IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review
Abstract
:1. Introduction
- (1)
- To inventory and classify various ML methods to process IMU data and locate the widely used and state-of-the-art methods regarding different application scenarios and tasks.
- (2)
- To inventory the target disorders that can benefit from IMU-ML systems based on movement-related medical conditions that regard specialized areas of rehabilitation.
- (3)
- To gauge the implementation details to build IMU-ML systems for assistive diagnosis and management, such as feature selection strategy, sensor attachment selection, and evaluation methods.
2. Methods and Taxonomy of Existing Approaches
Taxonomy Structure
- (1)
- Neurological disorders, which are the most common disorders that require rehabilitation, arise from people with diseases, injuries, or dysfunctions of the nervous system. Along this line, five kinds of conditions can benefit from neurological rehab: (a) degenerative disorders, such as Parkinson’s disease, multiple sclerosis, and Huntington’s disease, among which, Parkinson’s disease is the most common disorder in all the selected articles; (b) vascular disorder, which is mainly a stroke; (c) neurodevelopmental disorders, which are mainly cerebral palsy; (d) trauma, such as traumatic brain injury, spinal cord injury, and brachial plexus injury; and (e) functional disorders, such as seizure and vestibular system disorders. Additionally, there are other disorders and symptoms presented, and we categorized them as other neurological disorders.
- (2)
- Musculoskeletal disorders, including impairments or disabilities due to disease, disorders, or injuries to the muscles, tendons, ligaments, or bones. Three representative conditions can benefit from musculoskeletal rehab: (a) arthritis, which is mainly osteoarthritis, (b) back pain, and (c) Total Joint Replacement (TJR), such as total hip arthroplasty and total knee replacement.
- (3)
- Mental health disorders affect a person’s behaviors, feelings, and overall wellbeing, affecting many aspects of their daily lives. This mainly includes depression; however, illnesses such as bipolar disorder and schizophrenia are also represented in the studies included here.
- (4)
- Others consist of cardiac disorders, pulmonary disorders, and general rehabilitation, focusing on body parts such as the joints, upper limbs, and lower limbs.
3. IMUs for Monitoring Body Motion
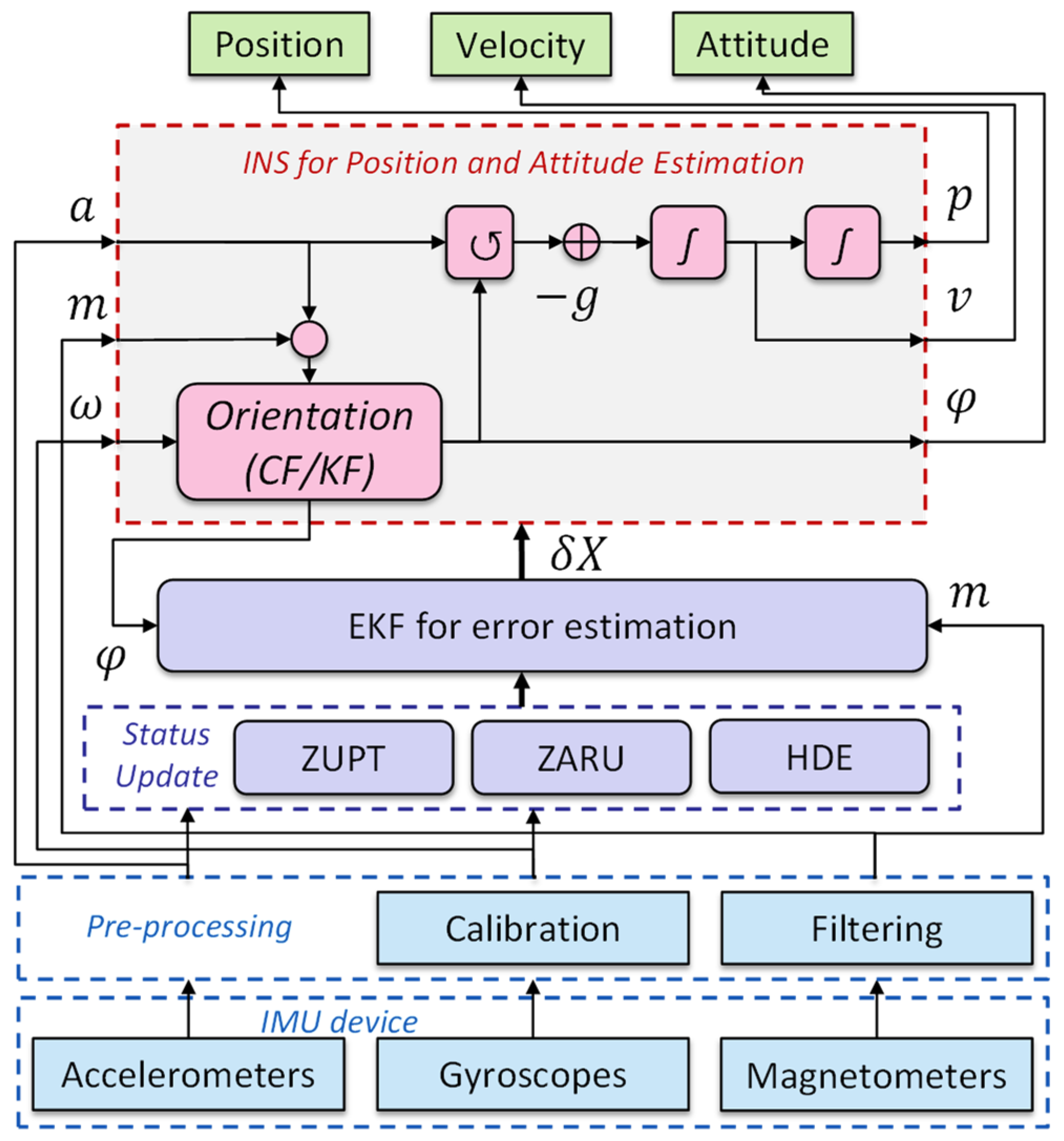
3.1. Numerical Methods
3.2. ML-Based Methods
3.2.1. Traditional ML Methods
3.2.2. Deep Learning Methods
3.2.3. Unsupervised Learning Methods
4. Results for Different Application Scenarios
4.1. Neurological Disorders
4.1.1. Parkinson’s Disease
4.1.2. Stroke
4.1.3. Cerebral Palsy
4.1.4. Cerebellar Ataxia
4.1.5. Others
| Disorders | Application | Sensor (n) | Placement | Model | Input Data/Features | Major Performance | Subjects/ Dataset | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| OA | SD | IMU (2) | 32, 33 | ANN | 100 samples | multiple | 14 h | 2020 | [111] |
| OA | SD | IMU (1) | 31 | LR | 63 features | MAE = 29% (left), 36% (right) | 10 p | 2020 | [25] |
| OA | CE | Accel (1) | 31 | RF | 26 features | Acc = 76.3% | 1198 p [112] | 2021 | [53] |
| OA | CE | Accel (3) | 2, 13, 35 | SVM | temporal features | Acc = 97.9% (initial), 90.6% (layer-1 SVM), 92.7% (layer-2 SVM) | 10 h | 2016 | [113] |
| OA | SD | Accel (4) | 13, 16, 29, 35 | LDA + PCA | 38 features | Acc = 81.7% | 39 p | 2017 | [65] |
| OA | PA | IMU (4) | 23, 24 | CNN | 200, 100, 40 ms window | Acc = 85%, 89–97%, 60–67% for 3 tasks | 18 p | 2021 | [114] |
| LBP | D | IMU (1) | 2 | SVM/MLP | 16 features | Acc = 75% | 94 p | 2020 | [115] |
| LBP | D | IMU (2) | 2, 11 | SVM | 52 features | Acc = 96% | 28 p, 24 h | 2017 | [48] |
| TJR | D | IMU (7) | 11, 23, 26, 34 | SVM | 2 feature sets | Acc = 87.2% (Set 1), 97.0% (Set 2) | 20 p, 24 h | 2019 | [46] |
| TJR | PA | IMU (4) | 13, 14, 16, 29 | DCNN | 100 samples | Acc = 98% | 12 p | 2021 | [116] |
| TJR | SA | Accel (2) IMU (1) | 6, 11 | k-means | Different for each PROM | TSS = 3.86, 3.56, 1.86 for each feature set | 22 p | 2019 | [117] |
| TJR | PA | IMU (1) | 14 | SOM | 356 features | Acc = 85.6–96.92% | 44 p, 10 h | 2018 | [118] |
4.2. Musculoskeletal Disorders
4.2.1. Osteoarthritis
4.2.2. Low Back Pain
4.2.3. Total Joint Replacement
| Disorders | Application | Sensor (n) | Placement | Model | Input Data/ Features | Major Performance | Subjects/ Dataset | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Depression | D | Accel (1) | 6 | RF | 14 features | Acc = 89.2% | 2112 p, 3783 h | 2019 | [119] |
| Depression | D | Accel (1), Light | 6 | Logistic Regression | 4 features | Acc = 91% | 18 p, 29 h | 2019 | [120] |
| Depression | D, SA | Accel (1), Health | 6 | XGBoost | 63 features | Acc = 76%, correlation coefficient = 0.61 | 45 p, 41 h | 2020 | [121] |
| Depression | D | Accel (1) | 6 | RF | 3 features | MCC= 0.44 | 23 p, 32 h | 2018 | [122] |
| Depression | SA | Accel (1) | 6 | RF, Adaboost, Theil-Sen | 3 sets of features | RMSE = 4.5 | 12 p | 2017 | [123] |
| Bipolar, ADHD | D | Accel (1) | 11 | SVM | 28 features | Acc = 83.1% | 92 p, 63 h | 2016 | [124] |
| Internalizing Disorders | D | IMU (1) | 11 | Logistic Regression | 39 features | Acc = 81% | 21 p, 41 h | 2019 | [125] |
4.3. Mental Illness
4.3.1. Depression
4.3.2. Other Mental Illness
| Disorders | Application | Sensor (n) | Placement | Model | Input Data/ Features | Major Performance | Subjects/ Dataset | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| COPD | SA | Acc (1) | - | SVM_rbf | 8 features | Acc = 99.2% | 55 p, 11 h | 2016 | [129] |
| COPD | CE | IMU (3) | 2, 11, 30 | PCA | Quaternion data | MAE < 2, R > 0.963 | 8 h | 2019 | [66] |
| Geriatrics | D | IMU (1) | 31 | CNN+LSTM | 500 samples | Acc = 95% | 20 p | 2021 | [130] |
| General | CE | IMU (2) | 13, 35 | Polynomial Regression | Orientation | RMSE = 4.81 (general), | 14 h | 2019 | [131] |
| General | PA | IMU (4) | 4, 5, 6, 7 | RF | 2 feature sets | 4.99 (personal) | 50 h | 2020 | [132] |
| General | PA | IMU (1) | 5 | RF/SVM | 237 features, ReliefF | Acc = 98.6% | 44 p, 10 h | 2019 | [133] |
| General | PA | IMU (3) | 3, 5, 6 | Conv+FSM | Raw | Acc = 97.2% (CV), 80.5% (LOSO) | 35 h | 2020 | [134] |
| General | PA | IMU (2) | 5, 6 | SVM | 144 features, PCA | Acc = 0.871 | 9 p, 9 n | 2021 | [135] |
4.4. Others
5. Discussion and Future Directions
5.1. Inertial Sensors and IoT Devices
5.1.1. Multi-Sensor Fusion
5.1.2. Self-Calibration
5.1.3. Consumer Grade IMU Devices
5.2. Data Processing Methods
- Disease Diagnosis, which means classifying patients from healthy controls;
- Symptom Detection, which means detecting a typical symptom of patients that indicates a detailed disease type and stages, such as the freezing of gait for PD patients;
- Characteristics Estimations, which means estimating disease-related characteristics such as stride length and joint loading value;
- Severity Assessment, which means regressing or classifying different severities of patients into certain estimating scales;
- Physical Activity Recognition for Patients.
5.2.1. Online and Edge Implementation
5.2.2. Open Dataset and Universal Model
5.2.3. Interpretable Model
5.2.4. Healthcare Representation and Digital Twin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 3D | 3-dimensional | FSR | Force-Sensitive Resistor | OA | Osteoarthritis |
| Acc | Accuracy | GPR | Gaussian Progress Regression | OFS | Optical Fiber Sensors |
| Accel | Accelerometer | Gyro | Gyroscope | p | patients |
| ADD | Attention Deficit Disorder | h | healthy controls | PA | Physical Activity |
| ADHD | Attention-Deficit/Hyperactivity Disorder | H&Y | Hoehn and Yahr | PCA | Principal Component Analysis |
| ADL | Activities of Daily Living | HD | Huntington’s Disease | PD | Parkinson’s Disease |
| ANN | Artificial Neural Networks | HDE | Heuristic Drift Elimination | PPV | Positive Predictive Values |
| AUC | Area Under Curve | HDRS | Hamilton Depression Rating Scale | PR | Polynomial Regression |
| BARS | Brief Ataxia Rating Scale | HMM | hidden Markov models | PSP | Progressive Supranuclear Palsy |
| BI | Brain Injury | IMU | Inertial Measurement Unit | R | Pearson Correlation Coefficient |
| BPI | Brachial Plexus Injury | IoHT | Internet of Health Things | RBF | Radial Basis Function |
| CA | Cerebellar Ataxia | IoT | Internet of Things | RF | Random Forest |
| CE | Characteristics Estimation | KAM | Knee Adduction Moments | RFID | Radio Frequency Identifications |
| CF | Complementary filter | KF | Kalman filter | RMSE | Root Mean Square Error |
| CFS | Correlation Feature Selection | KFM | Knee Flexion Moments | RNN | Recurrent Neural Network |
| CNN | Convolutional Neural Network | k-NN | k-Nearest Neighbor | RoM | Range of Motion |
| CoG | Center of Gravity | KOA | Knee Osteoarthritis | SA | Severity Assessment |
| COPD | Chronic Obstructive Pulmonary Disease | LBP | Low back pain | SARA | Scale for Assessment and Rating of Ataxia |
| CP | Cerebral Palsy | LDA | Linear Discriminant Analysis | SCI | Spinal Cord Injury |
| CV | Coefficient of Variation | LE | Lower Extremities | SD | Symptom Detection |
| D | Diagnosis | LIME | Local Interpretable Model-agnostic Explanations | Sen | Sensitivity |
| DNN | Deep Neural Network | LOSO | Leave-one-subject out | SOM | Self-Organizing Maps |
| DT | Decision Trees | LR | Linear Regression | Spec | Specificity |
| DTW | Dynamic Time Wrapping | LSTM | Long Short-Term Memory | SVM | Support Vector Machines |
| EHR | Electronic Health Records | MAE | Mean Absolute Error | SVR | Support Vector Regression |
| EM | Exaptation Maximization | MARG | Magnetic, Angular Rate, and Gravity | TJR | Total Joint Replacement |
| EMG | Electromyography | MCC | Mathew’s Correlation Coefficient | UE | Upper Extremity |
| EMI | Electromagnetic Interference | ML | Machine Learning | UPDRS | Unified Parkinson’s Disease Rating Scale |
| EMTS | Electromagnetic Tracking System | MMG | Mechanomyography | VS | Vestibular System |
| FMA | Fugl-Meyer Assessment | MS | Multiple Sclerosis | WMFT | Wolf Motor Function Test |
| FoG | Freezing of Gait | MSE | Mean Square Error | ZARU | Zero Angular Rate Update |
| FSM | Finite State Machine | NIHSS | National Institutes of Health Stroke Scale | ZUPT | Zero-velocity Update |
References
- Fan, Y.J.; Yin, Y.H.; Da Xu, L.; Zeng, Y.; Wu, F. IoT-Based Smart Rehabilitation System. IEEE Trans. Ind. Inform. 2014, 10, 1568–1577. [Google Scholar]
- Camara Gradim, L.C.; Archanjo Jose, M.; Marinho Cezar Da Cruz, D.; De Deus Lopes, R. IoT Services and Applications in Rehabilitation: An Interdisciplinary and Meta-Analysis Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2043–2052. [Google Scholar] [CrossRef]
- Life Sciences|Vicon Motion Capture for Biomechanics. Available online: https://www.vicon.com/applications/life-sciences/ (accessed on 27 May 2022).
- GAITRite|World Leader in Temporospatial Gait Analysis. Available online: https://www.gaitrite.com/ (accessed on 27 May 2022).
- Bhuiyan, M.N.; Rahman, M.M.; Billah, M.M.; Saha, D. Internet of Things (IoT): A Review of Its Enabling Technologies in Healthcare Applications, Standards Protocols, Security, and Market Opportunities. IEEE Internet Things J. 2021, 8, 10474–10498. [Google Scholar] [CrossRef]
- Hayyolalam, V.; Aloqaily, M.; Ozkasap, O.; Guizani, M. Edge-Assisted Solutions for IoT-Based Connected Healthcare Systems: A Literature Review. IEEE Internet Things J. 2021, 4662, 9419–9443. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An Emerging Era in the Management of Parkinson’s Disease: Wearable Technologies and the Internet of Things. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Habibzadeh, H.; Dinesh, K.; Rajabi Shishvan, O.; Boggio-Dandry, A.; Sharma, G.; Soyata, T. A Survey of Healthcare Internet of Things (HIoT): A Clinical Perspective. IEEE Internet Things J. 2020, 7, 53–71. [Google Scholar] [CrossRef]
- Gatouillat, A.; Badr, Y.; Massot, B.; Sejdic, E. Internet of Medical Things: A Review of Recent Contributions Dealing with Cyber-Physical Systems in Medicine. IEEE Internet Things J. 2018, 5, 3810–3822. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.M.R.; Kwak, D.; Kabir, M.H.; Hossain, M.; Kwak, K.S. The Internet of Things for Health Care: A Comprehensive Survey. IEEE Access 2015, 3, 678–708. [Google Scholar] [CrossRef]
- Dobkin, B.H. A Rehabilitation-Internet-of-Things in the Home to Augment Motor Skills and Exercise Training. Neurorehabilit. Neural Repair 2017, 31, 217–227. [Google Scholar] [CrossRef]
- Eskofier, B.M.; Lee, S.I.; Daneault, J.-F.; Golabchi, F.N.; Ferreira-Carvalho, G.; Vergara-Diaz, G.; Sapienza, S.; Costante, G.; Klucken, J.; Kautz, T.; et al. Recent Machine Learning Advancements in Sensor-Based Mobility Analysis: Deep Learning for Parkinson’s Disease Assessment. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 655–658. [Google Scholar]
- AbdulGhaffar, A.A.; Mostafa, S.M.; Alsaleh, A.; Sheltami, T.; Shakshuki, E.M. Internet of Things Based Multiple Disease Monitoring and Health Improvement System. J. Ambient. Intell. Humaniz. Comput. 2020, 11, 1021–1029. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Besier, T.F. An EMG-Driven Musculoskeletal Model to Estimate Muscle Forces and Knee Joint Moments in Vivo. J. Biomech. 2003, 36, 765–776. [Google Scholar] [CrossRef]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The Extraction of Neural Information from the Surface EMG for the Control of Upper-Limb Prostheses: Emerging Avenues and Challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef]
- Leal-Junior, A.G.; Frizera, A.; Avellar, L.M.; Marques, C.; Pontes, M.J. Polymer Optical Fiber for In-Shoe Monitoring of Ground Reaction Forces during the Gait. IEEE Sens. J. 2018, 18, 2362–2368. [Google Scholar] [CrossRef]
- Krigslund, R.; Dosen, S.; Popovski, P.; Dideriksen, J.L.; Pedersen, G.F.; Farina, D. A Novel Technology for Motion Capture Using Passive UHF RFID Tags. IEEE Trans. Biomed. Eng. 2013, 60, 1453–1457. [Google Scholar] [CrossRef]
- Homayounfar, S.Z.; Andrew, T.L. Wearable Sensors for Monitoring Human Motion: A Review on Mechanisms, Materials, and Challenges. SLAS Technol. 2020, 25, 9–24. [Google Scholar] [CrossRef]
- Haid, M.; Breitenbach, J. Low Cost Inertial Orientation Tracking with Kalman Filter. Appl. Math. Comput. 2004, 153, 567–575. [Google Scholar] [CrossRef]
- Sabatini, A.M. Quaternion-Based Extended Kalman Filter for Determining Orientation by Inertial and Magnetic Sensing. IEEE Trans. Biomed. Eng. 2006, 53, 1346–1356. [Google Scholar] [CrossRef]
- Mahony, R.; Hamel, T.; Morin, P.; Malis, E. Nonlinear Complementary Filters on the Special Linear Group. Int. J. Control 2012, 85, 1557–1573. [Google Scholar] [CrossRef] [Green Version]
- Lau, H.; Tong, K.; Zhu, H. Support Vector Machine for Classification of Walking Conditions of Persons after Stroke with Dropped Foot. Hum. Mov. Sci. 2009, 28, 504–514. [Google Scholar] [CrossRef]
- Lee, S.I.; Daneault, J.F.; Golabchi, F.N.; Patel, S.; Paganoni, S.; Shih, L.; Bonato, P. A Novel Method for Assessing the Severity of Levodopa-Induced Dyskinesia Using Wearable Sensors. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 8087–8090. [Google Scholar] [CrossRef]
- Sigcha, L.; Costa, N.; Pavón, I.; Costa, S.; Arezes, P.; López, J.M.; De Arcas, G. Deep Learning Approaches for Detecting Freezing of Gait in Parkinson’s Disease Patients through On-Body Acceleration Sensors. Sensors 2020, 20, 1895. [Google Scholar] [CrossRef] [Green Version]
- De Brabandere, A.; Emmerzaal, J.; Timmermans, A.; Jonkers, I.; Vanwanseele, B.; Davis, J. A Machine Learning Approach to Estimate Hip and Knee Joint Loading Using a Mobile Phone-Embedded IMU. Front. Bioeng. Biotechnol. 2020, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lach, J.; Lo, B.; Yang, G.-Z. Toward Pervasive Gait Analysis with Wearable Sensors: A Systematic Review. IEEE J. Biomed. Health Inform. 2016, 20, 1521–1537. [Google Scholar] [CrossRef]
- Prasanth, H.; Caban, M.; Keller, U.; Courtine, G.; Ijspeert, A.; Vallery, H.; Von Zitzewitz, J. Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review. Sensors 2021, 21, 2727. [Google Scholar] [CrossRef]
- Ainsworth, E.B. How Do I Measure Physical Activity in My Patients? Questionnaires and Objective Methods. Br. J. Sports Med. 2009, 43, 6. [Google Scholar] [CrossRef]
- Ahmed, H.; Tahir, M. Improving the Accuracy of Human Body Orientation Estimation with Wearable IMU Sensors. IEEE Trans. Instrum. Meas. 2017, 66, 535–542. [Google Scholar] [CrossRef]
- Li, Q.; Young, M.; Naing, V.; Donelan, J.M. Walking Speed Estimation Using a Shank-Mounted Inertial Measurement Unit. J. Biomech. 2010, 43, 1640–1643. [Google Scholar] [CrossRef]
- Rebula, J.R.; Ojeda, L.V.; Adamczyk, P.G.; Kuo, A.D. Measurement of Foot Placement and Its Variability with Inertial Sensors. Gait Posture 2013, 38, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Hu, H. Human Motion Tracking for Rehabilitation-A Survey. Biomed. Signal Process. Control 2008, 3, 1–18. [Google Scholar] [CrossRef]
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG Orientation Using a Gradient Descent Algorithm. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar] [CrossRef]
- Yun, X.; Bachmann, E.R. Design, Implementation, and Experimental Results of a Quaternion-Based Kalman Filter for Human Body Motion Tracking. IEEE Trans. Robot. 2006, 22, 1216–1227. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhao, H.; Qiu, S.; Gao, Q. Stance-Phase Detection for ZUPT-Aided Foot-Mounted Pedestrian Navigation System. IEEE/ASME Trans. Mechatron. 2015, 20, 3170–3181. [Google Scholar] [CrossRef]
- Rampp, A.; Barth, J.; Schülein, S.; Gaßmann, K.G.; Klucken, J.; Eskofier, B.M. Inertial Sensor-Based Stride Parameter Calculation from Gait Sequences in Geriatric Patients. IEEE Trans. Biomed. Eng. 2015, 62, 1089–1097. [Google Scholar] [CrossRef]
- Miyazaki, S. Long-Term Unrestrained Measurement of Stride Length and Walking Velocity Utilizing a Piezoelectric Gyroscope. IEEE Trans. Biomed. Eng. 1997, 44, 753–759. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, C.G.; Kim, J.W.; Hong, H.S.; Lee, J.M. Adaptive Step Length Estimation Algorithm Using Low-Cost MEMS Inertial Sensors. In Proceedings of the 2007 IEEE Sensors Applications Symposium SAS, San Diego, CA, USA, 6–8 February 2007; pp. 6–8. [Google Scholar] [CrossRef]
- Maqbool, H.F.; Husman, M.A.B.; Awad, M.I.; Abouhossein, A.; Iqbal, N.; Dehghani-Sanij, A.A. A Real-Time Gait Event Detection for Lower Limb Prosthesis Control and Evaluation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 1500–1509. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, L.; Fen, W.; Xu, X.; Liang, X.; Xu, X. A Robust Step Detection and Stride Length Estimation for Pedestrian Dead Reckoning Using a Smartphone. IEEE Sens. J. 2020, 20, 9685–9697. [Google Scholar] [CrossRef]
- Chau, T. A Review of Analytical Techniques for Gait Data. Part 1: Fuzzy, Statistical and Fractal Methods. Gait Posture 2001, 13, 49–66. [Google Scholar] [CrossRef]
- Aghanavesi, S.; Westin, J.; Bergquist, F.; Nyholm, D.; Askmark, H.; Aquilonius, S.M.; Constantinescu, R.; Medvedev, A.; Spira, J.; Ohlsson, F.; et al. A Multiple Motion Sensors Index for Motor State Quantification in Parkinson’s Disease. Comput. Methods Programs Biomed. 2020, 189, 105309. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Moreno Arostegui, J.M.; Cabestany, J.; Bayés, À.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home Detection of Freezing of Gait Using Support Vector Machines through a Single Waist-Worn Triaxial Accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar] [CrossRef]
- Ghoraani, B.; Hssayeni, M.D.; Bruack, M.M.; Jimenez-Shahed, J. Multilevel Features for Sensor-Based Assessment of Motor Fluctuation in Parkinson’s Disease Subjects. IEEE J. Biomed. Health Inform. 2020, 24, 1284–1295. [Google Scholar] [CrossRef]
- Albert, M.V.; Azeze, Y.; Courtois, M.; Jayaraman, A. In-Lab versus at-Home Activity Recognition in Ambulatory Subjects with Incomplete Spinal Cord Injury. J. NeuroEng. Rehabil. 2017, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Teufl, W.; Taetz, B.; Miezal, M.; Lorenz, M.; Pietschmann, J.; Jöllenbeck, T.; Fröhlich, M.; Bleser, G. Bleser Towards an Inertial Sensor-Based Wearable Feedback System for Patients after Total Hip Arthroplasty: Validity and Applicability for Gait Classification with Gait Kinematics-Based Features. Sensors 2019, 19, 5006. [Google Scholar] [CrossRef] [Green Version]
- Reches, T.; Dagan, M.; Herman, T.; Gazit, E.; Gouskova, N.; Giladi, N.; Manor, B.; Hausdorff, J. Using Wearable Sensors and Machine Learning to Automatically Detect Freezing of Gait during a FOG-Provoking Test. Sensors 2020, 20, 4474. [Google Scholar] [CrossRef]
- Ashouri, S.; Abedi, M.; Abdollahi, M.; Dehghan Manshadi, F.; Parnianpour, M.; Khalaf, K. A Novel Approach to Spinal 3-D Kinematic Assessment Using Inertial Sensors: Towards Effective Quantitative Evaluation of Low Back Pain in Clinical Settings. Comput. Biol. Med. 2017, 89, 144–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Yao, Y.; Ahmed, J.B.; Tan, Y.; Gu, D. Prediction of Freezing of Gait in Patients with Parkinson’s Disease by Identifying Impaired Gait Patterns. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 591–600. [Google Scholar] [CrossRef]
- Phienphanich, P.; Tankongchamruskul, N.; Akarathanawat, W.; Chutinet, A.; Nimnual, R.; Tantibundhit, C.; Suwanwela, N.C. Stroke Screening Feature Selection for Arm Weakness Using a Mobile Application. IEEE Access 2020, 8, 170898–170914. [Google Scholar] [CrossRef]
- Goodlich, B.I.; Armstrong, E.L.; Horan, S.A.; Baque, E.; Carty, C.P.; Ahmadi, M.N.; Trost, S.G. Machine Learning to Quantify Habitual Physical Activity in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2020, 62, 1054–1060. [Google Scholar] [CrossRef]
- Honoré, H.; Gade, R.; Nielsen, J.F.; Mechlenburg, I. Developing and Validating an Accelerometer-Based Algorithm with Machine Learning to Classify Physical Activity after Acquired Brain Injury. Brain Inj. 2021, 35, 460–467. [Google Scholar] [CrossRef]
- Sun, R.; Tomkins-Lane, C.; Muaremi, A.; Kuwabara, A.; Smuck, M. Physical Activity Thresholds for Predicting Longitudinal Gait Decline in Adults with Knee Osteoarthritis. Osteoarthr. Cartil. 2021, 29, 965–972. [Google Scholar] [CrossRef]
- Oubre, B.; Daneault, J.-F.; Jung, H.-T.; Whritenour, K.; Miranda, J.G.V.; Park, J.; Ryu, T.; Kim, Y.; Lee, S.I. Estimating Upper-Limb Impairment Level in Stroke Survivors Using Wearable Inertial Sensors and a Minimally-Burdensome Motor Task. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 601–611. [Google Scholar] [CrossRef]
- Derungs, A.; Amft, O. Synthesising Motion Sensor Data from Biomechanical Simulations to Investigate Motion Sensor Placement and Orientation Variations. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6391–6394. [Google Scholar]
- Lee, S.I.; Adans-Dester, C.P.; OBrien, A.T.; Vergara-Diaz, G.P.; Black-Schaffer, R.; Zafonte, R.; Dy, J.G.; Bonato, P. Predicting and Monitoring Upper-Limb Rehabilitation Outcomes Using Clinical and Wearable Sensor Data in Brain Injury Survivors. IEEE Trans. Biomed. Eng. 2021, 68, 1871–1881. [Google Scholar] [CrossRef]
- Caramia, C.; Torricelli, D.; Schmid, M.; Munoz-Gonzalez, A.; Gonzalez-Vargas, J.; Grandas, F.; Pons, J.L. IMU-Based Classification of Parkinson’s Disease from Gait: A Sensitivity Analysis on Sensor Location and Feature Selection. IEEE J. Biomed. Health Inform. 2018, 22, 1765–1774. [Google Scholar] [CrossRef]
- Rastegari, E.; Orn, D.; Ali, H. Smart Computational Approaches with Advanced Feature Selection Algorithms for Optimizing the Classification of Mobility Data in Health Informatics. In Proceedings of the 11th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics, Virtual Event, USA, 21 September 2020; pp. 1–9. [Google Scholar]
- Mirelman, A.; Ben Or Frank, M.; Melamed, M.; Granovsky, L.; Nieuwboer, A.; Rochester, L.; Del Din, S.; Avanzino, L.; Pelosin, E.; Bloem, B.R.; et al. Detecting Sensitive Mobility Features for Parkinson’s Disease Stages Via Machine Learning. Mov. Disord. 2021, 36, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Hssayeni, M.D.; Jimenez-Shahed, J.; Burack, M.A.; Ghoraani, B. Wearable Sensors for Estimation of Parkinsonian Tremor Severity during Free Body Movements. Sensors 2019, 19, 4215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwat, H.; Sarwat, H.; Awad, M.I.; Maged, S.A. Assessment of Post-Stroke Patients Using Smartphones and Gradient Boosting. In Proceedings of the 2020 15th International Conference on Computer Engineering and Systems (ICCES), Cairo, Egypt, 15–16 December 2020; pp. 1–6. [Google Scholar]
- İkizoğlu, S.; Heydarov, S. Accuracy Comparison of Dimensionality Reduction Techniques to Determine Significant Features from IMU Sensor-Based Data to Diagnose Vestibular System Disorders. Biomed. Signal Process. Control 2020, 61, 101963. [Google Scholar] [CrossRef]
- Bennett, T.R.; Wu, J. Inertial Measurement Unit-Based Wearable Computers for Assisted Living Applications. IEEE Signal Process. Mag. 2018, 33, 28–35. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Küderle, A.; Gaßner, H.; Klucken, J.; Eskofier, B.M.; Kluge, F. Inertial Sensor-Based Gait Parameters Reflect Patient-Reported Fatigue in Multiple Sclerosis. J. NeuroEng. Rehabil. 2020, 17, 165. [Google Scholar] [CrossRef]
- Kobsar, D.; Osis, S.T.; Boyd, J.E.; Hettinga, B.A.; Ferber, R. Wearable Sensors to Predict Improvement Following an Exercise Intervention in Patients with Knee Osteoarthritis. J. NeuroEng. Rehabil. 2017, 14, 94. [Google Scholar] [CrossRef] [Green Version]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters Using an Inertial Measurement Unit (IMU)-Based System. Sensors 2018, 19, 88. [Google Scholar] [CrossRef] [Green Version]
- Ravi, D.; Wong, C.; Deligianni, F.; Berthelot, M.; Andreu-Perez, J.; Lo, B.; Yang, G.Z. Deep Learning for Health Informatics. IEEE J. Biomed. Health Inform. 2017, 21, 4–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, Y.; Hao, S.; Peng, X.; Hu, L. Deep Learning for Sensor-Based Activity Recognition: A Survey. Pattern Recognit. Lett. 2019, 119, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-C.; Chen, S.-F.; Lin, C.-H.; Shih, C.-J.; Lin, A.-C.; Yuan, W.; Li, Y.-C.; Kuo, T.-Y. Detection and Classification of Stroke Gaits by Deep Neural Networks Employing Inertial Measurement Units. Sensors 2021, 21, 1864. [Google Scholar] [CrossRef]
- Butt, A.H.; Cavallo, F.; Maremmani, C.; Rovini, E. Biomechanical Parameters Assessment for the Classification of Parkinson Disease Using Bidirectional Long Short-Term Memory. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5761–5764. [Google Scholar]
- Rovini, E.; Fiorini, L.; Esposito, D.; Maremmani, C.; Cavallo, F. Fine Motor Assessment with Unsupervised Learning for Personalized Rehabilitation in Parkinson Disease. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 1167–1172. [Google Scholar]
- Som, A.; Krishnamurthi, N.; Buman, M.; Turaga, P. Unsupervised Pre-Trained Models from Healthy ADLs Improve Parkinson’s Disease Classification of Gait Patterns. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 784–788. [Google Scholar]
- Mohammadian Rad, N.; van Laarhoven, T.; Furlanello, C.; Marchiori, E. Novelty Detection Using Deep Normative Modeling for IMU-Based Abnormal Movement Monitoring in Parkinson’s Disease and Autism Spectrum Disorders. Sensors 2018, 18, 3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, L.; He, J.; Peng, L. CNN-Based PD Hand Tremor Detection Using Inertial Sensors. IEEE Sens. Lett. 2021, 5, 7002504. [Google Scholar] [CrossRef]
- Shawen, N.; O’Brien, M.K.; Venkatesan, S.; Lonini, L.; Simuni, T.; Hamilton, J.L.; Ghaffari, R.; Rogers, J.A.; Jayaraman, A. Role of Data Measurement Characteristics in the Accurate Detection of Parkinson’s Disease Symptoms Using Wearable Sensors. J. Neuroeng. Rehabil. 2020, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Cai, G.; Lin, Z.; Wang, Z.; Ye, Q. Validation of Inertial Sensing-Based Wearable Device for Tremor and Bradykinesia Quantification. IEEE J. Biomed. Health Inform. 2021, 25, 997–1005. [Google Scholar] [CrossRef]
- Mileti, I.; Germanotta, M.; Di Sipio, E.; Imbimbo, I.; Pacilli, A.; Erra, C.; Petracca, M.; Rossi, S.; Del Prete, Z.; Bentivoglio, A.; et al. Measuring Gait Quality in Parkinson’s Disease through Real-Time Gait Phase Recognition. Sensors 2018, 18, 919. [Google Scholar] [CrossRef] [Green Version]
- Perez-Ibarra, J.C.; Siqueira, A.A.G.; Krebs, H.I. Identification of Gait Events in Healthy Subjects and With Parkinson’s Disease Using Inertial Sensors: An Adaptive Unsupervised Learning Approach. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2933–2943. [Google Scholar] [CrossRef]
- Hannink, J.; Kautz, T.; Pasluosta, C.F.; Barth, J.; Schulein, S.; Gassmann, K.-G.; Klucken, J.; Eskofier, B.M. Mobile Stride Length Estimation with Deep Convolutional Neural Networks. IEEE J. Biomed. Health Inform. 2018, 22, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Barth, J.; Oberndorfer, C.; Pasluosta, C.; Schülein, S.; Gassner, H.; Reinfelder, S.; Kugler, P.; Schuldhaus, D.; Winkler, J.; Klucken, J. Stride Segmentation during Free Walk Movements Using Multi-Dimensional Subsequence Dynamic Time Warping on Inertial Sensor Data. Sensors 2015, 15, 6419–6440. [Google Scholar] [CrossRef]
- Bernad-Elazari, H.; Herman, T.; Mirelman, A.; Gazit, E.; Giladi, N.; Hausdorff, J.M. Objective Characterization of Daily Living Transitions in Patients with Parkinson’s Disease Using a Single Body-Fixed Sensor. J. Neurol. 2016, 263, 1544–1551. [Google Scholar] [CrossRef]
- Shi, B.; Yen, S.C.; Tay, A.; Tan, D.M.L.; Chia, N.S.Y.; Au, W.L. Convolutional Neural Network for Freezing of Gait Detection Leveraging the Continuous Wavelet Transform on Lower Extremities Wearable Sensors Data. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 5410–5415. [Google Scholar]
- Bachlin, M.; Plotnik, M.; Roggen, D.; Maidan, I.; Hausdorff, J.M.; Giladi, N.; Troster, G. Wearable Assistant for Parkinson’s Disease Patients with the Freezing of Gait Symptom. IEEE Trans. Inf. Technol. Biomed. 2009, 14, 436–446. [Google Scholar] [CrossRef]
- Tautan, A.-M.; Andrei, A.-G.; Ionescu, B. Freezing of Gait Detection for Parkinson’s Disease Patients Using Accelerometer Data: Case Study. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29–30 October 2020; pp. 1–4. [Google Scholar]
- Mazilu, S.; Calatroni, A.; Gazit, E.; Mirelman, A.; Hausdorff, J.M.; Tröster, G. Prediction of Freezing of Gait in Parkinson’s from Physiological Wearables: An Exploratory Study. IEEE J. Biomed. Health Inform. 2015, 19, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, L.; Rocchi, L.; Mazilu, S.; Gazit, E.; Hausdorff, J.M.; Chiari, L. Identification of Characteristic Motor Patterns Preceding Freezing of Gait in Parkinson’s Disease Using Wearable Sensors. Front. Neurol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lohit, S.; Toledo, M.J.; Buman, M.P.; Turaga, P. A Statistical Estimation Framework for Energy Expenditure of Physical Activities from a Wrist-Worn Accelerometer. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2631–2635. [Google Scholar]
- Goodwin, M.S.; Haghighi, M.; Tang, Q.; Akcakaya, M.; Erdogmus, D.; Intille, S. Moving towards a Real-Time System for Automatically Recognizing Stereotypical Motor Movements in Individuals on the Autism Spectrum Using Wireless Accelerometry. In Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Seattle, WA, USA, 13–17 September 2014; pp. 861–872. [Google Scholar]
- Guerra, J.; Uddin, J.; Nilsen, D.; Mclnerney, J.; Fadoo, A.; Omofuma, I.B.; Hughes, S.; Agrawal, S.; Allen, P.; Schambra, H.M. Capture, Learning, and Classification of Upper Extremity Movement Primitives in Healthy Controls and Stroke Patients. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 547–554. [Google Scholar]
- Parnandi, A.; Uddin, J.; Nilsen, D.M.; Schambra, H.M. The Pragmatic Classification of Upper Extremity Motion in Neurological Patients: A Primer. Front. Neurol. 2019, 10, 996. [Google Scholar] [CrossRef]
- He, J.; Chen, S.; Guo, Z.; Pirbhulal, S.; Wu, W.; Feng, J.; Dan, G. A Comparative Study of Motion Recognition Methods for Efficacy Assessment of Upper Limb Function. Int. J. Adapt. Control Signal Process. 2019, 33, 1248–1256. [Google Scholar] [CrossRef]
- Liu, X.; Rajan, S.; Ramasarma, N.; Bonato, P.; Lee, S.I. The Use of a Finger-Worn Accelerometer for Monitoring of Hand Use in Ambulatory Settings. IEEE J. Biomed. Health Inform. 2019, 23, 599–606. [Google Scholar] [CrossRef]
- Knarr, B.A.; Kesar, T.M.; Reisman, D.S.; Binder-Macleod, S.A.; Higginson, J.S. Changes in the Activation and Function of the Ankle Plantar Flexor Muscles Due to Gait Retraining in Chronic Stroke Survivors. J. Neuroeng. Rehabil. 2013, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.; Hermiz, J.; Labuzetta, J.; Arabadzhi, Y.; Karanjia, N.; Gilja, V. Use of Accelerometry for Long Term Monitoring of Stroke Patients. IEEE J. Transl. Eng. Health Med. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Ahmadi, M.N.; O’Neil, M.E.; Baque, E.; Boyd, R.N.; Trost, S.G. Machine Learning to Quantify Physical Activity in Children with Cerebral Palsy: Comparison of Group, Group-Personalized, and Fully-Personalized Activity Classification Models. Sensors 2020, 20, 3976. [Google Scholar] [CrossRef]
- Ahmadi, M.; O’Neil, M.; Fragala-Pinkham, M.; Lennon, N.; Trost, S. Machine Learning Algorithms for Activity Recognition in Ambulant Children and Adolescents with Cerebral Palsy. J. NeuroEng. Rehabil. 2018, 15, 105. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, J.; Nandy, A. Discrete Wavelet Transform Based Data Representation in Deep Neural Network for Gait Abnormality Detection. Biomed. Signal Process. Control 2020, 62, 102076. [Google Scholar] [CrossRef]
- Dostál, O.; Procházka, A.; Vyšata, O.; Ťupa, O.; Cejnar, P.; Vališ, M. Recognition of Motion Patterns Using Accelerometers for Ataxic Gait Assessment. Neural Comput. Appl. 2021, 33, 2207–2215. [Google Scholar] [CrossRef]
- Ngo, T.; Pathirana, P.N.; Horne, M.K.; Power, L.; Szmulewicz, D.J.; Milne, S.C.; Corben, L.A.; Roberts, M.; Delatycki, M.B. Balance Deficits Due to Cerebellar Ataxia: A Machine Learning and Cloud-Based Approach. IEEE Trans. Biomed. Eng. 2021, 68, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Oubre, B.; Daneault, J.-F.; Whritenour, K.; Khan, N.C.; Stephen, C.D.; Schmahmann, J.D.; Lee, S.I.; Gupta, A.S. Decomposition of Reaching Movements Enables Detection and Measurement of Ataxia. Cerebellum 2021, 20, 811–822. [Google Scholar] [CrossRef]
- Bennasar, M.; Hicks, Y.A.; Clinch, S.P.; Jones, P.; Holt, C.; Rosser, A.; Busse, M. Automated Assessment of Movement Impairment in Huntington’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2062–2069. [Google Scholar] [CrossRef]
- De Vos, M.; Prince, J.; Buchanan, T.; FitzGerald, J.J.; Antoniades, C.A. Discriminating Progressive Supranuclear Palsy from Parkinson’s Disease Using Wearable Technology and Machine Learning. Gait Posture 2020, 77, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Nazarahari, M.; Chan, K.M.; Rouhani, H. A Novel Instrumented Shoulder Functional Test Using Wearable Sensors in Patients with Brachial Plexus Injury. J. Shoulder Elb. Surg. 2021, 30, e493–e502. [Google Scholar] [CrossRef]
- Milošević, M.; Van de Vel, A.; Cuppens, K.; Bonroy, B.; Ceulemans, B.; Lagae, L.; Vanrumste, B.; Van Huffel, S. Feature Selection Methods for Accelerometry-Based Seizure Detection in Children. Med. Biol. Eng. Comput. 2017, 55, 151–165. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Li, Q.; Liu, T.; Yi, J. Two Shank-Mounted IMUs-Based Gait Analysis and Classification for Neurological Disease Patients. IEEE Robot. Autom. Lett. 2020, 5, 1970–1976. [Google Scholar] [CrossRef]
- Wang, X.; Ristic-Durrant, D.; Spranger, M.; Graser, A. Gait Assessment System Based on Novel Gait Variability Measures. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 467–472. [Google Scholar]
- Lemoyne, R.; Mastroianni, T. Implementation of a Smartphone as a Wearable and Wireless Gyroscope Platform for Machine Learning Classification of Hemiplegic Gait through a Multilayer Perceptron Neural Network. In Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), Orlando, FL, USA, 17–20 December 2018; pp. 946–950. [Google Scholar]
- Kim, J.-Y.; Park, G.; Lee, S.-A.; Nam, Y. Analysis of Machine Learning-Based Assessment for Elbow Spasticity Using Inertial Sensors. Sensors 2020, 20, 1622. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Gubbi, J.; Yan, B.; Palaniswami, M. Motor Recovery Monitoring in Post Acute Stroke Patients Using Wireless Accelerometer and Cross-Correlation. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6703–6706. [Google Scholar]
- Parnandi, A.; Wade, E.; Matarić, M. Motor Function Assessment Using Wearable Inertial Sensors. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 86–89. [Google Scholar]
- Stetter, B.J.; Krafft, F.C.; Ringhof, S.; Stein, T.; Sell, S. A Machine Learning and Wearable Sensor Based Approach to Estimate External Knee Flexion and Adduction Moments during Various Locomotion Tasks. Front. Bioeng. Biotechnol. 2020, 8, 9. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Hootman, J.M.; Semanik, P.A.; Chang, R.W.; Sharma, L.; Van Horn, L.; Bathon, J.M.; Eaton, C.B.; Hochberg, M.C. Obesity and Other Modifiable Factors for Physical Inactivity Measured by Accelerometer in Adults with Knee Osteoarthritis. Arthritis Care Res. 2013, 65, 53–61. [Google Scholar] [CrossRef]
- Chen, H.-P.; Chen, H.-C.; Liu, K.-C.; Chan, C.-T. Online Segmentation with Multi-Layer SVM for Knee Osteoarthritis Rehabilitation Monitoring. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 55–60. [Google Scholar]
- Tan, J.-S.; Beheshti, B.K.; Binnie, T.; Davey, P.; Caneiro, J.P.; Kent, P.; Smith, A.; O’Sullivan, P.; Campbell, A. Human Activity Recognition for People with Knee Osteoarthritis—A Proof-of-Concept. Sensors 2021, 21, 3381. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ashouri, S.; Abedi, M.; Azadeh-Fard, N.; Parnianpour, M.; Khalaf, K.; Rashedi, E. Using a Motion Sensor to Categorize Nonspecific Low Back Pain Patients: A Machine Learning Approach. Sensors 2020, 20, 3600. [Google Scholar] [CrossRef]
- Alcaraz, J.C.; Moghaddamnia, S.; Penner, M.; Peissig, J. Monitoring the Rehabilitation Progress Using a DCNN and Kinematic Data for Digital Healthcare. In Proceedings of the 2020 28th European Signal Processing Conference (EUSIPCO), Amsterdam, The Netherlands, 18–21 January 2021; pp. 1333–1337. [Google Scholar]
- Bini, S.A.; Shah, R.F.; Bendich, I.; Patterson, J.T.; Hwang, K.M.; Zaid, M.B. Machine Learning Algorithms Can Use Wearable Sensor Data to Accurately Predict Six-Week Patient-Reported Outcome Scores Following Joint Replacement in a Prospective Trial. J. Arthroplast. 2019, 34, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Huang, B.; Argent, R.; Caulfield, B.; Kechadi, T. Automatic Classification of Knee Rehabilitation Exercises Using a Single Inertial Sensor: A Case Study. In Proceedings of the 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV, USA, 4–7 March 2018; pp. 21–24. [Google Scholar]
- Zanella-Calzada, L.A.; Galván-Tejada, C.E.; Chávez-Lamas, N.M.; Gracia-Cortés, M.; Magallanes-Quintanar, R.; Celaya-Padilla, J.M.; Galván-Tejada, J.I.; Gamboa-Rosales, H. Feature Extraction in Motor Activity Signal: Towards a Depression Episodes Detection in Unipolar and Bipolar Patients. Diagnostics 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, S.; Lee, S.; Hong, S.; Kang, H.; Kim, N. Depression Prediction by Using Ecological Momentary Assessment, Actiwatch Data, and Machine Learning: Observational Study on Older Adults Living Alone. JMIR mHealth uHealth 2019, 7, e14149. [Google Scholar] [CrossRef] [Green Version]
- Tazawa, Y.; Liang, K.; Yoshimura, M.; Kitazawa, M.; Kaise, Y.; Takamiya, A.; Kishi, A.; Horigome, T.; Mitsukura, Y.; Mimura, M. Evaluating Depression with Multimodal Wristband-Type Wearable Device: Screening and Assessing Patient Severity Utilizing Machine-Learning. Heliyon 2020, 6, e03274. [Google Scholar] [CrossRef]
- Garcia-Ceja, E.; Riegler, M.; Jakobsen, P.; Torresen, J.; Nordgreen, T.; Oedegaard, K.J.; Fasmer, O.B. Motor Activity Based Classification of Depression in Unipolar and Bipolar Patients. In Proceedings of the 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS), Karlstad, Sweden, 18–21 June 2018; pp. 316–321. [Google Scholar]
- Ghandeharioun, A.; Fedor, S.; Sangermano, L.; Ionescu, D.; Alpert, J.; Dale, C.; Sontag, D.; Picard, R. Objective Assessment of Depressive Symptoms with Machine Learning and Wearable Sensors Data. In Proceedings of the 2017 Seventh International Conference on Affective Computing and Intelligent Interaction (ACII), San Antonio, TX, USA, 23–26 October 2017; pp. 325–332. [Google Scholar]
- Faedda, G.L.; Ohashi, K.; Hernandez, M.; McGreenery, C.E.; Grant, M.C.; Baroni, A.; Polcari, A.; Teicher, M.H. Actigraph Measures Discriminate Pediatric Bipolar Disorder from Attention-deficit/Hyperactivity Disorder and Typically Developing Controls. J. Child Psychol. Psychiatry 2016, 57, 706–716. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, R.S.; McGinnis, E.W.; Hruschak, J.; Lopez-Duran, N.L.; Fitzgerald, K.; Rosenblum, K.L.; Muzik, M. Rapid Detection of Internalizing Diagnosis in Young Children Enabled by Wearable Sensors and Machine Learning. PLoS ONE 2019, 14, e0210267. [Google Scholar] [CrossRef]
- Clark, L.A.; Cuthbert, B.; Lewis-Fernández, R.; Narrow, W.E.; Reed, G.M. Three Approaches to Understanding and Classifying Mental Disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest 2017, 18, 72–145. [Google Scholar] [CrossRef] [Green Version]
- Stafford, N.; Colom, F. Purpose and Effectiveness of Psychoeducation in Patients with Bipolar Disorder in a Bipolar Clinic Setting. Acta Psychiatr. Scand. 2013, 127, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ceja, E.; Riegler, M.; Jakobsen, P.; Tørresen, J.; Nordgreen, T.; Oedegaard, K.J.; Fasmer, O.B. Depresjon: A Motor Activity Database of Depression Episodes in Unipolar and Bipolar Patients. In Proceedings of the 9th ACM Multimedia Systems Conference, Amsterdam, The Netherlands, 12–15 June 2018; pp. 472–477. [Google Scholar]
- Cheng, Q.; Shang, J.; Juen, J.; Han, J.; Schatz, B. Mining Discriminative Patterns to Predict Health Status for Cardiopulmonary Patients. In Proceedings of the 7th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Seattle, WA, USA, 2–5 October 2016; pp. 41–49. [Google Scholar]
- Friedrich, B.; Lau, S.; Elgert, L.; Bauer, J.M.; Hein, A. A Deep Learning Approach for TUG and SPPB Score Prediction of (Pre-) Frail Older Adults on Real-Life IMU Data. Healthcare 2021, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Argent, R.; Drummond, S.; Remus, A.; O’Reilly, M.; Caulfield, B. Evaluating the Use of Machine Learning in the Assessment of Joint Angle Using a Single Inertial Sensor. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319868544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavan, L.; Surmacz, K.; Beard, D.; Mellon, S.; Rees, J. Adherence Monitoring of Rehabilitation Exercise with Inertial Sensors: A Clinical Validation Study. Gait Posture 2019, 70, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hua, A.; Chaudhari, P.; Johnson, N.; Quinton, J.; Schatz, B.; Buchner, D.; Hernandez, M.E. Evaluation of Machine Learning Models for Classifying Upper Extremity Exercises Using Inertial Measurement Unit-Based Kinematic Data. IEEE J. Biomed. Health Inform. 2020, 24, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Bevilacqua, A.; Kechadi, T.; Caulfield, B. Segmentation of Shoulder Rehabilitation Exercises for Single and Multiple Inertial Sensor Systems. J. Rehabil. Assist. Technol. Eng. 2020, 7, 2055668320915377. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hsieh, C.-Y.; Huang, H.-Y.; Wu, Y.-T.; Chen, L.-C.; Chan, C.-T.; Liu, K.-C. Automatic Functional Shoulder Task Identification and Sub-Task Segmentation Using Wearable Inertial Measurement Units for Frozen Shoulder Assessment. Sensors 2020, 21, 106. [Google Scholar] [CrossRef]
- Tedaldi, D.; Pretto, A.; Menegatti, E. A Robust and Easy to Implement Method for IMU Calibration without External Equipments. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 3042–3049. [Google Scholar]
- Milosevic, M.; Van de Vel, A.; Bonroy, B.; Ceulemans, B.; Lagae, L.; Vanrumste, B.; Huffel, S.V. Automated Detection of Tonic–Clonic Seizures Using 3-D Accelerometry and Surface Electromyography in Pediatric Patients. IEEE J. Biomed. Health Inform. 2016, 20, 1333–1341. [Google Scholar] [CrossRef]
- Huo, W.; Angeles, P.; Tai, Y.F.; Pavese, N.; Wilson, S.; Hu, M.T.; Vaidyanathan, R. A Heterogeneous Sensing Suite for Multisymptom Quantification of Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1397–1406. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Wang, J.; Zhang, J. Kinematic Analysis of Human Gait Based on Wearable Sensor System for Gait Rehabilitation. J. Med. Biol. Eng. 2016, 36, 843–856. [Google Scholar] [CrossRef]
- Hwangbo, M.; Kim, J.-S.; Kanade, T. IMU Self-Calibration Using Factorization. IEEE Trans. Robot. 2013, 29, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Wen, Z.; Zhou, W.; Ni, Z.; Li, X. Adaptive On-Line Self-Calibration of Errors in Multi-Stage-Noise-Shaping (MASH) Sigma-Delta Modulator Gyroscope. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 753–756. [Google Scholar]
- Prikhodko, I.P.; Merritt, C.; Gregory, J.A.; Geen, J.A.; Chang, J.; Bergeron, J.; Clark, W.; Judy, M.W. Continuous Self-Calibration Canceling Drive-Induced Errors in MEMS Vibratory Gyroscopes. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 35–38. [Google Scholar]
- Ren, C.; Liu, Q.; Fu, T. A Novel Self-Calibration Method for MIMU. IEEE Sens. J. 2015, 15, 5416–5422. [Google Scholar] [CrossRef]
- Dai, M.; Lu, J. A Full-Parameter Self-Calibration Method Based on Inertial Frame Filtering for Triaxis RINS under Swaying Base. IEEE Sens. J. 2018, 19, 2170–2180. [Google Scholar] [CrossRef]
- MTw Awinda. Available online: https://www.xsens.com/products/mtw-awinda (accessed on 28 May 2022).
- Wearable Sensor Products. Available online: https://shimmersensing.com/wearable-sensor-products/ (accessed on 28 May 2022).
- Morrison, C.; Huckvale, K.; Corish, B.; Banks, R.; Grayson, M.; Dorn, J.; Sellen, A.; Lindley, S. Visualizing Ubiquitously Sensed Measures of Motor Ability in Multiple Sclerosis: Reflections on Communicating Machine Learning in Practice. ACM Trans. Interact. Intell. Syst. (TiiS) 2018, 8, 1–28. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; Zhang, B.; Hu, X. Interpret Neural Networks by Identifying Critical Data Routing Paths. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 8906–8914. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why Should i Trust You?” Explaining the Predictions of Any Classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Choi, E.; Bahadori, M.T.; Song, L.; Stewart, W.F.; Sun, J. GRAM: Graph-Based Attention Model for Healthcare Representation Learning. In Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Halifax, NS, Canada, 13–17 August 2017; pp. 787–795. [Google Scholar]
- Lu, C.; Reddy, C.K.; Ning, Y. Self-Supervised Graph Learning with Hyperbolic Embedding for Temporal Health Event Prediction. IEEE Trans. Cybern. 2021, 1–13. [Google Scholar] [CrossRef]
- Tong, L.; Liu, R.; Peng, L. LSTM-Based Lower Limbs Motion Reconstruction Using Low-Dimensional Input of Inertial Motion Capture System. IEEE Sens. J. 2019, 20, 3667–3677. [Google Scholar] [CrossRef]
- Karakra, A.; Fontanili, F.; Lamine, E.; Lamothe, J. HospiT’Win: A Predictive Simulation-Based Digital Twin for Patients Pathways in Hospital. In Proceedings of the 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar]


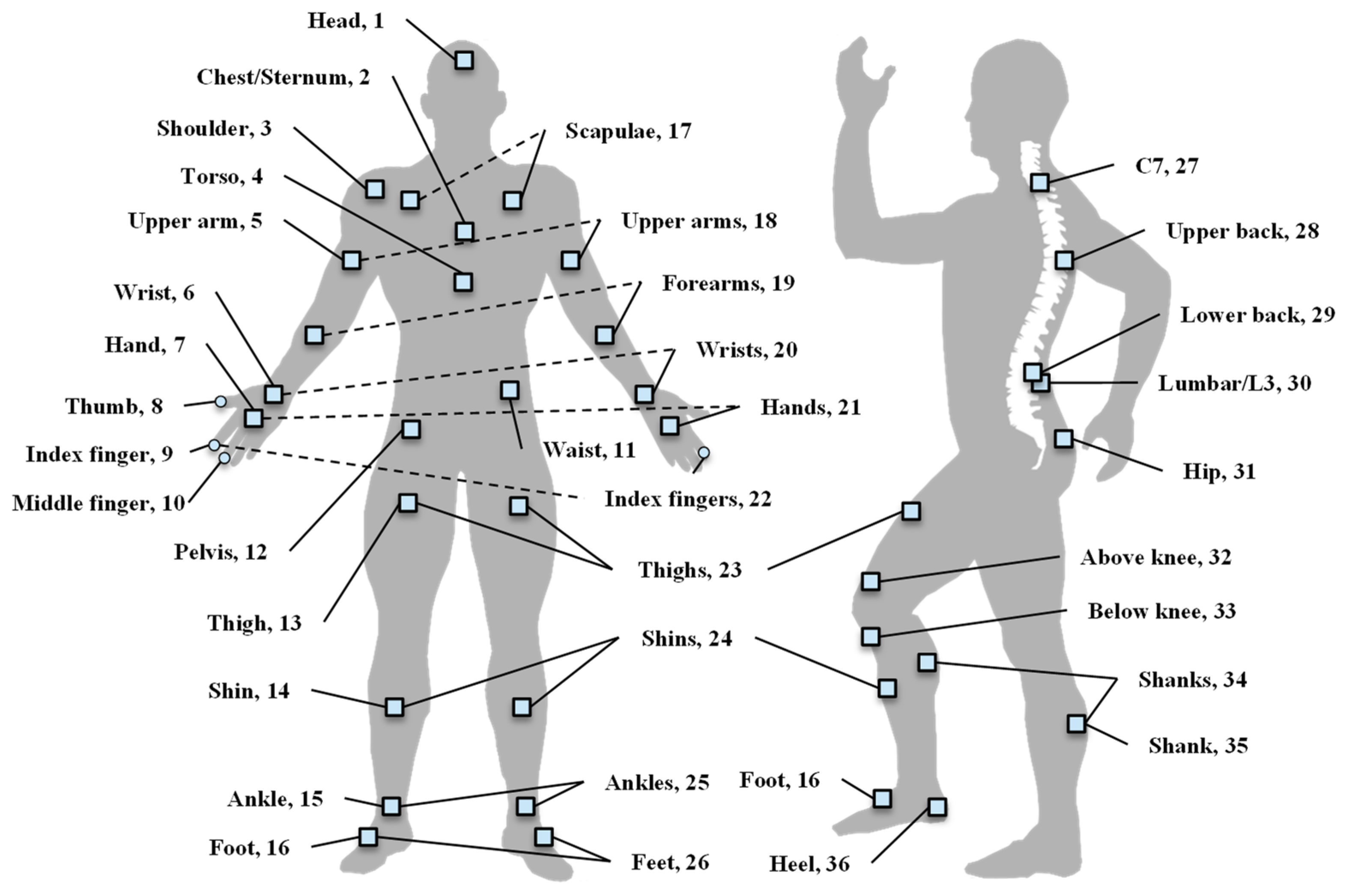


| Feature Categories | Features |
|---|---|
| Time | Standard deviation, mean, range, amplitude, root mean square, variance, skewness, kurtosis, coefficient of variation (CV), increment, power, energy, and jerk Segment time, zero-crossing ratio, number of peaks DTW coefficient, and autoregression coefficient |
| Frequency | Dominant frequency, power of dominant frequency, amplitude in certain bandwidth, moments of power spectral density, CV of frequency, and relative magnitude |
| Entropy | Sample entropy, spectral entropy, and approximate entropy |
| Correlation | Cross-correlation (peak and lag); autocorrelation (peaks, number, sum, amplitude, and lag) |
| High-order | Velocity, stride/step length, left and right asymmetry, range of motions, freezing index, and harmonic ratio |
| Disorders | Application | Sensor (n) | Placement | Model | Input Data/Features | Major Performance | Subjects/ Dataset | Year | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PD | D | IMU (1) | 6 | CNN | 28 samples | Acc = 97.32% | 5 p, 5 h | 2021 | [74] |
| PD | SD | IMU (2) | 19 | CNN | 5s window | Acc = 90.9% | 10 p | 2016 | [12] |
| PD | SD, SA | IMU (2) | 6, 7 | RF | 74 features | multiple | 13 p | 2020 | [75] |
| PD | SA | IMU (1) | 9 | SVM | 7 features | Acc = 96–97.33% | 45 p, 30 h | 2021 | [76] |
| PD | SD, SA | IMU (2) | 6, 15 | XGBoost | 78 features | R = 0.96 (ho), 0.93 (loso) | 24 p | 2019 | [60] |
| PD | CE | IMU (2) | 26 | HMM | raw | G < 0.25 | 26 p, 11 h | 2018 | [77] |
| PD | CE | IMU (2) | 16, 36 | HMM | raw | F1 ≥ 0.95 | 7 p, 5 h | 2020 | [78] |
| PD | CE | IMU (2) | 16, 36 | CNN | 256 samples | acc. ± prec. = 0.01 ± 5.37 cm | 116 p [36] | 2018 | [79] |
| PD | SA | IMU (8) | 2, 23, 24, 26, 30 | Meta-classifier | 18 feature sets | Acc = 84.00% ± 6.54% | 25 p | 2018 | [57] |
| PD | D | IMU (2) | 25 | Adaboost | 21 gait features | Acc = 85–95% | 20 p,10 h [80] | 2020 | [58] |
| PD | SA | IMU (6) | 6, 8, 9, 10, 26 | SOM | 41 features | Acc = 95% (2 classes), 81.7% (3 classes) | 30 p | 2019 | [71] |
| PD | SA | IMU (4) | 20, 25 | SVM | 178 features | R = 0.93, (0.85 (dys.), 0.84 (brady.), 0.79 (gait)) | 19 p | 2020 | [42] |
| PD | SA | IMU (5) | 20, 25, 29 | RUSBoost | 134 features | AUC = 0.76–0.90, Sen = 72–83%, Spec = 69–80% | 332 p, 100 h | 2021 | [59] |
| PD | SA | Accel (1) | 29 | SVM | temporal features | Acc = 92.3%, 89.3%, 85.9 for 3 binary classifications | 99 p, 38 h | 2016 | [81] |
| PD | SD | IMU (3) | 24, 29 | SVM_rbf | 86 features | Acc = 85.0%, Sen = 84.1% | 71 p | 2020 | [47] |
| PD | SD | IMU (3) | 25, 27 | CNN | 4s window | Acc = 89.2% | 67 p | 2020 | [82] |
| PD | SD | Accel (3) | 14, 15, 31 | CNN | 2–5s window | Sen = 93.44%, Spec = 87.38% | 10 p [83] | 2020 | [84] |
| PD | SD | Accel (1) | 29 | SVM | 55 features | GM = 76.8%, 84.0% (personal) | 21 p | 2017 | [43] |
| PD | SD | Accel (1) | 11 | CNN + LSTM | 4 features | AUC = 0.936 | 21 p [43] | 2020 | [24] |
| PD | SD | Accel (1) | 30 | C4.5 | 2 feature sets | Acc = 82.7%, 77.9% (2 modes) | 12 p | 2020 | [49] |
| PD | SD | IMU (3) | 24, 29 | LDA | 8 features | AUC = 0.76, Sen = 0.84 | 11 p [85] | 2017 | [86] |
| PD | D | IMU (6) | 6, 8, 9, 10, 26 | BiLSTM | 190 features | Acc = 82.4% | 64 p, 50 h | 2020 | [70] |
| PD | PA | Accel (6) | 2, 20, 25, 30 | Autoencoder | 250 samples | F1 = 73.89 ± 5.69 | 18 p, 16 h [87] | 2020 | [72] |
| PD | SD | Gyro (2) | 6, 15 | SVM | 3 feature sets | Acc = 83.56% | 19 p | 2020 | [44] |
| PD | D | Accel (3) | 4, 20 | Autoencoder | 1s window | AUC = 0.77 | [83], 6 p [88] | 2018 | [73] |
| Stroke | CE | IMU (11) | 1, 2, 12, 17, 18, 19, 21 | LDA | statistical features | Acc ≥ 93% | 10 h, 6 p [89] | 2019 | [90] |
| Stroke | SA | IMU (2) | 2, 6 | SVR | 109 features | RMSE = 18.2%, R = 0.70 | 36 p, 32 h | 2020 | [54] |
| Stroke | SA | IMU (1) | 6 | SVM | statistical features | Acc = 97.70% | 20 p | 2019 | [91] |
| Stroke | SA | IMU (1) | 6 | XGBoost | SMA feature | Acc = 95.56% | 10 p | 2020 | [61] |
| Stroke | CE | Accel (4) | 20, 22 | SVR | 271 features | nRMSE = 0.11, R = 0.78 | 10 p, 10 h | 2019 | [92] |
| Stroke | CE | IMU (1) | 7 | RF | 3 feature sets | Acc = 84.1%, Sen = 94.8% | 7 p | 2020 | [50] |
| Stroke | D | IMU (2) | 25 | DCNN | gait cycle | Acc = 99.35% (detection), | 30 p, 15 h | 2021 | [69] |
| Stroke | SA | Accel (1) | 13 | SVR | 20 features | 97.31% (classification) | 8 p [93] | 2019 | [55] |
| Stroke | SA | Accel (4) | 19, 24 | SVM | 9 features | nRMSE = 0.32% (affected), 0.36% (unaffected) | 18h | 2019 | [94] |
| CP | PA | Accel (3) | 6, 15, 31 | RF | 15 features | p < 0.05 | 38 p | 2020 | [95] |
| CP | PA | Accel (2) | 6, 31 | SVM | 27 features | Acc = 99.0–99.3% | 22 p | 2018 | [96] |
| CP | PA | IMU (3) | 6, 13, 31 | RF | 40 features | Acc = 82.0–89.0% | 11 p | 2020 | [51] |
| CP | D | IMU (2) | 13, 14 | CNN | 120 samples | Acc = 92% | 9 p, 9 h | 2020 | [97] |
| CA | D | Accel (6) | 1, 3, 13, 14, 16, 27 | ANN | DFT features | AUC = 0.98 | 25 p | 2021 | [98] |
| CA / PD | SA | IMU (2) | 15, 28 | Naive Bayes | 6 feature sets | Acc = 77.1%, 78.9%, 89.9%, 98.0%, 98.5% for 5 places | 62 p, 24 h | 2021 | [99] |
| CA | SA | IMU (1) | 6 | GPR + GPC | 53 features | Acc = 88.24% | 88 at, 44 pd, 34 h | 2021 | [100] |
| HD | SA | Accel (3) | 2, 20 | Meta-classifier | 234 features | RMSE = 3.6, R = 0.69 | 234 features | 2018 | [101] |
| PSP | D | IMU (6) | 2, 20, 26, 30 | RF | 17 features | Acc = 98.78%, R = 0.77, MAE = 12.41% | 21 psp, 20 pd, 39 h | 2020 | [102] |
| MS | SA | IMU (1) | 15 | RF | 6 gait features | Sen = 86% (PSP/PD), | 49 p | 2020 | [64] |
| BI | PA | Accel (1) | 32 | RF | statistical features | 90% (PSP/HC) | 25 p, 11 h | 2021 | [52] |
| SCI | PA | Accel (1) | 11 | SVM | temporal features | MAE = 1.38 | 13 p | 2017 | [45] |
| BI/Stroke | CE | Accel (5) | 2, 5, 6, 8, 9 | GPR | temporal features | Sen = 88.3–90.4% | 44 p | 2021 | [56] |
| BPI | CE | IMU (3) | 2, 18 | Ensemble | 20 features | Acc = 91.6%, 85.9% (at home) | 15 p, 15 h | 2021 | [103] |
| Seizure | D | Accel (4) | 20, 25 | LS-SVM | 140 features | RMSE = 6.9%, R = 0.94 | 51 p | 2017 | [104] |
| VS | D | IMU (5) | 11, 23, 26 | SVM | 22 features | Acc = 93%, R = 0.55–0.76 | 16 p, 21 h | 2020 | [62] |
| General | D | IMU (2) | 34 | SVM | 8 gait features | multiple | 36 p, 13 h | 2020 | [105] |
| General | CE | IMU (4) | 23, 24 | SVM | 16 gait features | Acc = 89.2% | 25 p, 24 h | 2017 | [106] |
| General | CE | IMU (1) | 6 | MLP | statistical features | Acc = 93.9% | 10 p | 2019 | [107] |
| Spasticity | SA | IMU (1) | 6 | RF | 2 feature sets | Acc = 91.61% | 50 p | 2020 | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, F.; Yerebakan, M.; Dai, Y.; Wang, W.; Li, J.; Hu, B.; Gao, S. IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare 2022, 10, 1210. https://doi.org/10.3390/healthcare10071210
Bo F, Yerebakan M, Dai Y, Wang W, Li J, Hu B, Gao S. IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare. 2022; 10(7):1210. https://doi.org/10.3390/healthcare10071210
Chicago/Turabian StyleBo, Fan, Mustafa Yerebakan, Yanning Dai, Weibing Wang, Jia Li, Boyi Hu, and Shuo Gao. 2022. "IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review" Healthcare 10, no. 7: 1210. https://doi.org/10.3390/healthcare10071210
APA StyleBo, F., Yerebakan, M., Dai, Y., Wang, W., Li, J., Hu, B., & Gao, S. (2022). IMU-Based Monitoring for Assistive Diagnosis and Management of IoHT: A Review. Healthcare, 10(7), 1210. https://doi.org/10.3390/healthcare10071210







