ± 0.03 | 9.03 ± 0.03 | 71.57 ± 0.03 | 1019.05 ± 11.49 | 16.29 ± 0.01 | |
| 14.25 ± 0.03 | 3.09 ± 0.03 | 8.88 ± 0.07 | 71.81 ± 0.02 | 1233.62 ± 12.13 | 3.57 ± 0.06 | |
| 2020 | 14.81 ± 0.01 | 3.09 ± 0.02 | 7.25 ± 0.05 | 72.33 ± 0.06 | 1081.71 ± 12.13 | 60.95 ± 0.01 |
| 14.32 ± 0.05 | 3.20 ± 0.02 | 9.78 ± 0.03 | 70.25 ± 0.03 | 904.14 ± 5.58 | 49.65 ± 0.01 | |
| 14.08 ± 0.05 | 3.39 ± 0.03 | 10.39 ± 0.06 | 69.96 ± 0.08 | 911.16 ± 14.20 | 7.82 ± 0.02 | |
| 13.66 ± 0.04 | 3.56 ± 0.04 | 10.26 ± 0.01 | 70.15 ± 0.07 | 710.95 ± 27.48 | 1.19 ± 0.01 | |
| 2021 | 13.95 ± 0.11 | 2.89 ± 0.03 | 7.29 ± 0.05 | 72.93 ± 0.07 | 501.17 ± 7.13 | 79.94 ± 0.22 |
| 13.89 ± 0.29 | 2.91 ± 0.03 | 7.99 ± 0.13 | 73.05 ± 0.03 | 526.18 ± 92.01 | 71.65 ± 0.05 | |
| 13.61 ± 0.04 | 2.96 ± 0.05 | 8.80 ± 0.01 | 72.64 ± 0.04 | 607.72 ± 8.79 | 33.39 ± 0.02 | |
| 13.60 ± 0.05 | 3.10 ± 0.01 | 9.39 ± 0.02 | 71.38 ± 0.10 | 662.80 ± 1.96 | 16.32 ± 0.01 |
| Cobrançosa Virgin Olive Oils | Galega Virgin Olive Oils | |||||
|---|---|---|---|---|---|---|
| Year | HYT (mg/kg) | TYR (mg/kg) | Health Claim (mg/20 g) | HYT (mg/kg) | TYR (mg/kg) | Health Claim (mg/20 g) |
| 2019 | 199.92 ± 14.58 b | 228.09 ± 4.21 b | 8.56 ± 0.13 b | 189.71 ± 1.09 a | 114.94 ± 5.98 a | 6.09 ± 0.14 a |
| 126.87 ± 3.39 c | 199.91 ± 1.61 c | 6.54 ± 0.04 c | 146.57 ± 10.03 b | 107.02 ± 9.87 a | 5.07 ± 0.03 b | |
| 223.43 ± 5.46 b | 224.90 ± 1.27 b | 8.97 ± 0.11 b | 120.29 ± 1.19 c | 85.94 ± 1.09 b | 4.12 ± 0.05 c | |
| 281.93 ± 9.91 a | 251.04 ± 1.84 a | 10.66 ± 0.20 a | 109.21 ± 3.27 d | 69.23 ± 0.13 c | 3.6 ± 0.08 d | |
| 2020 | 261.66 ± 35.13 a | 207.52 ± 3.51 a | 9.38 ± 0.77 a | 143.79 ± 12.02 a | 60.36 ± 4.70 a | 4.08 ± 0.16 a |
| 216.06 ± 5.04 b | 201.29 ± 26.93 a | 8.35 ± 0.63 b | 108.41 ± 8.71 b | 65.61 ± 5.03 a | 3.48 ± 0.27 b | |
| 213.85 ± 5.67 b | 169.39 ± 5.42 b | 7.66 ± 0.11 b | 56.39 ± 4.66 c | 40.62 ± 2.31 b | 1.94 ± 0.14 c | |
| 138.08 ± 2.00 c | 132.93 ± 4.05 c | 5.42 ± 0.07 c | 2.76 ± 0.97 d | 11.57 ± 2.62 c | 0.29 ± 0.09 d | |
| 2021 | 201.17 ± 2.25 a | 162.52 ± 0.86 a | 7.27 ± 0.06 a | 125.12 ± 3.03 a | 80.52 ± 0.73 a | 4.1 ± 0.45 a |
| 196.54 ± 11.71 a | 161.07 ± 0.89 a | 7.15 ± 0.25 a | 109.20 ± 0.53 b | 56.36 ± 0.31 b | 3.31 ± 0.02 b | |
| 195.98 ± 8.94 a | 165.94 ± 1.45 a | 7.24 ± 0.15 a | 41.20 ± 0.17 c | 30.82 ± 2.11 c | 1.44 ± 0.04 c | |
| 209.08 ± 0.10 a | 146.96 ± 0.56 b | 7.12 ± 0.01 a | 3.51 ± 0.98 d | 17.34 ± 0.24 d | 0.42 ± 0.02 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, F.; Gouveia, C.; Vitorino, C.; Oliveira, H.; Ferreira-Dias, S. How the “Olive Oil Polyphenols” Health Claim Depends on Anthracnose and Olive Fly on Fruits. Foods 2024, 13, 1734. https://doi.org/10.3390/foods13111734
Peres F, Gouveia C, Vitorino C, Oliveira H, Ferreira-Dias S. How the “Olive Oil Polyphenols” Health Claim Depends on Anthracnose and Olive Fly on Fruits. Foods. 2024; 13(11):1734. https://doi.org/10.3390/foods13111734
Chicago/Turabian StylePeres, Fátima, Cecília Gouveia, Conceição Vitorino, Helena Oliveira, and Suzana Ferreira-Dias. 2024. "How the “Olive Oil Polyphenols” Health Claim Depends on Anthracnose and Olive Fly on Fruits" Foods 13, no. 11: 1734. https://doi.org/10.3390/foods13111734








