Survey and Diversity of Grapevine Pinot gris virus in Algeria and Comprehensive High-Throughput Small RNA Sequencing Analysis of Two Isolates from Vitis vinifera cv. Sabel Revealing High Viral Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Collection of Viral Isolates
2.2. RNA Extraction and RT-PCR Detection of GPGV and Sequencing of PCR Amplicons
2.3. Phylogenic Analysis of Partial RdRp Domain
2.4. Small RNA Sequencing
2.5. Sequence Data Analysis
2.6. Determination of Presence of Grapevine Viruses
3. Results
3.1. Results of GPGV RT-PCR Detection
3.2. Phylogenetic Analysis
3.3. Detection of the Viruses and Viroids by Small RNA Sequencing
3.4. Description of Detected Viruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giampetruzzi, A.; Roumi, V.; Roberto, R.; Malossini, U.; Yoshikawa, N.; La Notte, P.; Terlizzi, F.; Credi, R.; Saldarelli, P. A new grapevine virus discovered by deep sequencing of virus- and viroid-derived small RNAs in Cv Pinot gris. Virus Res. 2012, 163, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Saldarelli, P.; Giampetruzzi, A.; Morelli, M.; Malossini, U.; Pirolo, C.; Bianchedi, P.; Gualandri, V. Genetic variability of Grapevine Pinot gris virus and its association with grapevine leaf mottling and deformation. Phytopathology 2015, 105, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichmeier, A.; Penazova, E.; Muljukina, N. Survey of Grapevine Pinot gris virus in certified grapevine stocks in Ukraine. Eur. J. Plant. Pathol. 2018, 152, 555–560. [Google Scholar] [CrossRef]
- Poojari, S.; Lowery, T.; Rott, M.; Schmidt, A.M.; Urbez-Torres, J.R. First report of Grapevine Pinot gris virus in British Columbia, Canada. Plant. Dis. 2016, 100, 1513. [Google Scholar] [CrossRef]
- Eichmeier, A.; Penazova, E.; Nebish, A. First Report of Grapevine Pinot gris virus on grapevines in Armenia. Plant. Dis. 2020, 104, 1000. [Google Scholar] [CrossRef]
- Wu, Q.; Habili, N. The recent importation of Grapevine Pinot gris virus into Australia. Virus Genes 2017, 53, 935–938. [Google Scholar] [CrossRef]
- Fajardo, T.V.M.; Eiras, M.; Nickel, O. First report of Grapevine Pinot gris virus infecting grapevine in Brazil. Australas. Plant Dis. Notes 2017, 12, 45. [Google Scholar] [CrossRef]
- Zamorano, A.; Medina, G.; Fernandez, C.; Cui, W.; Quiroga, N.; Fiore, N. First report of Grapevine Pinot gris virus in grapevine in Chile. Plant. Dis. 2019, 103, 1438–1439. [Google Scholar] [CrossRef]
- Rasool, S.; Naz, S.; Rowhani, A.; Golino, D.A.; Westrick, N.M.; Farrar, K.D.; Al Rwahnih, M. First Report of Grapevine Pinot gris virus infecting grapevine in Pakistan. Plant Dis. 2017, 101, 1958. [Google Scholar] [CrossRef]
- Eichmeier, A.; Penazova, E.; Pavelkova, R.; Mynarzova, Z.; Saldarelli, P. Detection of Grapevine Pinot gris girus in certified grapevine stocks in Moravia, Czech Republic. J. Plant Pathol. 2016, 98, 155–157. [Google Scholar]
- Bertazzon, N.; Filippin, L.; Forte, V.; Angelini, E. Grapevine Pinot gris virus seems to have recently been introduced to vineyards in Veneto, Italy. Arch. Virol. 2016, 161, 711–714. [Google Scholar] [CrossRef]
- Bertazzon, N.; Forte, V.; Filippin, L.; Causin, R.; Maixner, M.; Angelini, E. Association between genetic variability and titre of Grapevine Pinot gris virus with disease symptoms. Plant Pathol. 2017, 66, 949–959. [Google Scholar] [CrossRef]
- Malagnini, V.; de Lillo, E.; Saldarelli, P.; Beber, R.; Duso, C.; Raiola, A.; Zanotelli, L.; Valenzano, D.; Giampetruzzi, A.; Morelli, M.; et al. Transmission of grapevine Pinot gris virus by Colomerus vitis (Acari: Eriophyidae) to grapevine. Arch. Virol. 2016, 161, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- MADRP. Agricultural Statistics‒B Series, Ministère de l’Agriculture, du développement Rural et de la Pêche. Available online: http://madrp.gov.dz/agriculture/statistiques-agricoles/ (accessed on 21 September 2020).
- OIV. Statistical Report on World Vitiviniculture; International Organization Vine Wine: Paris, France, 2019. [Google Scholar]
- Hily, J.M.; Poulicard, N.; Candresse, T.; Vigne, E.; Beuve, M.; Renault, L.; Velt, A.; Spilmont, A.S.; Lemaire, O. Datamining, Genetic diversity analyses, and phylogeographic reconstructions redefine the worldwide evolutionary history of Grapevine Pinot gris virus and Grapevine berry inner necrosis virus. Phytobiomes J. 2020, 4, 165–177. [Google Scholar] [CrossRef] [Green Version]
- German, S.; Candresse, T.; Le Gall, O.; Lanneau, M.; Dunez, J. Analysis of the dsRNAs of Apple chlorotic leaf-spot virus. J. Gen. Virol. 1992, 73, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Eichmeier, A.; Kominkova, M.; Kominek, P.; Baranek, M. Comprehensive virus detection using next generation sequencing in grapevine vascular tissues of plants obtained from the wine regions of Bohemia and Moravia (Czech Republic). PLoS ONE 2016, 11, e0167966. [Google Scholar] [CrossRef] [PubMed]
- Glasa, M.; Predajna, L.; Kominek, P.; Nagyova, A.; Candresse, T.; Olmos, A. Molecular characterization of divergent grapevine Pinot gris virus isolates and their detection in Slovak and Czech grapevines. Arch. Virol. 2014, 159, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Lehad, A.; Selmi, I.; Louanchi, M.; Aitouada, M.; Mahfoudhi, N. Occurrence and diversity of Grapevine leafroll-associated virus 1 in Algeria. Phytopathol. Mediterr. 2019, 58, 277–281. [Google Scholar]
- Lehad, A.; Selmi, I.; Louanchi, M.; Aitouada, M.; Mahfoudhi, N. Survey and genetic diversity of grapevine leafroll associated virus 2 in Algeria. Int. J. Phytopathol. 2015, 4, 8. [Google Scholar] [CrossRef]
- Lehad, A.; Selmi, I.; Louanchi, M.; Aitouada, M.; Mahfoudhi, N. Genetic Diversity of Grapevine leafroll-associated virus 3 in Algeria. J. Plant. Pathol. 2015, 97, 203–207. [Google Scholar]
- Eichmeier, A.; Baranek, M.; Pidra, M. Analysis of genetic diversity and phylogeny of partial coat protein domain in Czech and Italian GFLV isolates. Plant. Prot. Sci. 2010, 46, 4. [Google Scholar] [CrossRef] [Green Version]
- Eichmeier, A.; Pieczonka, K.; Penazova, E.; Pecenka, J.; Gajewski, Z. Occurrence of Grapevine Pinot gris virus in Poland and description of asymptomatic exhibitions in grapevines. J. Plant. Dis. Prot. 2017, 124, 407–411. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kiss, T.; Penazova, E.; Pecenka, J.; Berraf-Tebbal, A.; Baranek, M.; Pokluda, R.; Cechova, J.; Gramaje, D.; Grzebelus, D. MicroRNAs in Vitis vinifera cv. Chardonnay are differentially expressed in response to Diaporthe species. Genes 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 21 September 2020).
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Eichmeier, A.; Kominkova, M.; Pecenka, J.; Kominek, P. High-throughput small RNA sequencing for evaluation of grapevine sanitation efficacy. J. Virol. Methods 2019, 267, 66–70. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial-DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Kominek, P.; Bryxiova, A. Comparison of three techniques for detection of grapevine leafroll-associated virus 1. Acta Virol. 2005, 49, 37–43. [Google Scholar]
- Gambino, G.; Gribaudo, I. Simultaneous detection of nine grapevine viruses by multiplex reverse transcription-polymerase chain reaction with coamplification of a plant RNA as internal control. Phytopathology 2006, 96, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Sabanadzovic, S.; Saldarelli, P.; Savino, V. Molecular diagnosis of grapevine fleck virus. Vitis 1996, 35, 137–140. [Google Scholar]
- Terlizzi, F.; Li, C.; Ratti, C.; Qiu, W.; Credi, R.; Meng, B. Detection of multiple sequence variants of Grapevine rupestris stem pitting-associated virus using primers targeting the polymerase domain and partial genome sequencing of a novel variant. Ann. Appl. Biol. 2011, 159, 478–490. [Google Scholar] [CrossRef]
- Ward, L.I.; Burnip, G.M.; Liefting, L.W.; Harper, S.J.; Clover, G.R.G. First report of Grapevine yellow speckle viroid 1 and Hop stunt viroid in Grapevine (Vitis vinifera) in New Zealand. Plant. Dis. 2011, 95, 617. [Google Scholar] [CrossRef]
- Tomitaka, Y.; Usugi, T.; Fukami, M.; Tsuda, S. First report of Grapevine Algerian latent virus infection on Alstroemeria spp. in Japan. Plant. Dis. 2016, 100, 1251. [Google Scholar] [CrossRef]
- Minafra, A.; Hadidi, A. Sensitive detection of Grapevine Virus-A, Virus-B, or Leafroll-Associated-III from viruliferous mealybugs and infected tissue by cDNA amplification. J. Virol. Methods 1994, 47, 175–187. [Google Scholar] [CrossRef]
- Lunden, S.; Qiu, W. First Report of Grapevine leafroll-associated virus 2 in a hybrid grape ’Vidal Blanc’ in Missouri. Plant. Dis. 2012, 96, 462. [Google Scholar] [CrossRef] [PubMed]
- Osman, F.; Rowhani, A. Application of a spotting sample preparation technique for the detection of pathogens in woody plants by RT-PCR and real-time PCR (TaqMan). J. Virol. Methods 2006, 133, 130–136. [Google Scholar] [CrossRef]
- Buoso, S.; Pagliari, L.; Musetti, R.; Fornasier, F.; Martini, M.; Loschi, A.; Fontanella, M.C.; Ermacora, P. With or without you: Altered plant response to boron-deficiency in hydroponically grown grapevines infected by Grapevine Pinot gris virus suggests a relation between grapevine leaf mottling and deformation symptom occurrence and boron plant availability. Front. Plant. Sci. 2020, 11, 226. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, G.L.; De Amicis, F.; De Sabbata, L.; Di Bernardo, N.; Governatori, G.; Notino, F. Occurrence of Grapevine Pinot gris virus in Friuli Venezia Giulia (Italy): Field monitoring and virus quantification by real-time RT-PCR. EPPO Bull. 2015, 45, 11. [Google Scholar] [CrossRef]
- Tarquini, G.; Ermacora, P.; Bianchi, G.L.; De Amicis, F.; Pagliari, L.; Martini, M.; Loschi, A.; Saldarelli, P.; Loi, N.; Musetti, R. Localization and subcellular association of Grapevine Pinot gris virus in grapevine leaf tissues. Protoplasma 2018, 255, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Al Rwahnih, M.; Golino, D.; Rowhani, A. First report of Grapevine Pinot gris virus infecting grapevine in the United States. Plant. Dis. 2016, 100, 1030. [Google Scholar] [CrossRef]
- Tarquini, G.; De Amicis, F.; Martini, M.; Ermacora, P.; Loi, N.; Musetti, R.; Bianchi, G.L.; Firrao, G. Analysis of new grapevine Pinot gris virus (GPGV) isolates from Northeast Italy provides clues to track the evolution of a newly emerging clade. Arch. Virol. 2019, 164, 1655–1660. [Google Scholar] [CrossRef] [PubMed]


| Sampling Regions | Altitude (m) | GPS Coordinates | Number of Plants | Cultivar |
|---|---|---|---|---|
| Medea (Benchicao) | 1006 | 36°12′4″ N 2°49′58″ E | 12 | Hmer Bouameur |
| Tipaza (Ahmer El Aïn) | 85 | 36°23′60″ N 2°51′35″ E | 12 | Carignan |
| Tipaza (Hadjout) | 72 | 36°31′36″ N 2°24′7″ E | 6 | Alphonse-Lavallée |
| Boumerdes (Bordj Menaiel) | 52 | 36°45′14″ N 3°40′28″ E | 12 | Sabel |
| Alger (Ain Benian) | 0 | 36°47′40″ N 2°55′53″ E | 12 | Cardinal, Alphonse-Lavallée |
| Sample No. | Region | Cultivar | RdRp Amplification | Partial RdRp, GenBank Acc. No. | Full-Length RdRp, GenBank Acc. No. |
|---|---|---|---|---|---|
| AL_1 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_2 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_3 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_4 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_5 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_6 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_7 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_8 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_9 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_10 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_11 | Médéa (Benchicao) | Hmer Bouameur | + | MT832147 | |
| AL_12 | Médéa (Benchicao) | Hmer Bouameur | − | ||
| AL_13 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_14 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_15 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_16 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_17 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_18 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_19 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_20 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_21 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_22 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_23 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_24 | Tipaza (Hamr El Ain) | Carignan | − | ||
| AL_25 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_26 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_27 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_28 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_29 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_30 | Tipaza (Hadjout) | Alphonse-Lavallée | − | ||
| AL_31 | Boumerdes | Sabel | + | MT832148 | |
| AL_32 | Boumerdes | Sabel | + | MT832149 | |
| AL_33 | Boumerdes | Sabel | + | MT832150 | |
| AL_34 | Boumerdes | Sabel | − | ||
| AL_35 | Boumerdes | Sabel | − | ||
| AL_36 | Boumerdes | Sabel | + | MT832151 | |
| AL_37 | Boumerdes | Sabel | − | ||
| AL_38 | Boumerdes | Sabel | − | ||
| AL_39 | Boumerdes | Sabel | − | ||
| AL_40 | Boumerdes | Sabel | − | ||
| AL_41 | Boumerdes | Sabel | + | MT832152 | MT843110 |
| AL_42 | Boumerdes | Sabel | + | MT832153 | MT843111 |
| AL_43 | Alger | Cardinal | − | ||
| AL_44 | Alger | Cardinal | − | ||
| AL_45 | Alger | Cardinal | − | ||
| AL_46 | Alger | Cardinal | − | ||
| AL_47 | Alger | Cardinal | − | ||
| AL_48 | Alger | Cardinal | − | ||
| AL_49 | Alger | Alphonse-Lavallée | − | ||
| AL_50 | Alger | Alphonse-Lavallée | − | ||
| AL_51 | Alger | Alphonse-Lavallée | − | ||
| AL_52 | Alger | Alphonse-Lavallée | − | ||
| AL_53 | Alger | Alphonse-Lavallée | + | MT832154 | |
| AL_54 | Alger | Alphonse-Lavallée | − |
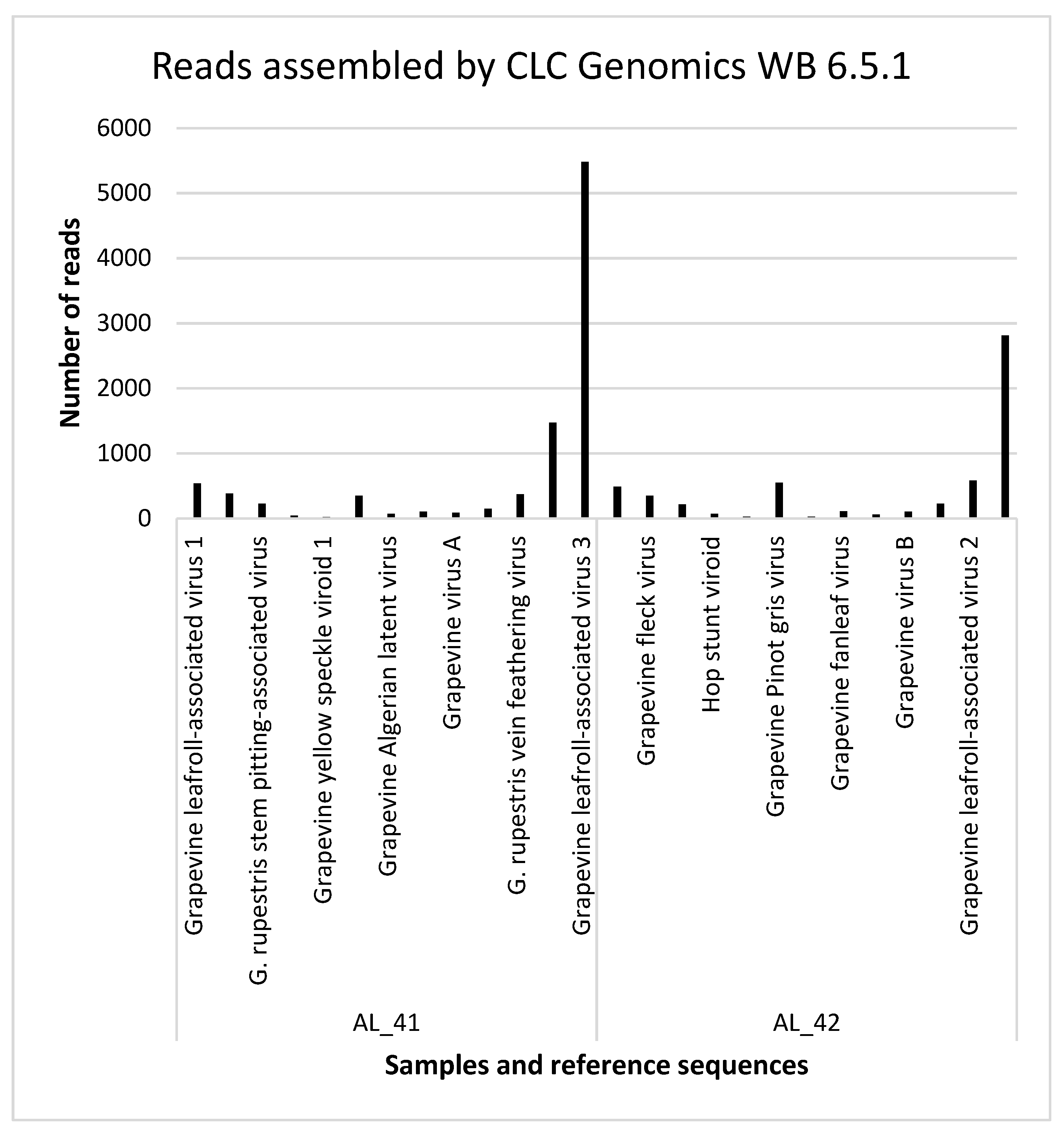
| Grapevine | Reference Accession Nr. | Virus | Contigs (Velvet, k-15) Identified by Blast (1E-5) | Reads Assembled by CLC Genomics WB 6.5.1 | Average Seq Depth | Genome Coverage | RT-PCR Result the Protocol Reference |
|---|---|---|---|---|---|---|---|
| AL_41 | NC_016509.1 | Grapevine leafroll-associated virus 1 | 0 | 538 | 0 | 13% | − (Kominek et al., 2005) [32], − (Gambino a Gribaudo 2006) [33] |
| NC_003347.1 | Grapevine fleck virus | 59 | 382 | 0.22 | 51% | + (Sabanadzovic et al., 1996) [34] | |
| NC_001948.1 | G. rupestris stem pitting-associated virus | 1 | 227 | 0.03 | 17% | + (Terlizzi et al., 2011) [35] | |
| NC_001351.1 | Hop stunt viroid | 0 | 40 | 0.15 | 26% | + (Eichmeier et al., 2016) [18] | |
| NC_001920.1 | Grapevine yellow speckle viroid 1 | 0 | 17 | 0.41 | 32% | + (Ward et al., 2011) [36] | |
| NC_015782.1 | Grapevine Pinot gris virus | 0 | 349 | 5.77E-3 | 74% | + (Saldarelli et al., 2015) [2] | |
| NC_011535.1 | Grapevine Algerian latent virus | 0 | 68 | 0 | 12% | − (Tomitaka et al., 2016) [37] | |
| NC_003623.1 | Grapevine fanleaf virus | 0 | 104 | 0 | 18% | − (Eichmeier et al., 2010) [23] | |
| NC_003604.1 | Grapevine virus A | 0 | 85 | 0 | 11% | − (Minafra and Hadidi 2004) [38] | |
| GU733707.1 | Grapevine virus B | 0 | 149 | 0 | 3% | + (Minafra and Hadidi 2004) [38] | |
| AY706994.1 | G. rupestris vein feathering virus | 0 | 371 | 0.03 | 13% | + (Eichmeier et al., 2016) [18] | |
| NC_007448.1 | Grapevine leafroll-associated virus 2 | 1768 | 1473 | 0.53 | 59% | + (Lunden and Qiu 2012) [39] | |
| NC_004667.1 | Grapevine leafroll-associated virus 3 | 2032 | 5479 | 1.29 | 79% | + (Osman et Rowhani, 2006) [40] | |
| AL_42 | NC_016509.1 | Grapevine leafroll-associated virus 1 | 0 | 489 | 0 | 9% | − (Kominek et al., 2005) [32], − (Gambino a Gribaudo 2006) [33] |
| NC_003347.1 | Grapevine fleck virus | 66 | 348 | 0.31 | 53% | + (Sabanadzovic et al., 1996) [34] | |
| NC_001948.1 | G. rupestris stem pitting-associated virus | 92 | 216 | 0.06 | 19% | + (Terlizzi et al., 2011) [35] | |
| NC_001351.1 | Hop stunt viroid | 0 | 67 | 0.3 | 54% | + (Eichmeier et al., 2016) [18] | |
| NC_001920.1 | Grapevine yellow speckle viroid 1 | 0 | 24 | 1.9 | 64% | + (Ward et al., 2011) [36] | |
| NC_015782.1 | Grapevine Pinot gris virus | 0 | 549 | 2.89E-3 | 89% | + (Saldarelli et al., 2015) [2] | |
| NC_011535.1 | Grapevine Algerian latent virus | 0 | 27 | 0 | 6% | − (Tomitaka et al., 2016) [37] | |
| NC_003623.1 | Grapevine fanleaf virus | 0 | 107 | 0 | 14% | − (Eichmeier et al., 2010) [23] | |
| NC_003604.1 | Grapevine virus A | 0 | 58 | 0 | 8% | − (Minafra and Hadidi 2004) [38] | |
| GU733707.1 | Grapevine virus B | 0 | 105 | 0 | 1% | − (Minafra and Hadidi 2004) [38] | |
| AY706994.1 | G. rupestris vein feathering virus | 0 | 228 | 0.07 | 8% | − (Eichmeier et al., 2016) [18] | |
| NC_007448.1 | Grapevine leafroll-associated virus 2 | 25 | 584 | 0.08 | 29% | + (Lunden and Qiu 2012) [39] | |
| NC_004667.1 | Grapevine leafroll-associated virus 3 | 327 | 2809 | 0.23 | 43% | + (Osman et Rowhani, 2006) [40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichmeier, A.; Peňázová, E.; Čechová, J.; Berraf-Tebbal, A. Survey and Diversity of Grapevine Pinot gris virus in Algeria and Comprehensive High-Throughput Small RNA Sequencing Analysis of Two Isolates from Vitis vinifera cv. Sabel Revealing High Viral Diversity. Genes 2020, 11, 1110. https://doi.org/10.3390/genes11091110
Eichmeier A, Peňázová E, Čechová J, Berraf-Tebbal A. Survey and Diversity of Grapevine Pinot gris virus in Algeria and Comprehensive High-Throughput Small RNA Sequencing Analysis of Two Isolates from Vitis vinifera cv. Sabel Revealing High Viral Diversity. Genes. 2020; 11(9):1110. https://doi.org/10.3390/genes11091110
Chicago/Turabian StyleEichmeier, Aleš, Eliška Peňázová, Jana Čechová, and Akila Berraf-Tebbal. 2020. "Survey and Diversity of Grapevine Pinot gris virus in Algeria and Comprehensive High-Throughput Small RNA Sequencing Analysis of Two Isolates from Vitis vinifera cv. Sabel Revealing High Viral Diversity" Genes 11, no. 9: 1110. https://doi.org/10.3390/genes11091110
APA StyleEichmeier, A., Peňázová, E., Čechová, J., & Berraf-Tebbal, A. (2020). Survey and Diversity of Grapevine Pinot gris virus in Algeria and Comprehensive High-Throughput Small RNA Sequencing Analysis of Two Isolates from Vitis vinifera cv. Sabel Revealing High Viral Diversity. Genes, 11(9), 1110. https://doi.org/10.3390/genes11091110






