Methods for Synthesis and Extraction of Resveratrol from Grapevine: Challenges and Advances in Compound Identification and Analysis
Abstract
:1. Introduction
2. Methods of Synthesis and Extraction
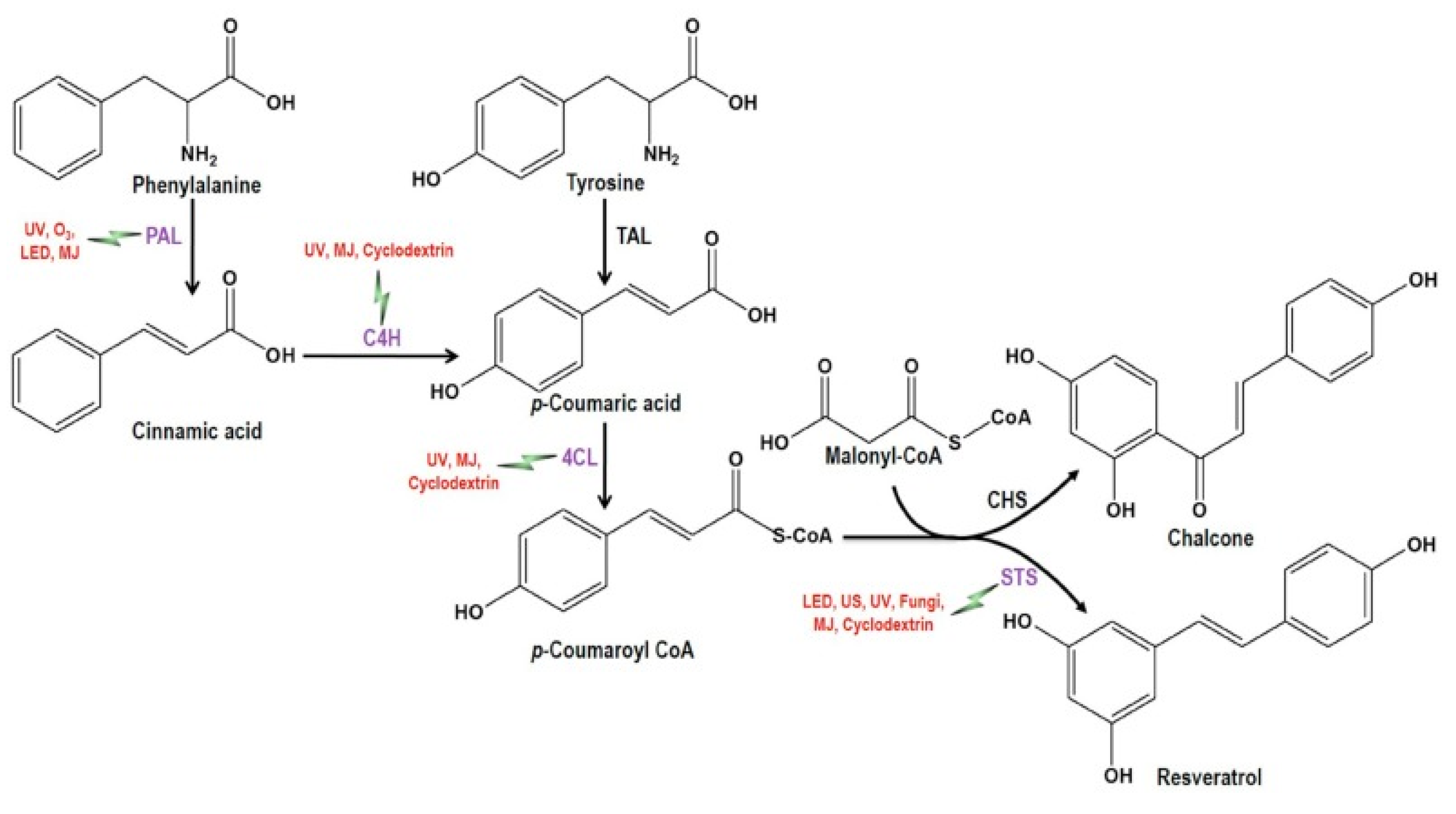
2.1. Synthesis and Chemical Extraction Methods
2.2. Synthesis and Natural Extraction Methods
2.2.1. Conventional Extraction (Maceration)
2.2.2. Ultrasound-Assisted Extraction (UAE)
2.2.3. Microwave-Assisted Extraction (MAE)
2.2.4. Membrane Extraction
2.2.5. Supercritical and Pressurized Fluid Extraction (SCFE)
2.2.6. Applying Electric Fields
2.2.7. Other Methods
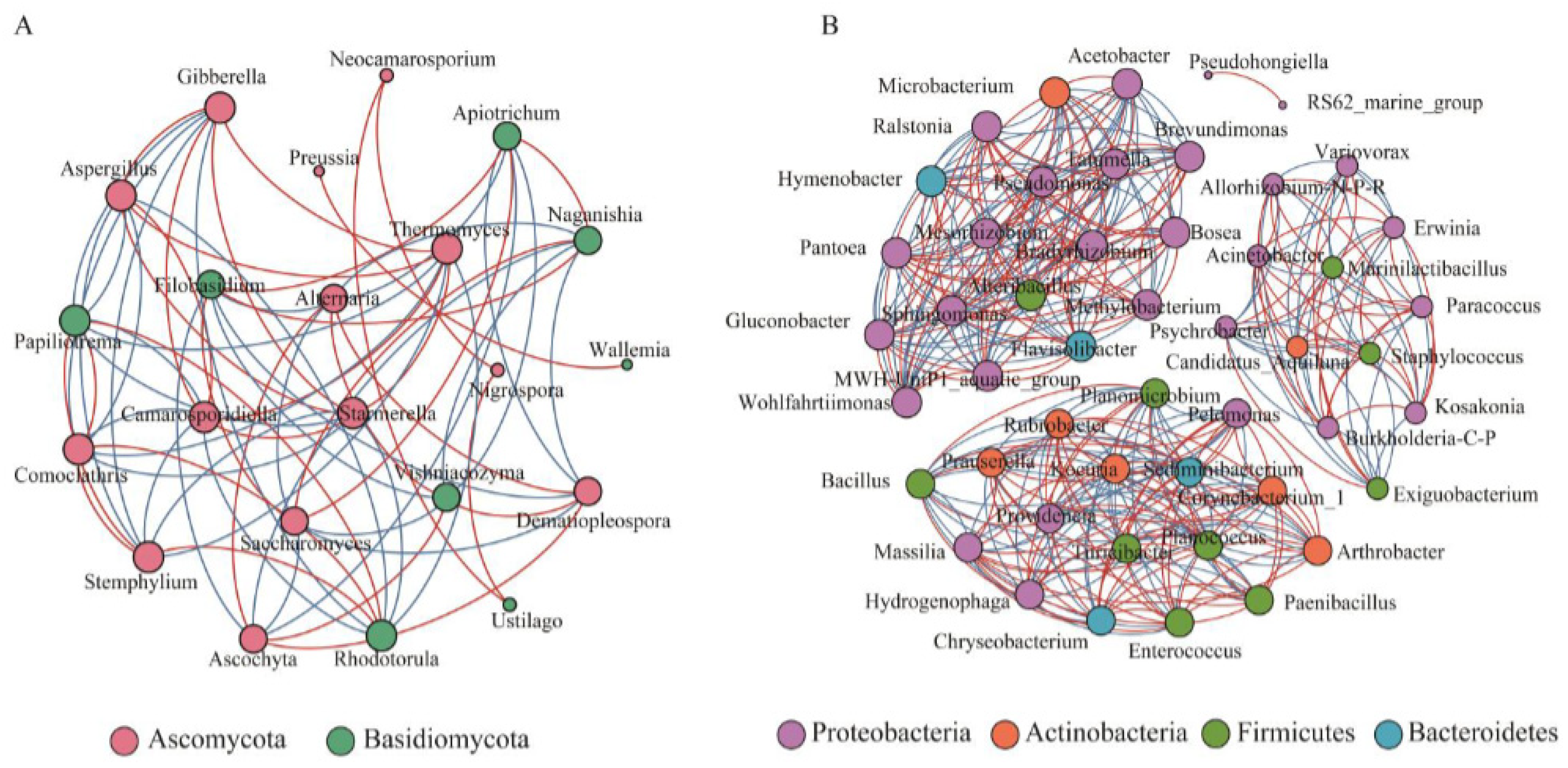
2.3. Biotechnological Synthesis and Extraction Methods
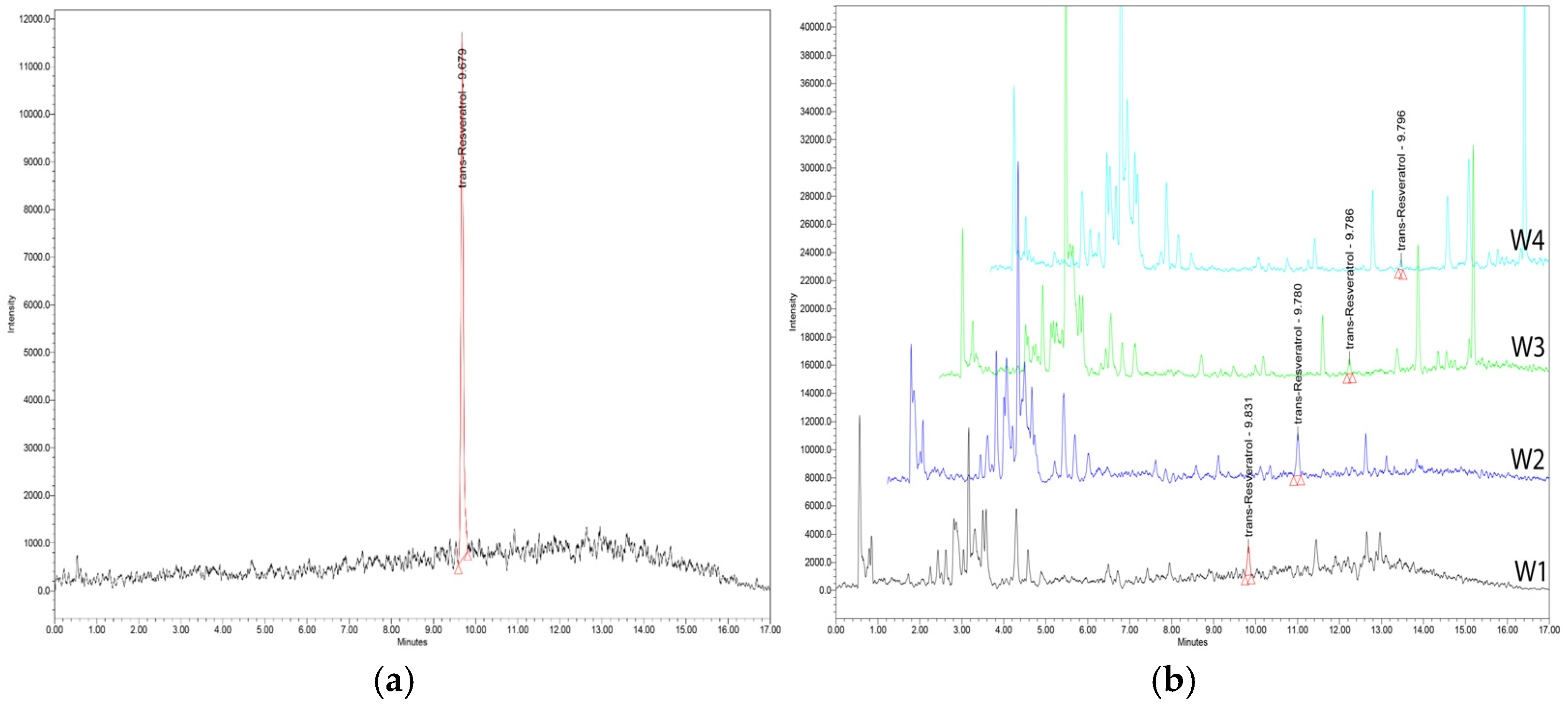
3. Identification Techniques
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPLC | High-performance liquid chromatography |
| HPLC-MS | HPLC–mass spectrometry |
| HPLC-UV | HPLC–ultraviolet |
| HPLC-GC/MS | HPLC–gas spectrometry/mass spectrometry |
| HPLC-DAD(UV)/CAD | HPLC–diode array detection (ultraviolet)/charged aerosol detector |
| HPLC-ESI-MS/MS | HPLC–electrospray ionization–mass spectrometry–mass spectrometry/mass spectrometry |
| HPLC-DAD-ESI-MSn | HPLC–diode array detection–electrospray ionization–mass spectrometry |
| HPLC-DAD-QToF | HPLC–diode array detection–quadrupole–time of flight mass spectrometry |
| UHPLC | Ultra-high-performance liquid chromatography |
| UPLC-FD | UPLC–fluorescence detection |
| UPLC-MS | UPLC–mass spectrometry |
| UHPLC-UV | UHPLC–ultraviolet |
| UHPLC-UV-DAD-MS | UHPLC–ultraviolet–diode array detection–mass spectrometry |
| UHPLC-(ESI+)-QToF-MS | UHPLC–(electrospray ionization-+)–quadrupole–time of flight mass spectrometry |
| UHPLC-Orbitrap MS4 | UHPLC–Orbitrap mass spectrometry |
| UPLC-VION-IMS-QToF | UPLC-VION-IMS–quadrupole–time of flight mass spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
| LC-ESI-QToF-MS/MS | Liquid chromatography–electrospray ionization–quadrupole–time of flight mass spectrometry/mass spectrometry |
| GSP/UV-A/HPLC | GSP/ultraviolet-A/HPLC |
| DW | Dry weight |
| FW | Fresh weight |
| SOX | Soxhlet extraction |
| DoE | Design of experiment |
| RSA | Radical scavenging activity |
| ACE | Angiotensin-converting enzyme |
| VCC | Vitamin C |
| TPC | The phenolic content |
| IPA | Isopropyl alcohol |
| cR | cis-resveratrol |
| tR | trans-resveratrol |
| HLM | Harvested at late maturity |
| PHWE | Pressurized hot water extraction |
| EHDs | Electrohydrodynamic methods |
| MAEs | Microwave-assisted processes |
| UAE | Ultrasound-assisted extraction method |
| DESs | Deep eutectic solvents |
| NaDESs | Deep natural solvent systems |
References
- Taillis, D.; Becissa, O.; Pébarthé-Courrouilh, A.; Renouf, E.; Palos-Pinto, A.; Richard, T.; Cluzet, S. Antifungal activities of a grapevine byproduct extract enriched in complex stilbenes and stilbenes metabolization by Botrytis cinerea. J. Agric. Food Chem. 2023, 71, 4488–4497. [Google Scholar] [CrossRef] [PubMed]
- Pébarthé-Courrouilh, A.; Jaa, A.; Valls-Fonayet, J.; Da Costa, G.; Palos-Pinto, A.; Richard, T.; Cluzet, S. UV-exposure decreases antimicrobial activities of a grapevine cane extract against Plasmopara viticola and Botrytis cinerea as a consequence of stilbene modifications—A kinetic study. Pest Manag. Sci. 2024, 80, 6389–6399. [Google Scholar] [CrossRef] [PubMed]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, C. trans-Resveratrol and trans-ε-Viniferin in Grape Canes and Stocks Originating from Savoie Mont Blanc Vineyard Region: Pre-extraction Parameters for Improved Recovery. ACS Sustain. Chem. Eng. 2019, 7, 8310–8316. [Google Scholar] [CrossRef]
- Labois, C.; Stempien, E.; Schneider, J.; Schaeffer-Reiss, C.; Bertsch, C.; Goddard, M.L.; Chong, J. Comparative study of secreted proteins, enzymatic activities of wood degradation and stilbene metabolization in grapevine botryosphaeria dieback fungi. J. Fungi 2021, 7, 568. [Google Scholar] [CrossRef]
- Pezet, R.; Cuenat, P. Resveratrol in wine: Extraction from skin during fermentation and post-fermentation standing of must from Gamay grapes. Am. J. Enol. Vitic. 1996, 47, 287–290. [Google Scholar] [CrossRef]
- Bavaresco, L.; Flamini, R.; Sansone, L.; Van Zeller de Macedo Basto Gonçalves, M.I.; Civardi, S.; Gatti, M.; Vezzulli, S. Improvement of healthy properties of grapes and wine with specific emphasis on resveratrol. J. Wine Res. 2011, 22, 135–138. [Google Scholar] [CrossRef]
- Căpruciu, R. Resveratrol in Grapevine Components, Products and By-Products—A Review. Horticulturae 2025, 11, 111. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Y.; Wang, B.; Xu, X.; Xu, X.; Hussain, D.; Ma, L.; Chen, D. Current analytical strategies for the determination of resveratrol in foods. Food Chem. 2024, 431, 137182. [Google Scholar] [CrossRef]
- Filipe, D.; Gonçalves, M.; Fernandes, H.; Oliva-Teles, A.; Peres, H.; Belo, I.; Salgado, J.M. Shelf-life performance of fish feed supplemented with bioactive extracts from fermented olive mill and winery by-products. Foods 2023, 12, 30. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and vascular health: Evidence from clinical studies and mechanisms of actions related to its metabolites produced by gut microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef]
- Robb, E.L.; Stuart, J.A. trans-resveratrol as a neuroprotectant. Molecules 2010, 15, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Saucier, C.; Bisbal, C.; Lambert, K. Grape polyphenols in the treatment of human skeletal muscle damage due to inflammation and oxidative stress during obesity and aging: Early outcomes and promises. Molecules 2022, 27, 6594. [Google Scholar] [CrossRef] [PubMed]
- Chinraj, V.; Raman, S. Neuroprotection by resveratrol: A review on brain delivery strategies for Alzheimer’s and Parkinson’s disease. J. Appl. Pharm. Sci. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Gui, J.; Sun, X.; Wen, S.; Liu, X.; Qin, B.; Sang, M. Resveratrol protects dopaminergic neurons in a mouse model of Parkinson’s disease by regulating the gut-brain axis via inhibiting the TLR4 signaling pathway. S. Med. J. 2024, 44, 270–279. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Das, D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Sy, B.; Krisa, S.; Richard, T.; Courtois, A. Resveratrol, ε-viniferin, and vitisin B from vine: Comparison of their in vitro antioxidant activities and study of their interactions. Molecules 2023, 28, 7521. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yang, Q.; Jiang, Y.X.; Chen, H.Q. Enhanced solubility, thermal stability and antioxidant activity of resveratrol by complexation with ovalbumin amyloid-like fibrils: Effect of pH. Food Hydrocoll. 2024, 148, 109463. [Google Scholar] [CrossRef]
- Petsini, F.; Detopoulou, M.; Choleva, M.; Kostakis, I.K.; Fragopoulou, E.; Antonopoulou, S. Exploring the effect of resveratrol, tyrosol, and their derivatives on platelet-activating factor biosynthesis in U937 cells. Molecules 2024, 29, 5419. [Google Scholar] [CrossRef]
- Pang, Q.; Wang, C.; Li, B.; Zhang, S.; Li, J.; Gu, S.; Shi, X. Resveratrol-loaded copolymer nanoparticles with anti-neurological impairment, antioxidant and anti-inflammatory activities against cerebral ischemia–reperfusion injury. Arab. J. Chem. 2024, 17, 105393. [Google Scholar] [CrossRef]
- Gál, R.; Halmosi, R.; Gallyas, F., Jr.; Tschida, M.; Mutirangura, P.; Tóth, K.; Alexy, T.; Czopf, L. Resveratrol and beyond: The effect of natural polyphenols on the cardiovascular system: A narrative review. Biomedicines 2023, 11, 2888. [Google Scholar] [CrossRef]
- Fernández Conde, M.E.; Cortiñas Rodriguez, J.A.; Taglinao, L.; Bisarya, D.; Rodríguez Pérez, L. Exploring the Versatile Nature of Resveratrol: A Comprehensive Review. arXiv 2024, arXiv:2024041394. [Google Scholar]
- Wang, Z.; Zhou, D.; Liu, D.; Zhu, B. Food-grade encapsulated polyphenols: Recent advances as novel additives in foodstuffs. Crit. Rev. Food Sci. Nutr. 2023, 63, 11545–11560. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies-A review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Zaitsev, G.P.; Grishin, Y.V.; Mosolkova, V.E.; Ogay, Y.A. Grape cane as a source of trans-Resveratrol and trans-Viniferin in the technology of biologically active compounds and its possible applications. In Advanced Bioactive Compounds Countering the Effects of Radiological, Chemical and Biological Agents, Proceedings of the NATO Science for Peace and Security Series A: Chemistry and Biology, 1 January 2013; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat-and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Richard, T.; Cantos-Villar, E. Grapevine cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape pomace as a natural source of phenolic compounds: Solvent screening and extraction optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef]
- Romero-Perez, A.I.; Lamuela-Raventos, R.M.; Andrés-Lacueva, C.; de la Torre-Boronat, M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Jiménez, J.B.; Orea, J.M.; Ureña, A.G.; Escribano, P.; Osa, P.L.D.L.; Guadarrama, A. Short anoxic treatments to enhance trans-resveratrol content in grapes and wine. Eur. Food Res. Technol. 2007, 224, 373–378. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; de Castro, M.D.L. Comparison of extraction methods for exploitation of grape skin residues from ethanol distillation. Talanta 2012, 101, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.; Roldán, M.; De Ory, A.I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-Gonzále, M.E.; Ferreira, C.F.R.I.; Barros, L. Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef]
- Guler, A. Effects of different maceration techniques on the colour, polyphenols and antioxidant capacity of grape juice. Food Chem. 2023, 404, 134603. [Google Scholar] [CrossRef]
- Junqua, R.; Carullo, D.; Ferrari, G.; Pataro, G.; Ghidossi, R. Ohmic heating for polyphenol extraction from grape berries: An innovative prefermentary process. Oeno One 2021, 55, 39–51. [Google Scholar] [CrossRef]
- Bianchi, A.; Santini, G.; Piombino, P.; Pittari, E.; Sanmartin, C.; Moio, L.; Modesti, M.; Bellincontro, A.; Mencarelli, F. Nitrogen maceration of wine grape: An alternative and sustainable technique to carbonic maceration. Food Chem. 2023, 404, 134138. [Google Scholar] [CrossRef]
- Beilankouhi, S.; Pourfarzad, A.; Ghanbarzadeh, B.; Rasouli, M.; Hamishekar, H. Identification of polyphenol composition in grape (Vitis vinifera cv. Bidaneh Sefid) stem using green extraction methods and LC-MS/MS analysis. Food Sci. Nutr. 2024, 12, 6789–6798. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Gambacorta, G.; Paradiso, V.M. Influence of prolonged maceration on phenolic compounds, volatile profile and sensory properties of wines from Minutolo and Verdeca, two Apulian white grape varieties. LWT 2024, 192, 115698. [Google Scholar] [CrossRef]
- Petit, E.; Rouger, C.; Griffault, E.; Ferrer, A.; Renouf, E.; Cluzet, S. Optimization of polyphenols extraction from grapevine canes using natural deep eutectic solvents. Biomass Convers. Biorefinery 2023, 14, 30545–30557. [Google Scholar] [CrossRef]
- Ramkumar, R.; Arun Prasath, V.; Karpoora Sundara Pandian, N.; Patra, A.; Sharma, P.; Arulkumar, M.; Sivaranjani, S.; Govindarasu, P. Investigating the influence of pin-to-plate atmospheric cold plasma on the physiochemical, nutritional, and shelf-life study of two raisins varieties during storage. J. Food Meas. Charact. 2024, 18, 7774–7793. [Google Scholar] [CrossRef]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Pulsed electric fields-assisted extraction of valuable compounds from red grape pomace: Process optimization using response surface methodology. Front. Nutr. 2023, 10, 1158019. [Google Scholar] [CrossRef]
- Peternel, L.; Sokač Cvetnić, T.; Gajdoš Kljusurić, J.; Jurina, T.; Benković, M.; Radojčić Redovniković, I.; Tušek, A.J.; Valinger, D. The effects of drying and grinding on the extraction efficiency of polyphenols from grape skin: Process optimization. Processes 2024, 12, 1100. [Google Scholar] [CrossRef]
- Marianne, L.C.; Lucía, A.G.; de Jesús, M.S.M.; Leonardo, H.M.E.; Mendoza-Sánchez, M. Optimization of the green extraction process of antioxidants derived from grape pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of identification and quantification of polyphenolic compounds in skins and seeds of four grape varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef] [PubMed]
- Candemir, A.; Çalışkan Koç, G.; Dirim, S.N.; Pandiselvam, R. Effect of ultrasound pretreatment and drying air temperature on the drying characteristics, physicochemical properties, and rehydration capacity of raisins. Biomass Convers. Biorefinery 2024, 14, 19623–19635. [Google Scholar] [CrossRef]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable extraction of polyphenols from vine shoots using deep eutectic solvents: Influence of the solvent, Vitis sp., and extraction technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Feriani, A.; Gómez-Cruz, I.; Hfaiedh, N.; Harrath, A.H.; Romero, I.; Castro, E.; Tlili, N. Grapevine shoot extract rich in trans-resveratrol and trans-ε-viniferin: Evaluation of their potential use for cardiac health. Foods 2023, 12, 4351. [Google Scholar] [CrossRef]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Ueda, J.M.; Griebler, K.R.; Finimundy, T.C.; Rodrigues, D.B.; Veríssimo, L.; Pires, T.C.; João Gonçalves, J.; Fernandes, I.P.; Pereira, E.; Barros, L.; et al. Polyphenol Composition by HPLC-DAD-(ESI-) MS/MS and Bioactivities of Extracts from Grape Agri-Food Wastes. Molecules 2023, 28, 7368. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Carretero, I.; Coves, J.R.; Pedrouso, A.; Castro-Barros, C.M.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Sentellas, S. Recovery of Phenolic Compounds from Wine Lees Using Green Processing: Identifying Target Molecules and Assessing Membrane Ultrafiltration Performance. Sci. Total Environ. 2023, 857, 159623. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, Y.J.; Park, S.C.; Kim, S.; Kim, Y.G.; Shin, G.; Jeong, H.J.; Ryu, Y.B.; Lee, J.; Lee, O.R.; et al. Highly efficient bioconversion of trans-resveratrol to δ-viniferin using conditioned medium of grapevine callus suspension cultures. Int. J. Mol. Sci. 2022, 23, 4403. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.M.; Conidi, C.; Cassano, A.A. Membrane-Assisted green strategy for purifying bioactive compounds from extracted white wine lees. Sep. Purif. Technol. 2024, 336, 126183. [Google Scholar] [CrossRef]
- Melo, F.D.O.; Ferreira, V.C.; Barbero, G.F.; Carrera, C.; Ferreira, E.D.S.; Umsza-Guez, M.A. Extraction of bioactive compounds from wine lees: A systematic and bibliometric review. Foods 2024, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Bertussi, R.A.; Agostini, G.; Atti Dos Santos, A.C.; Rossato, M.; Vanderlinde, R. Supercritical extraction from vinification residues: Fatty acids, α-tocopherol, and phenolic compounds in the oil seeds from different varieties of grape. Sci. World J. 2012, 2012, 790486. [Google Scholar] [CrossRef]
- Takács, K.; Pregi, E.; Vági, E.; Renkecz, T.; Tátraaljai, D.; Pukánszky, B. Processing stabilization of polyethylene with grape peel extract: Effect of extraction technology and composition. Molecules 2023, 28, 1011. [Google Scholar] [CrossRef]
- Bastos, K.V.L.D.S.; de Souza, A.B.; Tomé, A.C.; Souza, F.D.M. New Strategies for the Extraction of Antioxidants from Fruits and Their By-Products: A Systematic Review. Plants 2025, 14, 755. [Google Scholar] [CrossRef]
- Fu, T.; Feng, Y.; Zhang, S.; Sheng, Y.; Wang, C. Resveratrol-derived carbon dots integrated into gelatin/chitosan multifunctional films for intelligent packaging. Food Chem. X 2025, 25, 102182. [Google Scholar] [CrossRef]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of biomedical nanofibers/nanomembranes: Effects of process parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Michniak-Kohn, B.; Cielecka-Piontek, J. The antioxidant potential of resveratrol from red vine leaves delivered in an electrospun nanofiber system. Antioxidants 2023, 12, 1777. [Google Scholar] [CrossRef]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. Effects of polysaccharide-based coatings on postharvest storage life of grape: Measuring the changes in nutritional, antioxidant and phenolic compounds. J. Food Meas. Charact. 2022, 16, 1159–1170. [Google Scholar] [CrossRef]
- Markhali, F.S.; Teixeira, J.A. Extractability of oleuropein, hydroxytyrosol, tyrosol, verbascoside and flavonoid-derivatives from olive leaves using ohmic heating (a green process for value addition). Sustain. Food Prod. 2024, 2, 461–469. [Google Scholar] [CrossRef]
- Sazdanić, D.; Krstonošić, M.A.; Ćirin, D.; Cvejić, J.; Alamri, A.; Galanakis, C.M.; Krstonošić, V. Non-ionic surfactants-mediated green extraction of polyphenols from red grape pomace. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100439. [Google Scholar] [CrossRef]
- Vasilyeva, A.P.; Svinarev, A.V.; Ogurtsov, V.A.; Khodot, E.N.; Rakitin, O.A.; Trubnikova, E.V.; Shcherbakova, E.S.; Smirnova, M.S.; Shishkina, V.V.; Samoylenko, T.V.; et al. Study of the Wound-Healing Activity of a New Drug Derived from Cobalt Polyacrylate. Int. J. Mol. Sci. 2025, 26, 899. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.; Janaswamy, S. Porous starch granules loaded curcumin and resveratrol modulate starch digestibility of wheat bread: Toward developing novel functional foods. Food Hydrocoll. 2025, 162, 111006. [Google Scholar] [CrossRef]
- Liberatore, M.T.; Dilucia, F.; Rutigliano, M.; Viscecchia, R.; Spano, G.; Capozzi, V.; Bimbo, F.; di Luccia, A.; la Gatta, B. Polyphenolic characterization, nutritional and microbiological assessment of newly formulated semolina fresh pasta fortified with grape pomace. Food Chem. 2025, 463, 141531. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, X.; Yang, Y.; Zheng, L.; Xiao, D.; Ai, B.; Sheng, Z. Emerging technologies in reducing dietary advanced glycation end products in ultra-processed foods: Formation, health risks, and innovative mitigation strategies. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70130. [Google Scholar] [CrossRef]
- Laghari, F.; Chang, Q.; Zhang, H.; Zhang, J.; Pan, L.; Pu, Z.; Bao, J.; Zhang, R. Potential Mechanisms and Therapeutic Effect of Dietary Resveratrol Supplementation on the Spleen Organ of Chicken in Chronic Unpredictable Mild Stress Transcriptomic Analysis. Poult. Sci. 2025, 104, 104940. [Google Scholar] [CrossRef]
- Qin, X.; Niu, W.; Zhao, K.; Luo, Y.; Wang, W.; He, Y.; Yang, F.; Cao, B.; Min Du, M.; Su, H. Resveratrol enhances post-injury muscle regeneration by regulating antioxidant and mitochondrial biogenesis. Curr. Res. Food Sci. 2025, 10, 100972. [Google Scholar] [CrossRef]
- Mendes, I.; Ribeiro, M.G.C.; de Souza, L.F.; Rosa, C.D.O.B.; Hermsdorff, H.H.M.; Bressan, J. Effect of Polyphenol Supplementation on Adiposity: A Systematic Review of Randomized Clinical Trials. Curr. Nutr. Rep. 2025, 14, 36. [Google Scholar] [CrossRef]
- Iqbal, Y.; Amin, F.; Aziz, M.H.; Alhadlaq, H.A.; Alaizeri, Z.M. Biomimetic sodium alginate functionalized gold nanoparticles loaded with Trans-resveratrol for in-vitro cancer treatment and intercellular ROS studies against MCF-7 cell lines. Colloids Surf. A Physicochem. Eng. Asp. 2025, 712, 136448. [Google Scholar] [CrossRef]
- Yu, X.; Yao, Y.; Zhou, H.; Zhu, J.; Zhang, N.; Sang, S.; Zhou, H. Integrating network pharmacology and experimental validation to explore the potential mechanism by which resveratrol acts on osimertinib resistance in lung cancer. Oncol. Lett. 2025, 29, 192. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, X.; Zhang, S.; Luo, Z.; Song, L.; Chen, H.; Yao, X. Advances in the activity of resveratrol and its derivatives in cardiovascular diseases. Arch. Pharm. 2025, 358, e2400865. [Google Scholar] [CrossRef]
- Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W.; Sun, Y.; Ye, X.; He, Y.; Huang, X.; Wang, F.; Lu, Y.; Zhang, J. Anti-Inflammatory Resveratrol Protects Mice From Early Mortality After Haematopoietic Stem Cell Transplantation. J Cell. Mol. Med. JCMM 2025, 29, e70395. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, Y.; Bi, J.; Li, S.; Wu, H.; Zeng, M.; Pan, Y.; Lin, W.; Zhou, M.; Zhang, Z.; et al. An antioxidant composite film based on loquat seed starch incorporating resveratrol-loaded core-shell nanoparticles. Int. J. Biol. Macromol. 2025, 306, 141493. [Google Scholar] [CrossRef]
- Marant, B.; Crouzet, J.; Flourat, A.L.; Jeandet, P.; Aziz, A.; Courot, E. Key-enzymes involved in the biosynthesis of resveratrol-based stilbenes in Vitis spp.: A review. Phytochem. Rev. 2024, 24, 461–481. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Selles-Marchart, S.; Nájera, H.; Morante-Carriel, J.; Martínez-Esteso, M.J.; Bru-Martínez, R. Biosynthesis of piceatannol from resveratrol in grapevine can be mediated by cresolase-dependent ortho-hydroxylation activity of polyphenol oxidase. Plants 2024, 13, 2602. [Google Scholar] [CrossRef]
- Chang, C.H.; Lien, Y.T.; Lin, W.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Protective effects of piceatannol on DNA damage in Benzo [a] pyrene-induced human colon epithelial cells. J. Agric. Food Chem. 2023, 71, 7370–7381. [Google Scholar] [CrossRef]
- Piver, B.; Fer, M.; Vitrac, X.; Merillon, J.M.; Dreano, Y.; Berthou, F.; Lucas, D. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem. Pharmacol. 2004, 68, 773–782. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Kuban, W.; Daniel, W.A. The engagement of cytochrome P450 enzymes in tryptophan metabolism. Metabolites 2023, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ahn, T.; Jung, H.C.; Pan, J.G.; Yun, C.H. Generation of the human metabolite piceatannol from the anticancer-preventive agent resveratrol by bacterial cytochrome P450 BM3. Drug Metab. Dispos. 2009, 37, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Wally, O.; Rajcan, I.; Dhaubhadel, S. Comprehensive analysis of cytochrome P450 monooxygenases reveals insight into their role in partial resistance against Phytophthora sojae in soybean. Front. Plant Sci. 2022, 13, 862314. [Google Scholar] [CrossRef]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Dalla Vedova, A.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef]
- Gao, Y.; Fangel, J.U.; Willats, W.G.; Vivier, M.A.; Moore, J.P. Differences in berry skin and pulp cell wall polysaccharides from ripe and overripe Shiraz grapes evaluated using glycan profiling reveals extensin-rich flesh. Food Chem. 2021, 363, 130180. [Google Scholar] [CrossRef]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; de Bernonville, T.D.; Besseau, S.; Courdavault, V.; Oudin, A.; Pichon, O.; Clastre, M.; et al. Stilbenoid-enriched grape cane extracts for the biocontrol of grapevine diseases. In Plant Defence: Biological Control PIBC; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2020; Volume 22, pp. 215–239. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Wang, H.; Li, H. Dynamic succession of natural microbes during the Ecolly grape growth under extremely simplified eco-cultivation. Foods 2024, 13, 1580. [Google Scholar] [CrossRef]
- Sinrod, A.J.; Shah, I.M.; Surek, E.; Balanovarile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.E.; Radu, A.; Dumitru, A.; Ciocâlteu, M.V.; Mogoșeanu, G.D.; Bejenaru, C. Resveratrol: A Review on the biological activity and applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, S.K.; Kim, M.H.; Lee, H.B.; Lee, J.S. Analysis of trans-resveratrol contents of grape and grape products consumed in Korea. Korean J. Food Sci. Technol. 2003, 35, 764–768. [Google Scholar]
- Moreno, A.; Castro, M.; Falqué, E. Evolution of trans- and cis-resveratrol content in red grapes (Vitis vinifera L. cv Mencía, Albarello and Merenzao) during ripening. Eur. Food Res. Technol. 2008, 227, 667–674. [Google Scholar] [CrossRef]
- Wijekoon, C.; Netticadan, T.; Siow, Y.L.; Sabra, A.; Yu, L.; Raj, P.; Prashar, S. Potential associations among bioactive molecules, antioxidant activity and resveratrol production in Vitis vinifera fruits of North America. Molecules 2022, 27, 336. [Google Scholar] [CrossRef] [PubMed]
- Vo, G.T.; Liu, Z.; Chou, O.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Screening of phenolic compounds in australian grown grapes and their potential antioxidant activities. Food Biosci. 2022, 47, 101644. [Google Scholar] [CrossRef]
- Kaya, O. Harmony in the vineyard: Exploring the eco-chemical interplay of Bozcaada Çavuşu (Vitis vinifera L.) grape cultivar and pollinator varieties on some phytochemicals. Eur. Food Res. Technol. 2024, 250, 1327–1339. [Google Scholar] [CrossRef]
- Ji, M.; Li, Q.; Ji, H.; Lou, H. Investigation of the distribution and season regularity of resveratrol in Vitis amurensis via HPLC–DAD–MS/MS. Food Chem. 2014, 142, 61–65. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanassova, S.; Atanasov, V.; Grozeva, N. Content of polyphenolic compounds and antioxidant potential of some Bulgarian red grape varieties and red wines, determined by HPLC, UV, and NIR spectroscopy. Agriculture 2020, 10, 193. [Google Scholar] [CrossRef]
- Erte, E.; Vural, N.; Mehmetoğlu, Ü.; Güvenç, A. Optimization of an abiotic elicitor (ultrasound) treatment conditions on trans-resveratrol production from Kalecik Karası (Vitis vinifera L.) grape skin. J. Food Sci. Technol. 2021, 58, 2121–2132. [Google Scholar] [CrossRef]
- Serni, E.; Tomada, S.; Haas, F.; Robatscher, P. Characterization of phenolic profile in dried grape skin of Vitis vinifera L. cv. Pinot Blanc with UHPLC-MS/MS and its development during ripening. J. Food Compos. Anal. 2022, 114, 104731. [Google Scholar] [CrossRef]
- Capruciu, R.; Cichi, D.D.; Mărăcineanu, L.C.; Costea, D.C. The resveratrol content in black grapes skins at different development stages. Sci. Papers. Ser. B. Hortic. 2022, LXVI, 245–252. [Google Scholar]
- Sun, Y.; Xi, B.; Dai, H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’Grape Berries. Agronomy 2023, 13, 633. [Google Scholar] [CrossRef]
- Lai, G.; Fu, P.; He, L.; Che, J.; Wang, Q.; Lai, P.; Lu, J.; Lai, C. CRISPR/Cas9 mediated CHS2 mutation provides a new insight into resveratrol biosynthesis by causing a metabolic pathway shift from flavonoids to stilbenoids in Vitis davidii cells. Hortic. Res. 2024, 12, uhae268. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, M.; Cheng, Y.; Yang, B.; Kuang, H.; Wang, Z.; Yi, S.; Wang, B.; Fu, Y. Surfactant-assisted and ionic liquid aqueous system pretreatment for biocatalysis of resveratrol from grape seed residue using an immobilized microbial consortia. J. Food Process. Preserv. 2021, 45, e15279. [Google Scholar] [CrossRef]
- Kavgacı, M.; Yukunc, G.O.; Keskin, M.; Can, Z.; Kolaylı, S. Comparison of Phenolic Profile and Antioxidant Properties of Pulp and Seeds of Two Different Grapes Types (Vitis vinifera L. and Vitis labrusca L.) Grown in Anatolia: The Amount of Resveratrol of Grape Samples. Chem. Afr. 2023, 6, 2463–2469. [Google Scholar] [CrossRef]
- Hernández, M.D.M.; Castillo Río, C.; Blanco González, S.I.; Menéndez, C.M. Phenolic profile changes of grapevine leaves infected with Erysiphe necator. Pest. Manag. Sci. 2024, 80, 397–403. [Google Scholar] [CrossRef]
- Tahmaz, H.; Küskü, D.Y. Investigation of some physiological and chemical changes in shoots and leaves caused by UV-C radiation as an abiotic stress source in grapevine cuttings. Sci. Hortic. 2024, 336, 113383. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Puerto, M.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Protection and reversion role of a pure stilbene extract from grapevine shoot and its major compounds against an induced oxidative stress. J. Funct. Foods 2021, 79, 104393. [Google Scholar] [CrossRef]
- Kiene, M.; Zaremba, M.; Januschewski, E.; Juadjur, A.; Jerz, G.; Winterhalter, P. Sustainable in silico-supported ultrasonic-assisted extraction of oligomeric stilbenoids from grapevine roots using natural deep eutectic solvents (NADES) and stability study of potential ready-to-use extracts. Foods 2024, 13, 324. [Google Scholar] [CrossRef]
- Noronha, H.; Silva, A.; Garcia, V.; Billet, K.; Dias, A.C.; Lanoue, A.; Gallusci, P.; Gerós, H. Grapevine woody tissues accumulate stilbenoids following bud burst. Planta 2023, 258, 118. [Google Scholar] [CrossRef]
- Fermo, P.; Comite, V.; Sredojević, M.; Ćirić, I.; Gašić, U.; Mutić, J.; Baošić, R.; Tešić, Ž. Elemental analysis and phenolic profiles of selected italian wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The role of resveratrol in eye diseases—A review of the literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Hoferer, L.; Rodrigues Guimarães Abreu, V.L.; Graßl, F.; Fischer, O.; Heinrich, M.R.; Gensberger-Reigl, S. Identification and quantification of resveratrol and its derivatives in franconian wines by comprehensive liquid chromatography–tandem mass spectrometry. ACS Food Sci. Technol. 2023, 3, 1057–1065. [Google Scholar] [CrossRef]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 247, p. 01063. [Google Scholar] [CrossRef]
- Rai, R.; Merrell, C.; Yokoyama, W.; Nitin, N. Infusion of trans-resveratrol in micron-scale grape skin powder for enhanced stability and bioaccessibility. Food Chem. 2021, 340, 127894. [Google Scholar] [CrossRef]
- Liu, F.; Lei, J.; Shao, X.; Fan, Y.; Huang, W.; Lian, W.; Wang, C. Effect of Pretreatment and Drying on the Nutritional and Functional Quality of Raisins Produced with Seedless Purple Grapes. Foods 2024, 13, 1138. [Google Scholar] [CrossRef]
- Guamán-Balcázar, M.C.; Setyaningsih, W.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of resveratrol from functional foods: Cookies and jams. Appl. Acoust. 2016, 103, 207–213. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Guamán-Balcázar, M.d.C.; Oktaviani, N.M.D.; Palma, M. Response Surface Methodology Optimization for Analytical Microwave-Assisted Extraction of Resveratrol from Functional Marmalade and Cookies. Foods 2023, 12, 233. [Google Scholar] [CrossRef]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Avendaño-Godoy, J.; Ortega, E.; Urrutia, M.; Escobar-Avello, D.; Luengo, J.; von Baer, D.; Mardones, C.; Gómez-Gaete, C. Prototypes of nutraceutical products from microparticles loaded with stilbenes extracted from grape cane. Food Bioprod. Process. 2022, 134, 19–29. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of some stilbenoids extracted from two cultivars of Lambrusco—Vitis vinifera Species: An opportunity to valorize pruning canes for a more sustainable viticulture. Molecules 2023, 28, 4074. [Google Scholar] [CrossRef]
- Kiene, M.; Zaremba, M.; Fellensiek, H.; Januschewski, E.; Juadjur, A.; Jerz, G.; Winterhalter, P. In silico-assisted isolation of trans-resveratrol and trans-ε-viniferin from grapevine canes and their sustainable extraction using natural deep eutectic solvents (NADES). Foods 2023, 12, 4184. [Google Scholar] [CrossRef]
- Ingrà, C.; Del Frari, G.; Favole, M.; Tumminelli, E.; Rossi, D.; Collina, S.; Ferrandino, A. Effects of growing areas, pruning wound protection products, and phenological stage on the stilbene composition of grapevine (Vitis vinifera L.) canes. J. Agric. Food Chem. 2024, 72, 11465–11479. [Google Scholar] [CrossRef]
- Kosović, E.; Topiař, M.; Cuřínová, P.; Sajfrtová, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Sci. Rep. 2020, 10, 5564. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Rajko Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; et al. Non-thermal ultrasonic extraction of polyphenolic compounds from red wine lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-assisted extraction to obtain phenolic-enriched wine lees with enhanced bioactivity in hypertensive rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef]
- Gaudin, K.; Valls-Fonayet, J.; Cordazzo, R.; Serafin, W.; Lafon, E.; Gaubert, A.; Richard, T.; Cluzet, S. Separation of polyphenols by HILIC methods with diode array detection, charged aerosol detection and mass spectrometry: Application to grapevine extracts rich in stilbenoids. J. Chromatogr. A 2024, 1736, 465422. [Google Scholar] [CrossRef]
- Pezet, R.; Pont, V.; Cuenat, P. Method to determine resveratrol and pterostilbene in grape berries and wines using High-performance liquid chromatography and highly sensitive fluorimetric detection. J. Chromatogr. A 1994, 663, 191–197. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.M. Analysis of several phenolic compounds with potential antioxidant properties in grape extracts and wines by HPLC photodiode array detection without sample preparation. J. Chromatogr. A 2000, 881, 461–469. [Google Scholar] [CrossRef]
- Gambuti, A.; Strollo, D.; Ugliano, M.; Lecce, L.; Moio, L. trans-Resveratrol, quercetin, (+)-catechin, and (−)-epicatechin content in south Italian monovarietal wines: Relationship with maceration time and marc pressing during winemaking. J. Agric. Food Chem. 2004, 52, 5747–5751. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Phenolic composition of the Brazilian seedless table grape varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011, 59, 8314–8323. [Google Scholar] [CrossRef]
- Giuffrè, A.M. High performance liquid chromatography-diode array detector (HPLC-DAD) detection of trans-resveratrol: Evolution during ripening in grape berry skins. Afr. J. Agric. Res 2013, 8, 224–229. [Google Scholar]
- Göksel, Z.; Kayahan, S.; Atak, A.; Şen, A.; Tunçkal, C. Phenolic contents of some disease-resistant raisins (Vitis spp. In Proceedings of the XIII International Conference on Grapevine Breeding, Genetics and Management 1385, Cappadocia, Turkey, 21–24 August 2023. [Google Scholar] [CrossRef]
- Mohammadparast, B.; Rasouli, M.; Eyni, M. Resveratrol contents of 27 grape cultivars. Appl. Fruit Sci. 2024, 66, 1053–1060. [Google Scholar] [CrossRef]
- Medouni-Adrar, S.; Medouni-Haroune, L.; Cadot, Y.; Soufi-Maddi, O.; Makhoukhe, A.; Boukhalfa, F.; Boulekbache-Makhlouf, L.; Madani, K. Phenolic compounds profiling in Ahmar Bou-Amar grapes extracted using two statistically optimized extraction methods. J. Food Meas. Charact. 2024, 18, 6986–7002. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Costa, C.E.; Romaní, A.; Domingues, L. Overview of resveratrol properties, applications, and advances in microbial precision fermentation. Crit. Rev. Biotechnol. 2024, 44, 1–17. [Google Scholar] [CrossRef]
- Jiang, X.; Zuo, L.; Gao, S.; Yang, Q.; Li, Y.; Chen, Y.; Xie, X.; Peng, C. Green Production Pathways, Instability, and Stability of Resveratrol: A Systematic Review. J. Food Biochem. 2025, 1, 8210896. [Google Scholar] [CrossRef]
- Naryal, S.; Rana, M.; Sharma, N.; Ahmed, B.; Sharma, P.; Kujur, S.; Jaiswal, S.; Gupta, S.D.; Patel, S.M.B.; Kaushal, S.; et al. Navigating Sustainable and Healthy Future: Green Nanotechnology, Regulatory Priorities, and Challenges. In Role of Science and Technology for Sustainable Future; Sobti, R.C., Ed.; Springer: Singapore, 2024; pp. 353–369. [Google Scholar] [CrossRef]
- Krstonošić, M.A.; Sazdanić, D.; Ćirin, D.; Maravić, N.; Mikulić, M.; Cvejić, J.; Krstonošić, V. Aqueous solutions of non-ionic surfactant mixtures as mediums for green extraction of polyphenols from red grape pomace. Sustain. Chem. Pharm. 2023, 33, 101069. [Google Scholar] [CrossRef]

| Fractions | Methods/ Extracting Substances | Detection Technique * | Content in Resveratrol | References | |
|---|---|---|---|---|---|
| Grapevine components | Whole grape | C2H3N/H2O (40:60, v/v) | HPLC-UV | 0.09235 mg/L, DW | [92] |
| C2H3N–CH3COOH | HPLC-FL | 7–24 mg/L, DW | [93] | ||
| acidified water (0.1% H3PO4)/C2H3N | HPLC-GC/MS | 13.9 ± 2.87 mg/L, DW | [94] | ||
| 70% C2H5OH | LC-ESI-QToF-MS/MS | 227,000 mg/L, FW | [95] | ||
| MeOH (70%)/H2O (8:2, v/v) | HPLC-DAD-ESI-MSn | 4.04 mg/L, DW | [96] | ||
| Skin | MeOH | HPLC-ESI-MS/MS | 30.6 ± 1.7 mg/L, DW | [97] | |
| 1% HCl in MeOH | HPLC | 3.13 ± 0.33 to 14.57 ± 1.34 mg/L, FW | [98] | ||
| incubation time—24 h, US application method-(P01), US frequency—20 kHz, US treatment time—60 min and ultrasonic intensity (UI)—1.15 W cm−2 | HPLC | 180 ± 10 mg/L to 3580 ± 80 mg/L, DW | [99] | ||
| MeOH–deionized water (1:1) with 1% CH2O2 (v/v) | UHPLC | 0.05 mg/L, FW | [100] | ||
| MeOH | HPLC | 0.065 to 7.119 mg/L, DW (cis-resveratrol-cR) 0.633 to 9.152 mg/L, DW (tR) | [101] | ||
| MeOH/C4H8O2 (1:1, v/v) | HPLC | 0.667 mg/L, DW | [102] | ||
| 70% MeOH | UPLC-MS-MS | 2.76 mg/L, FW | [103] | ||
| Seed | MeOH | HPLC-ESI-MS/MS | 20.4 ± 0.7 mg/L, DW | [97] | |
| H2O-CH2O2-C2H3N (76.935/0.065/23, v/v/v) | UHPLC-MS/MS | 305.98 ± 0.23 mg/L, DW | [104] | ||
| Pulp | MeOH | HPLC-UV | 45 to 1018.9 mg/L, DW | [105] | |
| Stem | C2H5OH (5%, v/v) | HPLC | 680 to 1870 mg/L, DW | [33] | |
| 1. (H2O + MAE + UAE + atmospheric pressure); 2. (H2O + MAE + UAE + reduced pressure). | HPLC-ESI-MS/MS | 1121 ± 4.8 mg/L, DW | [38] | ||
| Leaf | MeOH | HPLC-ESI-MS/MS | 6.2 ± 0.1 mg/L, DW | [97] | |
| The DoE technique applied to red vine leaf c (50% MeOH, temperature 70 °C and three replicates per one hour) | HPLC | 0.306 ± 0.009 mg/L DW | [60] | ||
| Two dark sonication replicates two dark sonication cycles (10 mL of 0.1 m HCl 80% MeOH solution) (10 mL of 0.1 m HCl 80% MeOH solution, at 4 °C in 15 min) | UPLC | 30–40 mg/L FW−1 × 10−1 | [106] | ||
| UV-C treatment/MeOH | LC-MS/MS | 0.01997718–0.3578911798 mg/L, FW | [107] | ||
| 70% MeOH | UPLC-MS-MS | 4.22 mg/L, FW | [103] | ||
| Shoot | EC50 Caco-2 /EC50 HepG2-H2O2 | HPLC | 14.74 and 29.47 mg/L, DW | [108] | |
| MeOH-H2O (80:20, v/v) | HPLC | 148.53 mg/L−1, DW | [47] | ||
| Root | MeOH | HPLC-ESI-MS/MS | 86.3 ± 2.5 mg/L, DW | [97] | |
| COSMO-RS-NADES | UHPLC-UV | 520–2470 mg/L, DW | [109] | ||
| Wood | Botrytis cinerea secretome | UHPLC-UV-DAD-MS | 9541 ± 16,800 mg/L, DW | [1] | |
| Woody tissues | 80% MeOH | UPLC-MS | 69.1 to 436.5 mg/L, DW−1 | [110] | |
| Bud | 80% MeOH | UPLC-MS | 150 mg/L, DW−1 | [110] | |
| Grapes product | Wine | C2H3N/H2O (40:60, v/v) | HPLC-UV | 0.1047 mg/L, DW | [97] |
| MeOH | UHPLC-Orbitrap MS4 | 4.00 mg/L, DW (red wine) | [111] | ||
| Transepithelial diffusion | LC-MS | 0–1.089 mg/L, FW (white wine); 0.29 mg/L, FW (rosé wine); 0.361–1.972 mg/L, FW (red wine). | [112] | ||
| MeOH | UHPLC- MS/MS | 0.07–2.61 mg/L, DW (cR) 0.05–3.82 mg/L, DW (tR) | [113] | ||
| Juice | C2H3N/H2O (40:60, v/v) | HPLC-UV | 0.000091 mg/L, DW | [102] | |
| C2H6O/water solution (60:40, v/v) | HPLC | 4.4 to 7.0 mg/L, DW | [114] | ||
| Concentrated juice | C2H6O/water solution (60:40, v/v) | HPLC | 12.4 to 21.3 mg/L, DW | [114] | |
| Grape skin powder | C2H6O/H2O (50%, v/v) | GSP/UV-A/HPLC | 250 mg/L, DW | [115] | |
| Raisin | HCl/MeOH/H2O, 1:80:19, v/v/v) | UPLC-VION-IMS-QToF | 16,544,000 ± 44,000 mg/L, DW | [116] | |
| Jam | UP200S ultrasonic system optimized with solvent composition (10–70% and 30–90% MeOH in H2O; solvent-to-solid ratio (10:1–40:1); ultrasonic probe diameter | UPLC-FD | 0.027 ± 0.01 to 1.760 ± 0.04 mg/L, DW | [117] | |
| Marmalade | BBD optimized with solvent composition (60–100% and 10–70% MeOH in H2O); MAE power (250–750 W); solvent-to-solid ratio (20:5–60:5) | UHPLC-FD | 1.74 mg/L−1, DW | [118] | |
| By-products | Grape canes | C2H6O/H2O (80:20, v/v) | HPLC-DAD-Q-ToF | 227.07 mg/L−1, DW | [119] |
| The microencapsulation (by spray drying) using maltodextrin (MD) (10% w/v) and UV irradiation (254 nm) | HPLC | 679.6 ± 51.6 mg/L, DW | [120] | ||
| Sonicate/macerate 96% C2H6O (v/v) | HPLC-MS | 815.9 ± 153 mg/L, DW | [121] | ||
| NADES evaluation combined with HPCC biphasic solvent by COSMO-RS calculations | UHPLC-UV | 1.50 mg/L, DW | [122] | ||
| HPLC-UV-DAD | HPLC-ESI/MS | 890 ± 20 mg/L−1, DW (dormant bud) 610 ± 10 mg/L−1, DW (second extended leaf) 200 ± 70 mg/L−1, DW (sixth extended leaf and visible inflorescence) | [123] | ||
| Grape pomace | C2H6O (5%, v/v) | HPLC | 190 to 1073 mg/L, DW | [33] | |
| Extracted by SOX and MAC in IPA | HPLC-DAD/MS | 0.042–0.653 mg/L, DW (tR) 0.05–0.35 mg/L, DW (cRl) | [124] | ||
| UAE for one hour (80% MeOH solution (100 mL) acidified with 0.1% CH2O2) | HPLC/DAD/TOF | 100 ± 20 mg/L, DW | [125] | ||
| Wine lees | Conventional aqueous (CE) and non-conventional UAE | HPLC | 36,360 mg/L, DW | [126] | |
| Enzyme-assisted extraction based on the hydrolysis of WL proteins | UHPLC-(ESI+)-Q-ToF-MS | 164.00 ± 0.80 mg/L, DW | [127] | ||
| Grapevine extracts | MeOH/H2O (50:50, v/v) | HPLC-DAD(UV)/CAD | 36.75 mg/L−1, DW (CAD) 211.25 mg/L−1, DW (DAD/UV) | [128] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căpruciu, R.; Gheorghiu, C.N. Methods for Synthesis and Extraction of Resveratrol from Grapevine: Challenges and Advances in Compound Identification and Analysis. Foods 2025, 14, 1091. https://doi.org/10.3390/foods14071091
Căpruciu R, Gheorghiu CN. Methods for Synthesis and Extraction of Resveratrol from Grapevine: Challenges and Advances in Compound Identification and Analysis. Foods. 2025; 14(7):1091. https://doi.org/10.3390/foods14071091
Chicago/Turabian StyleCăpruciu, Ramona, and Constantin Nicolae Gheorghiu. 2025. "Methods for Synthesis and Extraction of Resveratrol from Grapevine: Challenges and Advances in Compound Identification and Analysis" Foods 14, no. 7: 1091. https://doi.org/10.3390/foods14071091
APA StyleCăpruciu, R., & Gheorghiu, C. N. (2025). Methods for Synthesis and Extraction of Resveratrol from Grapevine: Challenges and Advances in Compound Identification and Analysis. Foods, 14(7), 1091. https://doi.org/10.3390/foods14071091




